Pain is a complex experience shaped by biological, emotional, cognitive and social factors that all can be influenced by ethnicity/race [37]. Race is associated with altered pain responsiveness in adults [10]. In the clinical setting, African-American adults report greater pain unpleasantness [28] and pain intensity [9] than Non-Hispanic Whites with comparable pain conditions. Similarly, in the laboratory setting, African-Americans consistently exhibit greater evoked pain responsiveness compared to Non-Hispanic Whites [28]. Such racial differences in pain responsiveness might help explain, in part, the higher prevalence of chronic pain found in African-Americans compared to Non-Hispanic Whites [27].
Two significant gaps in this literature are notable. First, few controlled evoked pain studies have been conducted in healthy youth [3,21,25]. The only such study addressing race/ethnicity found that African-American youth reported lower evoked pain intensity than Non-Hispanic White youth [21]. It remains unclear at what point in development observed racial differences in pain responding emerge. Second, there is a dearth of studies on racial differences in temporal summation [28]. Temporal summation of second pain (TSSP) refers to increases in perceived pain intensity evoked by repetitive nociceptive stimuli administered at constant intensity. Whereas pain tolerance may be influenced by nociceptive stimulus intensity, pain sensitivity [15], and descending pain inhibition [2], TSSP provides a more specific index of pain sensitivity [35], one factor potentially contributing to chronic pain risk [14,44]. Elevated TSSP has been demonstrated in chronic pain conditions including functional abdominal pain [9], fibromyalgia [35], low back pain [6], and temporomandibular disorders [24]. In one large prospective study, higher heat pain TSSP was associated with increased risk for subsequent temporomandibular disorder onset [14].
Few studies have investigated race effects on TSSP. One recent study showed higher TSSP in older African-Americans compared to older Non-Hispanic Whites [30]. Although not directly addressing racial differences in TSSP levels, another recent study in adults showed that higher TSSP predicted greater clinical pain ratings for Non-Hispanic White but not African-American participants [13]. To our knowledge, whether racial differences in TSSP are present in pain-free youth has not previously been examined. If healthy African-American youth demonstrate elevated TSSP relative to Non-Hispanic White youth, this might suggest that racial differences in pain responsiveness emerge relatively early in life and may be linked to differences in spinal cord processing of nociceptive stimuli.
Our primary hypotheses in the current study were that African-American youth would display both elevated evoked pain responsiveness and greater TSSP compared to Non-Hispanic White youth. Prior work [1] had demonstrated substantial response variability to TSSP protocols in healthy individuals, revealing discrete subgroups showing TSSP, no TSSP, or temporal decrease in second pain. Although the mechanisms contributing to temporal decrease in second pain are unclear, it may be driven by high first pulse pain ratings (i.e., ceiling effects) [1] or by habituation. We therefore adopted a two-stage analytic approach, examining race effects on TSSP first as a continuous measure over the temporal summation trials, and then as a categorical measure based on the three response groups described above.
Method
Participants
Participants were recruited from the Adolescent and Young Adult Health Clinic at the Monroe Carrell Jr. Children’s Hospital at Vanderbilt University and from a research recruitment website through the Vanderbilt Kennedy Center. Study procedures were approved by Meharry Medical College and Vanderbilt University Institutional Review Boards. All subjects and their parents provided written informed assent/consent prior to beginning study procedures.
Exclusion criteria were as follows: chronic pain (defined as clinical pain ≥ three months in duration), use of prescription opioid analgesics, learning difficulties requiring full-time special education services, sunburn or painful dermatological conditions at the time of the laboratory assessment, and pregnancy. All females who reported having had menarche provided urine samples for a pregnancy test prior to the pain-testing protocol (no female participants were excluded due to pregnancy). Of the 78 participants who enrolled in the study, one could not complete the laboratory portion due to an equipment malfunction and two more did not contribute data to the 48°C temporal summation protocol due to experimenter error. Thus, the final sample included in multilevel analyses consisted of 75 participants.
Measures
Pubertal maturation
Tanner staging was conducted based on pictorial representations of genital/breast development provided by youth self-report [22,23]. Tanner stages one and two reflect development up to the onset of puberty and tanner stages three to five reflect post-pubertal development. A dichotomous score was derived for each participant (stages one or two = 0; stages three to five = 1).
Somatic symptoms
The Children’s Somatization Inventory revised form (CSI) [39] was used to determine the perceived severity of somatic symptoms (e.g., headache, dizziness, nausea, back pain) in the past 2 weeks. Participants reported how much they were bothered by 24 specific somatic symptoms on a 5-point scale ranging from “not at all” (0) to “a whole lot” (4). Items were summed and total scores ranged between 0 and 96. In this sample, coefficient alpha for the CSI was .84.
Functional disability
The Functional Disability Inventory (FDI) [7,41] was used to determine the perceived impact of general physical health on psychosocial and physical functioning. Participants reported the degree of difficulty they would have performing 15 specific activities due to their physical health on a 5-point scale ranging from 0 to 4. Items were summed and total scores ranged between 0 and 60. In this sample, coefficient alpha for the FDI was .91.
Pain Testing Procedures
Pain tolerance and TSSP were assessed with a Medoc TSA-II Neurosensory Analyzer using commercially available software (TPS-CoVAS version 3.19, Medoc Inc.). To determine pain tolerance, a thermode (30 × 30 mm) was attached to the ventral forearm of the participant’s non-dominant arm and moved upwards on the forearm to a new location for each trial. Participants were instructed to terminate the stimulus by clicking on a computer mouse “when you can’t stand the heat pain any longer.” The protocol started at an adaptation temperature of 40°C and the temperature increased at a ramp rate of 0.5°C per second until the participant indicated that the maximum tolerance was reached. The maximum temperature limit was 51°C. Four pain tolerance trials were conducted, with a 25 second interstimulus interval during which the thermode was moved to a new, non-overlapping location. TSSP was then assessed using a standardized oscillating thermal stimulation protocol employed previously by the authors [6,9] and others [12]. Anticipatory anxiety prior to this protocol (i.e., “how nervous, afraid, or worried are you about the activity?”) was assessed by participant self-report using a numerical rating scale (0 = “Not at all” and 10 = “Worst imaginable”). A sequence of ten heat pulses with a 48°C target stimulus intensity was applied to the ventral forearm. Each pulse was 0.5 seconds in duration, started at a temperature of 40°C, with sequential pulses administered at a frequency of 0.4 Hz. During temporal summation procedures, participants rated the intensity of pain sensation shortly after the peak of each heat pulse using a numerical rating scale (0 = “No Pain” and 10 = “Worst Possible Pain”). The current protocol has been used to demonstrate chronic pain-related differences in temporal summation between healthy controls and both chronic low back pain patients [6] and functional abdominal pain patients [9].
Data Analytic Plan
Racial differences in pain tolerance to the four tolerance trials were examined using t-tests. Normality assumptions for pain tolerance analyses were tested using Levene’s test for equality of variances. Temporal summation outcomes were first examined as a continuous measure. To address hypotheses regarding within- and between-individual changes simultaneously, we specified a series of multilevel models (MLM) using hierarchical linear models (HLM 6) [29] consisting of a within-person (i.e., level-1) sub-model describing how each individual’s pain ratings changed over successive pulses, and a between-person (i.e., level-2) sub-model describing how these changes varied across individuals [4,34].
The Level 1 model was as follows:
The Level 2 model was as follows:
In this equation, Painti indicates the numerical pain rating (0 to 10) at pulse t for person i, Pulse denotes the pulse number (1 to 10), and Race denotes African-American (0) or Non-Hispanic White (1). Of primary interest was the interaction between race and pulse (β11). Normality assumptions were checked using a Q-Q plot of residuals.
Temporal summation group assignment for categorical analyses was determined based on regression coefficients estimated individually for each participant’s pain ratings according to procedures described by Pfister and colleagues [26]. Participants with positive and significant regression slopes were assigned to the TSSP group; those with negative and significant regression slopes were assigned to the temporal decrease in second pain group; those with non-significant regression slopes were assigned to the no TSSP group. Racial differences in the frequency distribution of temporal summation group assignment were examined using χ2 analyses.
Results
Table 1 presents descriptive data across the African-American and Non-Hispanic White groups. The sample was comprised of 78 healthy youth, ages 10 to 17 (mean age = 14.8, SD = 1.8). Recruitment focused on this age range to capture pain experiences across the pubertal transition. The sample was relatively balanced in terms of both race and gender, consisting of 20 African-American males (26%), 20 African-American females (26%; two identified as Hispanic), 18 Non-Hispanic White males (22%), and 20 Non-Hispanic White females (26%). Race and sex were based on self-report. Two participants discontinued the temporal summation protocol prior to the 10th pulse because they provided a maximum pain intensity rating of 10; their data were included in MLM and categorical analyses. No racial differences were observed in sex distribution, age, Tanner stage, anticipatory anxiety prior to temporal summation protocol, somatic symptoms, or functional disability due to physical health. Preliminary MLM analyses examined the impact of baseline pain rating and anticipatory anxiety rating during the temporal summation protocol, tanner stage, sex, and the race X sex interaction on within-individual changes in pain ratings during the temporal summation task. Neither baseline pain rating (p > .10), anticipatory anxiety rating (p > .80), tanner stage (p > .40), sex (p > .05), nor the sex X race interaction (p > .10) were associated with the degree of TSSP; including these variables in MLM analyses did not significantly alter findings reported below for race effects.
Table 1.
| African-American (n = 40) |
Non-Hispanic White (n = 38) |
African-American vs. Non-Hispanic White |
|
|---|---|---|---|
| N (%) | N (%) | X2 | |
|
|
|||
| Sex | 0.05 | ||
| Male | 20 (50) | 18 (47) | |
| Female | 20 (50) | 20 (53) | |
| Tanner Stage† | 0.13 | ||
| 1,2 | 3 (8) | 4 (11) | |
| 3-5 | 34 (92) | 34 (89) | |
|
|
|||
| M (SD) | M (SD) | t | |
|
|
|||
| Age | 14.6 (2.0) | 15.0 (1.6) | 1.19 |
| Somatic symptoms | 7.3 (5.9) | 9.8 (8.8) | 1.45 |
| Functional Disability | 3.6 (6.6) | 3.7 (7.0) | 0.07 |
| Anticipatory anxiety* | 2.3 (2.2) | 2.0 (2.1) | 0.67 |
Three African-American participants chose not to answer this question.
Assessed prior to temporal summation protocol.
Race and Pain Tolerance
Means and standard error of the mean (SEM) for pain tolerance are presented for African-Americans and Non-Hispanic Whites in Figure 1. Comparisons for each trial revealed no group differences for the first three trials, but that African-Americans had lower pain tolerance compared to Non-Hispanic Whites in the fourth trial (mean tolerance in African-Americans = 46.6°C, mean tolerance in Non-Hispanic White = 47.4°C, t = 1.98, p = .05; Levene’s test indicated unequal variances on the fourth trial [F = 5.27, p = .03], so degrees of freedom were adjusted from 75 to 72.5).
Figure 1.
Pain Tolerances for African-Americans and Non-Hispanic Whites. *p = .05.
Race and Temporal Summation
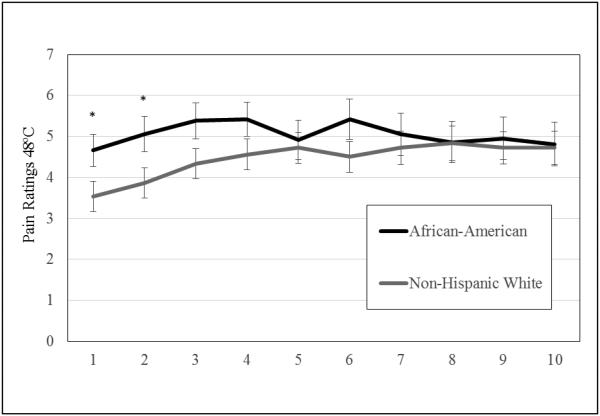
Changes in mean pain ratings across pulses in African-Americans and Non-Hispanic Whites are presented in Figure 2. African-American participants reported higher pain ratings for the baseline (mean rating in African-Americans = 4.67, mean rating in Non-Hispanic Whites = 3.53, t = 2.13, p = .04) and second pulses (mean rating in African-Americans = 5.05, mean rating in Non-Hispanic Whites = 3.86, t = 2.08, p = .04), but not subsequent pulses.
Figure 2.
Temporal Summation Pain Ratings for African-Americans and Non-Hispanic Whites. *p < .05.
The race X pulse interaction was significantly associated with changes in pain ratings (b = .11, SE = .03, p < .001) (Figure 3). Simple slope analysis revealed that pain ratings increased across successive pulses for Non-Hispanic Whites (b = .12, SE = .02, p < .001), but did not change significantly for African-Americans (b = .01, SE = .02, p = .76). Baseline pain ratings were significantly higher in African-Americans compared to Non-Hispanic Whites (b = −1.20, SE = .59, p = .04). Hence, African-Americans started with higher pain ratings at baseline and showed minimal TSSP, whereas Non-Hispanic Whites had lower pain ratings at baseline and did show evidence of TSSP. As noted above, adjustment for baseline pain differences did not alter the findings regarding racial differences in TSSP, nor did adjustment for anticipatory anxiety ratings.
Figure 3.
Multilevel Model Testing the Race by Time Interaction as a Predictor of Temporal Summation Pain Ratings. *p < .001.
Racial Distribution of Temporal Summation Groups
Individual regression slope analyses resulted in the assignment of 22 participants to the TSSP group (29.3%), 6 participants to the temporal decrease in second pain group (8.0%), and 47 participants to the no TSSP group (62.7%). There was a significant relationship between race and response group assignment [χ2 (2) = 6.09, p = .05]. Whereas similar numbers of African-Americans and Non-Hispanic Whites were assigned to the TSSP (n’s = 10 and 12, respectively) and no TSSP groups (n’s = 23 and 24, respectively), the temporal decrease in second pain group consisted entirely of African-Americans (n = 6, 15% of this racial group).
Discussion
African-American adults exhibit higher evoked pain responsiveness and report greater chronic pain intensity and unpleasantness than Non-Hispanic White adults with comparable pain conditions [10,31]. This study examined whether similar racial differences in evoked pain responsiveness are present in youth and whether these differences might be linked to differences in TSSP. Results were to some extent consistent with meta-analytic evidence of elevated pain responsiveness in African-American adults [28]: African-American youth gave higher initial pain ratings during a standardized thermal pain stimulus (TSSP) protocol and exhibited marginally lower heat pain tolerance than Non-Hispanic White youth. The only other known study to investigate the impact of race on evoked pain responsiveness in youth did not detect differences in pain tolerance among African-American, Caucasian, Hispanic and Asian participants [21]. The authors speculated that this null finding may have been linked to their use of a heat stimulus (infrared light) that differed from the direct skin contact heat stimuli more commonly utilized in adult protocols [21]. A second possibility is that racial differences in pain responsiveness may only emerge later in adolescence (mean ages of the current sample and the sample described by Lu and colleagues [21] were 14.8 and 12.7 years, respectively).
Contrary to our hypothesis, TSSP was lower in African-American than Non-Hispanic White youth. One prior study of healthy young adults (n = 188) found that heat pain tolerance and temporal summation loaded on different factors, with African-Americans (n = 11) being proportionally more represented in the high pain sensitivity cluster relative to other ethnic groups and no African-Americans assigned to the temporal summation cluster [17]. A second study conducted in middle-aged and older adults revealed higher TSSP in older compared to younger African-Americans, and the reverse pattern for Non-Hispanic Whites [30]. In light of these latter findings, the current results suggest that race could impact the developmental course of TSSP: whereas African-Americans may exhibit lower TSSP than Non-Hispanic Whites in adolescence and young adulthood, they may have higher TSSP later in life compared to Non-Hispanic Whites. Prospective studies are needed to determine whether developmental factors influence TSSP in African-Americans, and if changes in TSSP impact on age-related increases in the prevalence of chronic pain conditions in this population [18].
TSSP protocols are especially sensitive to age-related changes in pain perception [11,16,20] due to increasing reliance on C-fiber-mediated pain responding in older individuals [6]. Temporal decreases in second pain at the leg have previously been reported in pain-free older adults and were interpreted as reflecting an age-related decrease in central nervous system nociceptive processing [13]. Researchers have also speculated that diminishing TSSP in the elderly could reflect age-related changes in peripheral nervous system morphology (e.g., loss of unmyelinated nerve fibers) and function (e.g., decrease in nerve conduction velocity and sensory discrimination) [30,38]. However, the mechanisms that contribute to potential increases in TSSP in African-Americans over the life span have yet to be examined.
African-Americans consistently exhibit higher chronic pain ratings [10,31] and greater evoked pain responsiveness [28] than Non-Hispanic Whites. The current work may extend these findings to a younger population, suggesting elevated evoked pain responsiveness in African-American youth, although findings in this regard were not consistently observed across measures nor large in magnitude. Intriguingly, the present study also suggests that African-American youth show lower TSSP than Non-Hispanic Whites. This result appears to be driven in part by greater variability in TSSP slopes among African-American as compared to Non-Hispanic youth, who were distributed across all three response groups. Although the mechanisms contributing to lower overall TSSP and greater incidence of temporal decrease in second pain in African-American relative to Non-Hispanic White youth are unknown, the current study suggests that elevated TSSP cannot account for racial differences in evoked pain responsiveness, at least among younger persons, given that approximately equal numbers of African-American (n = 10) and Non-Hispanic White youth (n = 12) were assigned to the TSSP group.
Across racial groups, the distribution of TSSP, no TSSP, and temporal decrease in second pain groups for youth in the current study was similar to the distribution reported for pain-free adults by Anderson and colleagues [1]: 28.7% in TSSP group; 58.7% in no TSSP group; 12.6% in the temporal decrease in second pain group. However, analyses examining racial differences in the distribution of temporal summation groups revealed that temporal decrease to second pain occurred exclusively in African-American youth. One possible interpretation is that temporal decreases in second pain may reflect habituation effects, with current findings suggesting racial differences in habituation to pain among youth. A study examining neural correlates of pain perception found diminishing pain intensity ratings over repeated heat pain stimuli in women with interpersonal violence exposure (IPV) and a link between right anterior insula deactivation and avoidance symptoms [36]. Given the high prevalence of IPV in African-Americans and associated use of avoidant coping strategies [43], future studies should examine whether prior IPV exposure and/or avoidance could facilitate habituation to pain. A second possibility is that skin pigmentation could impact heat pain responsivity, as has been reported in animal studies showing shorter response latencies for pigmented regions in radiant heat tail flick assays [42]. Additional work is required to address these possibilities.
Limitations of the present study provide directions for future research. First, this sample consisted of adolescents without chronic pain who reported low levels of somatic symptoms and functional disability. It is not clear whether similar racial differences in TSSP would be observed in youth with chronic pain. Second, this study was cross sectional; prospective studies following youth into emerging adulthood will be necessary to determine whether age and race interact to determine evoked pain responsiveness and temporal summation, and to bridge the child and adult experimental pain literatures. Third, marginal differences between African-American and Non-Hispanic White youth in pain tolerance should be interpreted with caution given the possibility of Type I error and may not be clinically meaningful. Fourth, although studies suggest that experimental pain indices such as temporal summation are relatively stable when assessed over a period of one week [19], it remains to be seen whether these indices reliably discriminate between African-American and Non-Hispanic White youth over time. Future studies should investigate whether racial differences in experimental pain perception are linked mechanistically to racial differences in medication response and adverse effects [32,33]. In addition, whether racial differences in heat pain TSSP can be detected in healthy youth using other pain modalities (e.g., pressure) remains to be seen.
Despite these limitations, the current study demonstrated differences between African-American and Non-Hispanic White youth in evoked pain responsiveness and temporal summation, with the latter in an unanticipated direction. These findings highlight the importance of employing both static and dynamic pain assessment modalities. In addition, they identify important avenues for future research examining factors (e.g., socioeconomic, cultural, developmental, parenting, coping, neuroendocrine, genetic) that could explain racial differences in experimental pain responses. Childhood pain experiences increase the likelihood of developing chronic pain in adulthood [8,40], making early identification of at-risk youth a critical feature of targeted interventions. To the extent that these interventions include experimental pain tasks as indicators of risk or treatment response, the current findings underscore the importance of considering both developmental factors and race.
Acknowledgements
Research was funded in part by grants from the National Institute of Health (UL1 RR024975/TR000445, U54 RR026140/MD007593, G12 RR003032/MD007586, R01 MH068391, K01 MH101403, RO1 DA017805, R24 DA021471, R24 DA036420, R01 DA031726), by NICHD Grant P30HD15052 to the Vanderbilt Kennedy Center for Research on Human Development, by the Endowed Chair in Brain and Behavior Research at Meharry Medical College (Uma Rao) and by the Betsey R. Bush Endowed Professorship in Behavioral Health at the University of Tennessee (Uma Rao). These funding agencies had no further role in the study design, data collection, analysis or interpretation of data, writing of the report, or the decision to submit the manuscript for publication. The authors report no conflicts of interest. We gratefully acknowledge all individuals who participated in this study.
References
- [1].Anderson RJ, Craggs JG, Bialosky JE, Bishop MD, George SZ, Staud R, Robinson ME. Temporal summation of second pain: Variability in responses to a fixed protocol. Eur J Pain. 2013;17:67–74. doi: 10.1002/j.1532-2149.2012.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10:556–72. doi: 10.1016/j.jpain.2009.02.002. [DOI] [PubMed] [Google Scholar]
- [3].Blankenburg M, Boekens H, Hechler T, Maier C, Krumova E, Scherens A, Magerl W, Aksu F, Zernikow B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain. 2010;149:76–88. doi: 10.1016/j.pain.2010.01.011. [DOI] [PubMed] [Google Scholar]
- [4].Bryk AS, Raudenbush SW. Applications and data analysis methods. Sage; Thousand Oaks: 1992. Hierarchical linear models. [Google Scholar]
- [5].Chakour MC, Gibson SJ, Bradbeer M, Helme RD. The effect of age on A-delta and C-fibre thermal pain perception. Pain. 1996;64:143–52. doi: 10.1016/0304-3959(95)00102-6. [DOI] [PubMed] [Google Scholar]
- [6].Chung OY, Bruehl S, Diedrich L, Diedrich A. The impact of blood pressure and baroreflex sensitivity on wind-up. Anesth Analg. 2008;107:1018–25. doi: 10.1213/ane.0b013e31817f8dfe. [DOI] [PubMed] [Google Scholar]
- [7].Claar RL, Walker LS. Functional assessment of pediatric pain patients: Psychometric properties of the Functional Disability Inventory. Pain. 2006;121:77–84. doi: 10.1016/j.pain.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- [9].Dengler-Crish CM, Bruehl S, Walker LS. Increased wind-up to heat pain in women with a childhood history of functional abdominal pain. Pain. 2011;152:802–08. doi: 10.1016/j.pain.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerence: Clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–23. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- [11].Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: Clinical relevance in healthy older and younger adults. J Pain. 2001;2:307–17. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- [12].Fillingim RB, Edwards RR. Is self-reported childhood abuse history associated with pain perception among healthy young women and men? Clin J Pain. 2005;21:387–97. doi: 10.1097/01.ajp.0000149801.46864.39. [DOI] [PubMed] [Google Scholar]
- [13].Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R, Fessler BJ, Redden DT, Bradley LA, Fillingim RB. Temporal summation of pain as a prospective predictive of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: Ethnic differencies. Psychosom Med. 2014;76:302–10. doi: 10.1097/PSY.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Greenspan JD, Slade GD, Bair E, Dubner R, Fillingim RB, Ohrbach R, Knott C, Diatchenko L, Liu Q, Maixner W. Pain sensitivity and autonomic factors associated with development of TMD: The OPPERA prospective cohort study. J Pain. 2013;14:T63–T74. doi: 10.1016/j.jpain.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hansson P. Translational aspects of central sensitization induced by primary afferent activity: What it is and what it is not. Pain. 2014;155:1932–1934. doi: 10.1016/j.pain.2014.07.016. [DOI] [PubMed] [Google Scholar]
- [16].Harkins SW, Davis MD, Bush FM, Kasberger J. Suppression of first pain and slow temporal summation of second pain in relation to age. J Gerontol. 1996;51A:M260–M265. doi: 10.1093/gerona/51a.5.m260. [DOI] [PubMed] [Google Scholar]
- [17].Hastie BA, Riley JL, III, Robinson ME, Glover T, Campbell CM, Staud R, Fillingim RB. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–37. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [18].Helme RD, Gibson SJ. The epidemiology of pain in elderly people. Clin Geriatr Med. 2001;17:417–31. doi: 10.1016/s0749-0690(05)70078-1. [DOI] [PubMed] [Google Scholar]
- [19].Kong J-T, Johnson KA, Balise RR, Mackey S. Test-retest reliability of thermal temporal summation using an individualized protocol. J Pain. 2013;14:79–88. doi: 10.1016/j.jpain.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410–18. doi: 10.1016/j.pain.2005.03.025. [DOI] [PubMed] [Google Scholar]
- [21].Lu Q, Zeltzer L, Tsao J. Multiethnic differences in responses to laboratory pain stimuli among children. Health Psychol. 2013;32:905–14. doi: 10.1037/a0032428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maixner W, Fillingim R, Sigurdsson A, Shelly K, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: Evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- [25].Meier PM, Berde CB, DiCanzio J, Zurakowski D, Sethna NF. Thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve. 2001;24:1339–1345. doi: 10.1002/mus.1153. [DOI] [PubMed] [Google Scholar]
- [26].Pfister R, Schwarz K, Carson R, Jancyzk M. Easy methods for extracting individual regression slopes: Comparing SPSS, R, and Excel. Quant Meth Psych. 2013;9:72–78. [Google Scholar]
- [27].Plesh O, Adams SH, Gansky SA. Racial/Ethnic and gender prevalence in reported common pains in a national sample. J Orofac Pain. 2011;25:25–31. [PMC free article] [PubMed] [Google Scholar]
- [28].Rahim-Williams FB, Riley JL, III, Williams AKK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: Do biology, psychology, and culture matter? Pain Med. 2012;13:522–40. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Raudenbush SW, Bryk A, Cheong YF, Congdon R, du Toit M. HLM 6: Hierarchical linear and nonlinear modeling. Scientific Software International; Lincoln, IL: 2004. [Google Scholar]
- [30].Riley JL, III, Cruz-Almeida Y, Glover TL, King CD, Goodin BR, Sibille KT, Bartley EJ, Herbert MS, Sotolongo A, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Age and race effects on pain sensitivity and modulation among middle-aged and older adults. J Pain. 2014;15:272–82. doi: 10.1016/j.jpain.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Riley JL, III, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD. Racial/ethnic differences in the experience of chronic pain. Pain. 2002;100:291–98. doi: 10.1016/S0304-3959(02)00306-8. [DOI] [PubMed] [Google Scholar]
- [32].Sadhasivam S, Chidambaran V, Ngamprasertwong P, Esslinger HR, Prows C, Zhang X, Martin LJ, McAuliffe J. Race and unequal burden of perioperative pain and opioid related adverse effects in children. Pediatrics. 2012;129:832–38. doi: 10.1542/peds.2011-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sibille KT, Kindler LL, Glover TL, Gonzalez RD, Staud R, Riley JL, III, Fillingim RB. Individual differences in morphine and butorphanol analgesia: A laboratory pain study. Pain Med. 2011;12:1076–85. doi: 10.1111/j.1526-4637.2011.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- [35].Staud R, Bovee CE, Robinson ME, Price DD. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain. 2008;139:315–23. doi: 10.1016/j.pain.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Strigo IA, Simmons AN, Matthews SC, Grimes EM, Allard CB, Reinhardt LE, Paulus MP, Stein MB. Neural correlateds of altered pain response in women with posttraumatic stress disorder from intimate partner violence. Biol Psychiatry. 2010;68:442–50. doi: 10.1016/j.biopsych.2010.03.034. [DOI] [PubMed] [Google Scholar]
- [37].Turk DC. Biopsychosocial perspective on chronic pain. In: Gatchel RJ, Turk DC, editors. Psychological approaches to pain management: A practitioner’s handbook. Guilford Press; New York: 1996. pp. 3–32. [Google Scholar]
- [38].Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- [39].Walker LS, Beck JE, Garber J, Lambert W. The Children’s Somatization Inventory: Psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34:430–40. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Walker LS, Dengler-Crish CM, Rippel S, Bruehl S. Functional abdominal pain in childhood and adolescence increases risk for chronic pain in adulthood. Pain. 2010;150:568–72. doi: 10.1016/j.pain.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Walker LS, Greene JW. The Functional Disability Inventory: Measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;1991;16:39–58. doi: 10.1093/jpepsy/16.1.39. [DOI] [PubMed] [Google Scholar]
- [42].Wen T, Ansonoff MA, Pintar JE. The tail pigmentation pattern of C57BL/6J mice affects nociception/pain quantification in the tail flick test. Eur J Pain. 2009;13:564–67. doi: 10.1016/j.ejpain.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].West CM. Black women and intimate partner violence: New directions for research. J Interpers Violence. 2004;19:1487–93. doi: 10.1177/0886260504269700. [DOI] [PubMed] [Google Scholar]
- [44].Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheu. 2008;37:339–52. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]