Abstract
Regulatory T cells (Tregs) are a subset of CD4+ T cells that maintain immune tolerance in part by their ability to inhibit the proliferation of conventional CD4+ T cells (Tconvs). The role of the T cell receptor (TCR) and the downstream signaling pathways required for this suppressive function of Tregs are not fully understood. To yield insight into how TCR-mediated signals influence Treg suppressive function, we assessed the ability of Tregs with altered TCR-mediated signaling capacity to inhibit Tconv proliferation. Mature Tregs deficient in SLP-76, an adaptor protein that nucleates the proximal signaling complex downstream of the TCR, were unable to inhibit Tconv proliferation, suggesting that TCR signaling is required for Treg suppressive function. Moreover, Tregs with defective PLCγ activation due to a Y145F mutation of SLP-76 were also defective in their suppressive function. Conversely, enhancement of diacylglycerol-mediated signaling downstream of PLCγ by genetic ablation of a negative regulator of diacylglycerol kinase ζ increased the suppressive ability of Tregs. Since SLP-76 is also important for integrin activation and signaling, we tested the role of integrin activation in Treg-mediated suppression. Tregs lacking the adaptor proteins ADAP or Crk/CrkL, which are required for TCR-mediated integrin activation, inhibited Tconv proliferation to a similar extent as wildtype Tregs. Together, these data suggest that TCR-mediated PLCγ activation but not integrin activation is required for Tregs to inhibit Tconv proliferation.
Introduction
Regulatory T cells (Tregs) are a subset of CD4+ T cells with the ability to both limit immune responses during infection and inhibit autoimmunity in the steady state. The critical role of these cells is exemplified in the setting of Treg deficiency in both mice and humans, where a lack of functional Tregs results in lethal autoimmune pathology (1–4). Tregs additionally mediate peripheral tolerance through suppression of immune responses against food antigens, inhaled particles, and microbiota at barrier surfaces (5–8). For these reasons, an understanding of the suppressive capabilities of these cells is of extreme importance.
Like conventional T cells, TCR stimulation of Tregs results in the activation of protein tyrosine kinases that phosphorylate immunoreceptor tyrosine-based activation motifs within CD3 chains of the TCR (9). This phosphorylation allows the tyrosine kinase ZAP-70 to be recruited to the proximal TCR signaling complex, where it phosphorylates two key adaptor proteins, linker for activated T cells (LAT) and Src homology 2 (SH2) domain-containing leukocyte protein of 76 kDa (SLP-76) (10). Phosphorylation of LAT and SLP-76 allows them to form a stable signaling complex that serves as a scaffold for several downstream molecules, including phospholipase C γ1 (PLCγ1). Production of the second messengers inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG) by activated PLCγ1 leads to downstream activation of key transcription factors including NFAT, AP-1 and NF-κB that are required for cellular responses including activation, proliferation, and survival (11).
While thymic Treg are derived from developing thymocytes that receive relatively strong TCR signals through high-affinity TCR/self-MHC interactions (12–16), the role of TCR signaling during peripheral functional responses remains less clear. Several groups have shown that TCR signals are required by Tregs for full suppressive function (12–14, 16, 17). However, some have argued that TCR signals are not an absolute requirement for Treg suppressive function, since molecules such as CTLA-4 and CD25 that are constitutively expressed by Tregs could mediate suppression (18). CTLA-4 molecules on the surface of Tregs compete with costimulatory molecules on other T cells for interactions with B7 receptors on DCs (19) and actively remove CD80/CD86 from DCs through trans-endocytosis (20). CTLA-4 can also induce production of the tryptophan-catabolizing enzyme IDO by DCs, which depletes tryptophan from the environment to impede T cell activation and proliferation (21). In addition to CTLA-4, Tregs express the high-affinity IL-2 receptor CD25, which has been suggested to act as an “IL-2 sink,” binding up IL-2 released during an immune response to prevent IL-2-mediated division and survival of nearby Tconvs (22–24).
More recently, it has been shown that the catalytic function of ZAP-70 is not requisite for Treg suppressive function, as long as scaffolding aspects of the protein remain intact (25). This is somewhat surprising, since the ability of ZAP-70 to phosphorylate LAT and SLP-76 has long been considered an absolute requirement for TCR-mediated signaling, and indeed, ZAP-70 catalytic function was necessary for several other TCR-mediated functions assessed in the study (10, 25). Interestingly, downstream activation of integrins remained intact in cells harboring catalytically inactive ZAP-70, and adhesion to ICAM-1 was instead dependent upon the phosphorylation of ZAP-70 at sites unrelated to its catalytic function. Additionally, mutation of these same phosphorylation sites abrogated Treg suppressive function (25). These findings suggest ZAP-70 to have scaffolding properties that are both required and sufficient for integrin activation and Treg suppressor function. Since disruption of surface integrin expression has also been shown to impede Treg function (26, 27), it seems that TCR-driven suppression may occur at least partly through TCR-driven activation of integrins that facilitate interactions with APCs.
In this study, we assessed the role of TCR-mediated signal transduction in the suppressive function of Tregs. Our data suggest that TCR-mediated signals downstream of PLCγ1 but not those leading to integrin activation are required for the suppressive function of Tregs.
Materials and Methods
Mice
C57BL/6 (B6), B6.SJL (CD45.1+), TCRβ/δ DKO, and B6 Foxp3.GFP reporter mice were purchased from The Jackson Laboratory. B6.SJL Foxp3.GFP reporter mice were generated by crossing B6.SJL mice to B6 Foxp3.GFP reporter mice. UBC-CreERT2 used for acute deletion has been described (28). SLP-76 conditional heterozygous (cHet), SLP-76 conditional knockout (cKO), and SLP-76 Y145F mice have been previously described (29, 30). Bcl-xL transgenic mice were a gift from Dr. Craig Thompson (31). SLP-76 cKO Bcl-xL mice were generated by crossing Bcl-xL transgenic mice to SLP-76 cKO mice. SLP-76 conditional Y145F (cY145F) mice were generated by crossing SLP Y145F mice to SLP-76 cKO mice. DGKζ KO mice were a gift from Drs. Gary Koretzky and Xiao-ping Zhong (32). Adhesion and degranulation promoting adapter protein (ADAP) KO mice were graciously provided by Dr. Gary Koretzky (33).
Conditional DGKζ KO (cDGKζ KO) mice were created by inGenious Targeting Laboratory (Ronkonkoma, NY). Briefly, DGKζ genomic DNA was replaced with a construct containing an intronic 5’ loxp site flanking a neomycin cassette inserted between exons 6 and 7, and a 3’ loxp site inserted between exons 11 and 12. Upon expression of cre recombinase, a truncated form of DGKζ is expressed that contains the first 187 amino acids of DGKζ followed by 8 unrelated amino acids expressed from frameshifted codons in exon 12, followed by a stop codon in exon 12. The expressed region of DGKζ contains one of the DAG-binding C1 domains and a portion of the second C1 domains, but no other functional or enzymatic components. For the inducible deletion of DGKζ, mice were subsequently bred to C57Bl/6 mice that express ER-cre recombinase fusion protein, such that Cre activity was induced in cells exposed to Tamoxifen. CT10 regulator of kinase (Crk)/Crk-like (CrkL) loxp-flanked (floxed) × CD4-cre mice (Crk/CrkL DKO) and CD4-cre negative littermate controls on a mixed B6×129 genetic background were generated by breeding Crk floxed/floxed:CrkL floxed/floxed mice to CD4-cre mice. All mice were at least 6 weeks of age at time of use and were housed in pathogen-free conditions and treated in strict compliance with Institutional Animal Care and Use Committee regulations of the University of Pennsylvania and the Children’s Hospital of Philadelphia.
Reagents and antibodies
Tamoxifen was purchased from Sigma-Aldrich (St. Louis, MO). Carboxyfluorescein succinimidyl ester (CFSE) and LIVE/DEAD Fixable Dead Cell stain was purchased from Molecular Probes, Invitrogen (Carlsbad, CA). Antibodies were purchased from either BD Pharmingen (San Diego, CA): anti-CD4 (RM4-5), anti-CD25 (PC61), anti-CD3 (2C11), and anti-CD28 (37.51); Biolegend (San Diego, CA): anti-CD45.2 (104), anti-CD45.1 (A20), and anti-CD4 (GK1.5), or eBioscience (San Diego, CA): anti-Foxp3 (FJK-16s), anti-CD69 (H1.2F3), anti-glucocorticoid-induced TNFR family related gene (GITR) (DTA-1), and anti-CTLA-4 (UC10-4B9).
Flow cytometry, cell sorting, and data analysis
For flow cytometric analyses, cells were stained with antibodies against surface antigens at 4°C for 20 minutes in PBS. Intracellular Foxp3 staining was performed with the Foxp3 Staining Set (eBioscience) according to the manufacturer’s protocol. Flow cytometry was performed with an LSR II or FACSCanto (BD Biosciences). For cell sorting, T cells were purified with either CD4 or CD90.2 magnetic beads using MACS columns (Miltenyi Biotec, Auburn, CA) according to the manufacturer’s protocol prior to cell surface staining. FACS was performed with a FACSAria cell sorter (BD Biosciences). FACS-sorted populations were typically of 95–99% purity. Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Cell division data from Treg CFSE dilution profiles, gated on live CD4+Foxp3− cells, were transformed into “Division Index” data using Flowjo’s “Proliferation” function. The “Division Index” measures the average number of division per cell. Suppressive ability of Tregs was normalized for each assay by calculating the “percent suppression” at each Treg dilution. Since baseline Tconv division (no cocultured Tregs, 0% suppression) can vary between experimental repeats, this calculation allowed for comparison of multiple assays. The division index of Tconvs cultured at a particular Tconv to Treg ratio was divided by baseline Tconv division for the same assay. This fraction was multiplied by 100 and subtracted from 100 to achieve the “% Suppression of Tconv Division” for the particular Treg genotype across all experimental repeats.
Tamoxifen treatment
Tamoxifen was resuspended in ethanol (1 g/mL), diluted in corn oil to a final concentration of 20 mg/mL, and heated on a 37°C cell shaker until dissolved. Mice were weighed on the first day of Tamoxifen administration and were treated with 200 µg Tamoxifen per gram body weight by oral gavage. Tamoxifen administration was repeated each day for five consecutive days.
Western blot analysis
YFP+CD4+ T cells were sorted from Tamoxifen-treated T2-Cre+ WT or cDGKζ KO mice (with a YFP-based Cre reporter) by flow cytometry. The cells were then lysed in 1% Ipegal in Tris-buffered saline with protease/phosphatase inhibitors (protease inhibitor cocktail solution [Roche, Sigma], 1 mM sodium orthovanadate, 50 mM sodium fluoride, 50 mM sodium pyrophosphate, 0.2 mM dichloroisocoumarin, and 1 mM benzamidine), and the proteins were resolved by SDS-PAGE (Bio-Rad Laboratories, Hercules, CA). Total DGKζ and β-actin were analyzed by Western blot. The antibodies against β-actin and DGKζ were obtained from Santa Cruz Biotechnology (Dallas, TX).
Treg suppression assays
MACS-enriched CD90.2+ or CD4+ T cells were FACS-sorted for CD4+CD25+ Tregs (CD45.2+), or were FACS-sorted for CD4+Foxp3− Tconvs (CD45.1+) from WT B6.SJL Foxp3.GFP reporter mice. The Tconvs were carboxyfluorescein succinimidyl ester (CFSE)-labeled and cultured at various ratios with Tregs in the presence of irradiated T cell-depleted CD45.2+ feeder cells and soluble anti-CD3 (0.5 – 1 µg/ml). CFSE labeling was performed by resuspending cells with PBS containing CFSE (5 mM) at 37°C followed by continuous shaking for 9 minutes. The reaction was then immediately quenched with 100% FBS, and the cells were washed prior to culture. CFSE dilution of Tconvs (CD4+CD45.1+) was assessed by flow cytometry after 4 days in culture. For in vivo suppression assays, T cell-deficient (TCRβ/δ DKO) mice were adoptively transferred with 7.5 × 105 Tconvs (CD4+CD25−GITR−CD44lo) from B6.SJL (CD45.1+) mice with or without 2.5 × 105 Tregs (CD4+CD25hiGITRhi) from B6 or Y145F KI mice (CD45.2+). 7 days later, peripheral lymph nodes were harvested and the absolute number of CD45.1+ Tconvs was determined by flow cytometry.
Generation of mixed bone marrow (BM) chimeras
Donor BM was depleted of T cells by CD4 and CD8 magnetic bead depletion (Miltenyi). T cell-depleted BM from CD45.1+CD45.2+ WT donor (competitor) mice were mixed at a 1:1 ratio with CD45.2+ donor BM (WT or DGKζ KO) and a total of 3–4 × 106 BM cells were injected i.v. into lethally irradiated (11 Gy) CD45.1+ recipient mice. Splenic Tregs were FACS-sorted from mixed BM chimeric mice 9–11 weeks post BM transplant.
Statistical analyses
All values were graphed and analyzed for statistical significance using Prism software. Paired or unpaired two-tailed Student’s t-test or the Mann Whitney test was used to calculate each p value as indicated in the figure legends. P values < 0.05 were considered statistically significant.
Results
Tregs require TCR signaling through SLP-76 to mediate suppressive function
To test the role of TCR signaling in Treg function, we assessed the in vitro suppressive function of Tregs lacking SLP-76. SLP-76-deficient T cells exhibit almost no measurable activation of signal transduction pathways emanating from the TCR (34). Since SLP-76 is necessary for T cell development, we utilized a previously described Cre-lox conditional deletion strategy (SLP-76 cKO) to delete a floxed copy of SLP-76 in mature mouse Tregs using a Tamoxifen-inducible system with a YFP-based Cre reporter (30). SLP-76 cKO and SLP-76 conditional heterozygous littermates (SLP-76 cHet mice) were treated with Tamoxifen for 5 days, followed by a 7 day rest period (Supplemental Fig. 1A), after which residual SLP-76 protein can no longer be detected in T cells (30). TCR-mediated CD69 upregulation was seen on peripheral blood YFP+CD4+ T cells taken from SLP-76 cHet but not SLP-76 cKO mice, suggesting that TCR signaling was indeed ablated in CD4+ T cells from SLP-76 cKO mice (Supplemental Fig. 1B). To assess the suppressive ability of mature Tregs lacking SLP-76, splenic YFP+CD25+CD4+ T cells from either SLP-76 cKO or SLP-76 cHet mice were sorted by flow cytometry. FACS-sorted Tregs were co-cultured with CFSE-labeled CD4+ Tconvs and irradiated splenocytes in the presence of anti-CD3 stimulation. After 4 days of co-culture, SLP-76 cHet Tregs but not SLP-76 cKO Tregs suppressed anti-CD3-mediated division of Tconvs, indicating that SLP-76 is required for Treg-mediated inhibition of Tconv proliferation (Fig. 1, A and B).
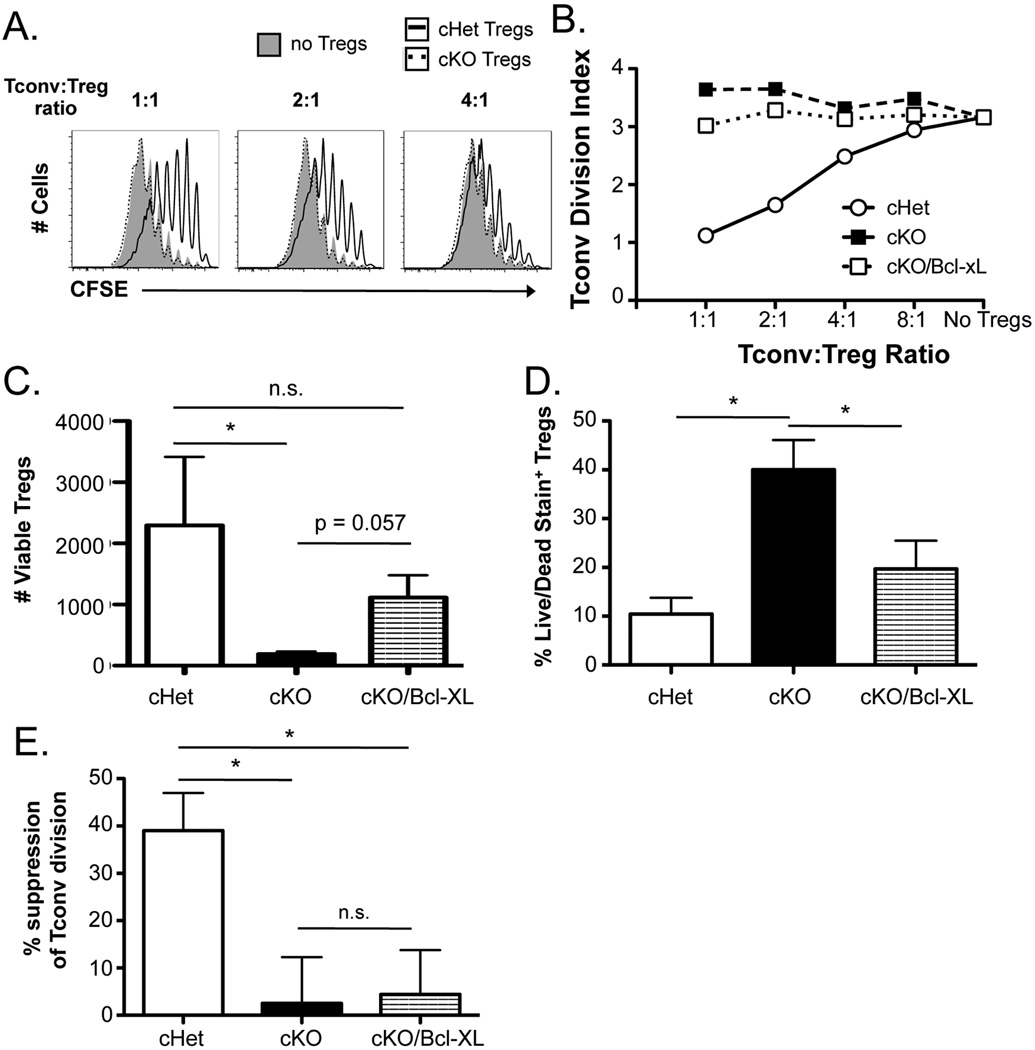
Figure 1. SLP-76-deficient Tregs display no suppressive ability in vitro.
(A) CFSE-labeled splenic CD4+Foxp3− cells (Tconvs) were co-cultured with YFP+CD4+CD25+ Tregs from Tamoxifen-treated SLP-76 cHet, SLP-76 cKO, or SLP-76 cKO/Bcl-xL mice for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. Representative CFSE plots from cultures lacking Tregs or containing a 1:1, 2:1, or 4:1 Tconv:Treg ratio are shown, and (B) the division index of Tconvs at various Tconv:Treg ratios is shown. (C) The absolute number of live and (D) the fraction of live/dead viability stain-positive SLP-76 cHet, SLP-76 cKO, and SLP-76 cKO/Bcl-xL Tregs from 4 independent experiments were determined using a viability dye in the 4-day co-cultures. (E) The division index of Tconvs at a 2:1 Tconv:Treg ratio was normalized to the baseline Tconv division (no Tregs) for each experiment (n = 4 experiments) and represented as mean % suppression ± SEM. n.s. = not significant, * indicates statistical significance of p < 0.05 by Mann Whitney test or unpaired two-tailed Student t-test.
Since SLP-76 cKO naïve and effector T cells display a survival defect (35, 36), we tested if a lack of fitness was responsible for the functional defect observed in SLP-76 cKO Tregs. The number of Tregs recovered from the suppression assays was significantly decreased in cultures with SLP-76 cKO compared to SLP-76 cHet Tregs (Fig. 1C). Moreover, the proportion of live/dead stain-positive SLP-76 cKO Tregs was significantly elevated compared to SLP-76 cHet Tregs (Fig. 1D). To enhance the survival of SLP-76 cKO Tregs, SLP-76 cKO mice were bred to mice transgenically expressing the pro-survival protein Bcl-xL in all T cells (31). Although forced Bcl-xL expression increased the in vitro survival of SLP-76 cKO Tregs (Fig. 1C, D), the suppressive function of SLP-76 cKO Bcl-xL Tregs was similar to SLP-76 cKO Tregs, which was significantly diminished compared to SLP-76 cHet Tregs (Fig. 1, A, B, and E).
It was recently shown that the acute deletion of the TCR leads to various phenotypic changes in the effector/differentiation state of Tregs in vivo (37, 38). To test the impact of SLP-76 ablation on the phenotype of Tregs used in our study, we measured the expression of various co-stimulatory molecules and effector/differentiation markers on SLP-76 cKO Tregs. The expression of CD62L, GITR, CTLA4, CD25, ICOS, CD39, CD103, and CXCR3 were similar between SLP-76 cHet and cKO Tregs while, of the markers examined, CD69 and KLRG1 were decreased in SLP-76 cKO Tregs compared to cHet Tregs (Supplemental Fig. 2). These data indicate that acute loss of TCR signaling in SLP-76 cKO Tregs impacts the expression of some, but not all Treg surface markers. Moreover, compared to SLP-76+/+ controls, the loss of one allele of SLP-76 (SLP-76 cHet) was sufficient to cause Tregs to variable extents display reduced expression of GITR, CTLA4, ICOS, KLRG1, CXCR3, and CD103 (Supplemental Fig. 2). Of the markers tested, a reduction in ICOS and KLRG1 was most consistently observed. This suggests that even partial attenuation of TCR signaling caused by the acute loss of one allele of SLP-76 can impact effector/differentiation markers on Tregs.
Tyrosine 145 of SLP-76 is required for optimal suppressive function of Tregs
TCR-induced activation of PLCγ1 is dependent on the phosphorylation of SLP-76 at tyrosine 145. To more selectively investigate the role of TCR-mediated PLCγ1 activation in Treg function, we utilized mice expressing a Y→F mutation at tyrosine 145 of SLP-76 (SLP-76 Y145F), which exhibit greatly diminished TCR-mediated activation of PLCγ1 (29). Similar to SLP-76 cKO Tregs, SLP-76 Y145F Tregs were significantly impaired in their ability to suppress Tconv division (Fig. 2, A and B). The viability of SLP-76 Y145F Tregs was similar to that of WT Tregs (Fig. 2C), suggesting that the defect in suppression was not due to decreased number of Tregs in the cultures. SLP-76 Y145F Tregs displayed some suppressive capacity at Treg to Tconv ratios of 2:1 or higher, suggesting that they were slightly more functional than SLP-76 cKO Tregs (data not shown).
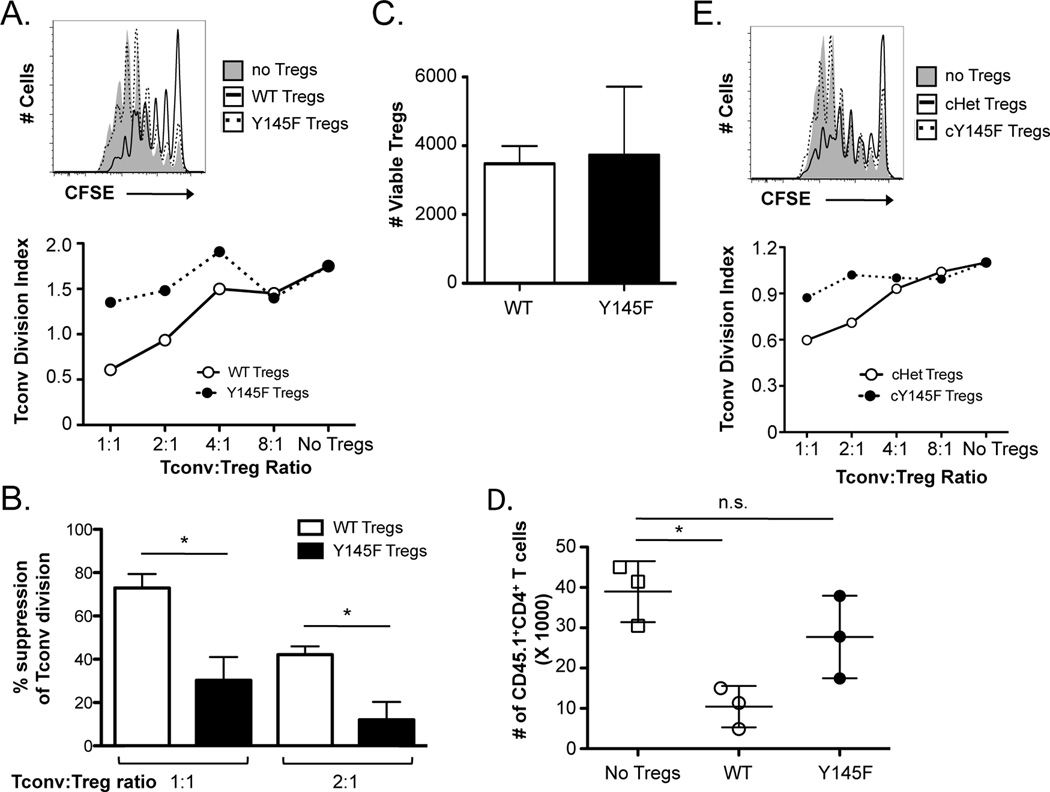
Figure 2. SLP-76 Y145F Tregs demonstrate greatly diminished in vitro and in vivo suppressive function.
(A) CD4+CD25+ Tregs were sorted from SLP-76 Y145F mice and co-cultured with CFSE-labeled CD4+Foxp3− cells (Tconvs) at various ratios for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. A representative CFSE plot from cultures lacking Tregs or at a 1:1 Treg:Tconv ratio is shown (top). A representative plot of the division index of Tconvs at various Treg:Tconv ratios is shown (bottom). (B) The percent suppression at a 1:1 and 1:2 Treg:Tconv ratio were compiled from 3 independent experiments and represented as mean ± SEM. (C) The absolute number of live WT and SLP-76 Y145F Tregs from 3 independent experiments was determined using a viability dye in the 4-day co-cultures. (D) TCRβ/δ DKO mice were adoptively transferred with Tconvs with or without Tregs from B6 or SLP-76 Y145F mice. The absolute number of Tconvs recovered is shown. One representative of two independent experiments is shown. (E) YFP+CD4+CD25+ Tregs from Tamoxifen-treated SLP-76 cHet or SLP-76 cY145F mice were co-cultured with CFSE-labeled Tconvs for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. A CFSE plot from cultures lacking Tregs or at a 1:1 Treg:Tconv ratio (top) and a plot of the division index of Tconvs at various Treg:Tconv ratios are shown (bottom). One representative of 2 independent experiments is shown. * indicates statistical significance of p < 0.05 by unpaired two-tailed Student t-test.
We next tested the in vivo function of SLP-76 Y145F Tregs. T cell-deficient mice were adoptively transferred with Tconvs with or without Tregs from WT or SLP-76 Y145F mice and 7 days later we assessed the number of T cells present in peripheral LNs. We found that the absolute number of Tconvs was significantly decreased in mice receiving WT Tregs compared to no Tregs (Fig. 2D). In contrast, the absolute number of Tconvs was not significantly different between mice receiving SLP-76 Y145F Tregs and no Tregs (Fig. 2D). Thus, SLP-76 Y145F Tregs also display functional defects in suppressing Tconv proliferation in vivo.
The SLP-76 Y145F mutation alters T cell selection in the thymus (29), which could contribute to the defective Treg function that was observed. Thus, we additionally tested the function of Tregs that express a floxed WT copy of SLP-76 in addition to SLP-76 Y145F (SLP-76 cY145F mice), such that a WT SLP-76 copy was present during T cell development but could be inducibly deleted in the periphery through a Tamoxifen-inducible Cre. Using a YFP-based cre reporter to detect cells with SLP-76 deletion, YFP+CD25+CD4+ T cells were FACS-sorted from SLP-76 cY145F mice and SLP-76 cHet mice that had been treated with Tamoxifen for 5 days and rested for 7 days. Similar to SLP-76 Y145F Tregs, SLP-76 cY145F Tregs also exhibited an attenuated ability to suppress Tconv division (Fig. 2E). Together, these results demonstrate that phosphorylation of SLP-76 is critical for optimal Treg suppressive function and suggest that PLCγ1 is an important downstream mediator of this process.
Enhancement of DAG signals increases the suppressive function of Tregs
One major outcome of PLCγ1 activation is the generation of the potent second messenger, DAG. To determine whether the selective enhancement of DAG signaling downstream of PLCγ1 augments the suppressive capacity of Tregs, we examined the function of Tregs from mice lacking the DAG-metabolizing kinase DGKζ, which exhibit prolonged DAG-mediated signaling downstream of PLCγ1 activation (32, 39, 40). To exclude cell-extrinsic effects that could be potentially brought out by germline DGKζ deficiency, mixed BM chimeric mice (CD45.1+ WT host) were generated with WT BM (CD45.1+/CD45.2+) mixed at a 1:1 ratio with either WT or DGKζ KO BM (CD45.2+). After reconstitution of the T cell compartment (9 – 11 weeks post-BM transplant), splenic Tregs (CD25+CD4+) or WT competitor (CD45.1+/CD45.2+) and of WT or DGKζ KO experimental (CD45.2+) origin were sorted by flow cytometry and their inhibitory activity against Tconv proliferation was tested. As expected, the suppressive ability was similar between WT competitor and WT experimental Treg populations sorted from WT/WT mixed BM chimeras (Fig. 3A). In contrast, Tregs of DGKζ KO BM origin exhibited significantly increased suppressive capacity compared Tregs of WT competitor origin from the same mouse (Fig. 3A). Since thymic selection is impacted by DGKζ deficiency (41), we generated mice with two floxed DGKζ alleles that were additionally crossed to a Tamoxifen-inducible Cre and YFP-based Cre reporter (cDGKζ KO mice). Treatment of these mice with Tamoxifen led to efficient deletion of DGKζ in T cells (Fig. 3B). Similar to DGKζ KO Tregs, FACS-sorted Tregs (YFP+CD25+CD4+) from DGKζ KO Tregs exhibited an enhanced ability to limit Tconv proliferation in vitro (Fig. 3, C and D). Especially striking was that the inhibition of Tconv division was still apparent by cDGKζ KO Tregs at a 1:8 and 1:16 Treg to Tconv ratio, dilutions at which WT Tregs are consistently unable to effectively mediate suppression (Fig. 3, C and D). Together, these data suggest that DAG pathways play a positive role in the suppressive function of Tregs.
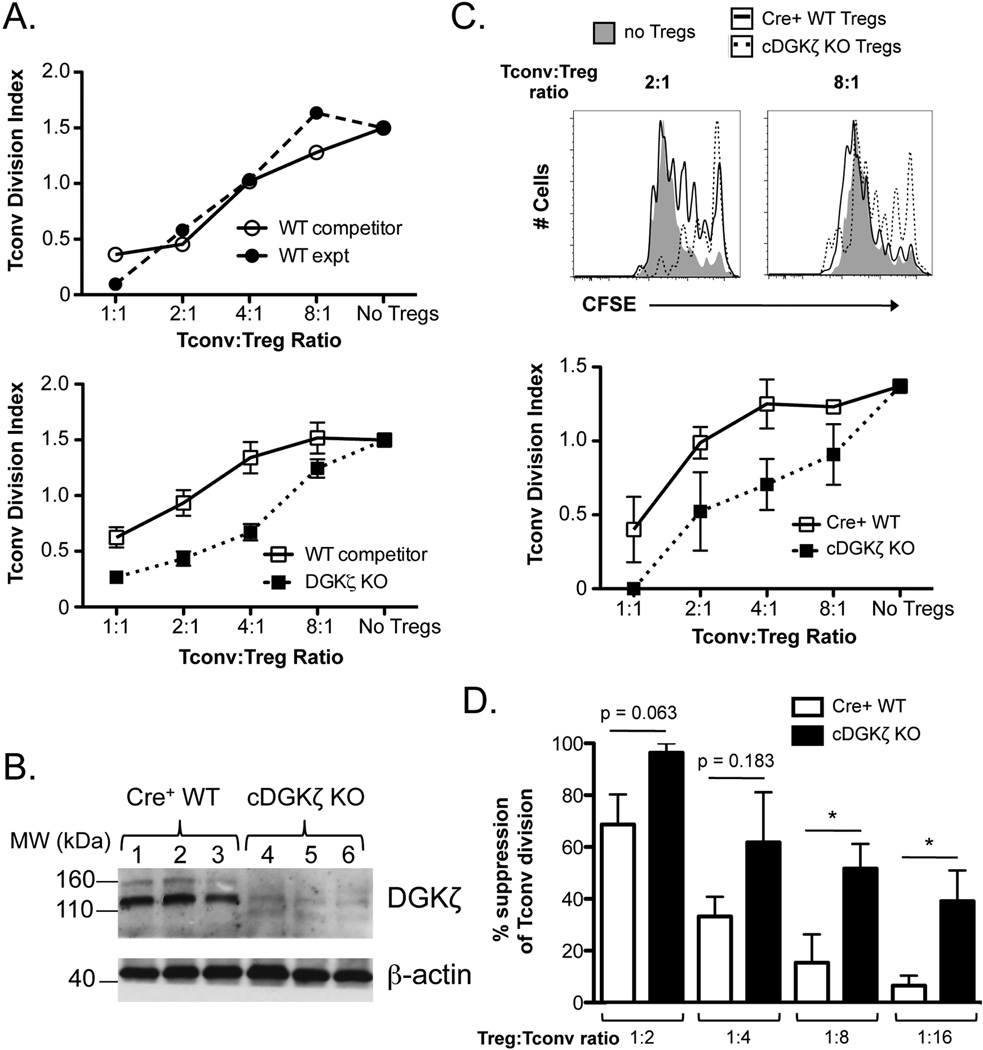
Figure 3. DGKζ KO Tregs exhibit enhanced suppressive capacity.
(A) CD4+CD25+ Tregs were sorted from WT/WT or WT/DGKζ KO mixed BM chimeras. WT competitor Tregs (CD45.1+/CD45.2+) or experimental Tregs (CD45.2+; WT or DGKζ KO) were cultured with CFSE-labeled Tconvs for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. The division index of Tconvs at various Treg:Tconv ratios is shown for cells sorted from WT/WT (top) and WT/DGKζ KO mixed BM chimeras (bottom). The data are represented as mean ± SEM of n = 3 mixed BM chimeras. One representative of 2 independent experiments is shown. (B) FACS-sorted YFP+CD4+ T cells from Tamoxifen-treated T2-Cre+ WT (lanes 1–3) or cDGKζ KO mice (lanes 4–6) were FACS-sorted, lysed, and analyzed for expression for DGKζ and β-actin by Western blot analysis. (C) YFP+CD4+CD25+ Tregs from Tamoxifen-treated T2-Cre+ WT and cDGKζ KO mice were co-cultured with CFSE-labeled Tconvs for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. Representative CFSE plots from four-day cultures lacking Tregs or at a 2:1 or 8:1 Tconv:Treg ratio of either Cre+ WT or cDGKζ KO Tregs (top) and a plot of the division index of Tconvs at various Tconv:Treg ratios are shown (bottom). The data are represented as mean ± SEM of n = 3 separate mice (bottom). One representative of 2 independent experiments is shown. (D) The division index of Tconvs at various Tconv:Treg ratios were normalized to the baseline Tconv division (no Tregs) for each experiment (n = 2 experiments) and represented as mean % suppression ± SEM (n = 4 mice total). * indicates statistical significance of p<0.05 by unpaired two-tailed Student t-test.
To test the impact of DGKζ ablation on the phenotype of Tregs used in our study, we measured the expression of various co-stimulatory molecules and effector/differentiation markers on DGKζ cKO Tregs. The expression of CD62L, GITR, CTLA4, CD25, ICOS, CD39, CD103, KLRG1, CD69, and CXCR3 were similar between DGKζ cKO and DGKζ+/+ Tregs (Supplemental Fig. 3). These data suggest that although the loss of TCR signaling by SLP-76 deficiency impacts the expression of Treg effector/differentiation markers, the specific increase in DAG-mediated signaling does not alter these markers on Tregs.
Tregs lacking proteins involved in TCR-mediated inside-out integrin activation display intact suppressive function
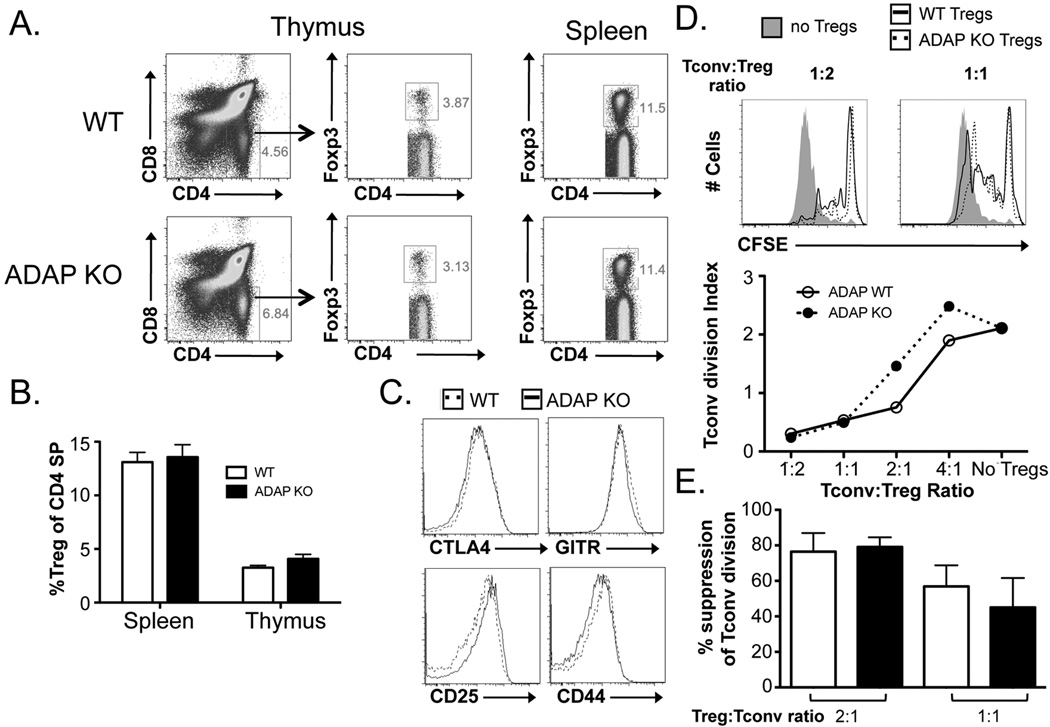
Signals from the TCR induce a conformational change whereby integrins achieve a high-affinity state in a process known as “inside-out” signaling (42). As integrin activation is important for Treg function (26, 27), we assessed the requirement of TCR-mediated inside-out integrin signaling in the ability of Tregs to inhibit Tconv division. We first tested the role of ADAP in Treg function, as ADAP is required for TCR-driven inside-out integrin signaling and is recruited to the immunological synapse by phosphorylated SLP-76 (43). ADAP KO mice exhibited normal Treg frequencies and numbers in both the thymus and spleen (Fig. 4A). Moreover, the expression of Treg cell-surface molecules associated with function, including CTLA-4, CD25, and GITR, appeared grossly normal upon flow cytometric analysis (Fig. 4). The function of FACS-sorted splenic ADAP KO and WT littermate control Tregs were compared. Surprisingly, in comparison to WT Tregs, ADAP KO Tregs exhibited normal ability to suppress Tconv division (Fig. 4C).
Figure 4. ADAP KO mice harbor Tregs with normal development and suppressive function.
(A) Representative flow cytometric plots and (B) the fraction of Tregs (out of CD4+ T cells) from the thymus and spleen of WT and ADAP KO Tregs are shown. (C) The expression of CTLA-4, CD25, GITR, and CD44 on Tregs from WT and ADAP KO Tregs is shown. (D) CFSE-labeled splenic CD4+Foxp3− Tconvs were cultured with either WT or ADAP KO Tregs for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. Representative CFSE plots from four-day cultures lacking Tregs or containing a 1:2 or 1:1 Tconv to Treg ratio of either WT or ADAP KO Tregs are shown (top). A plot of the division index of Tconvs at various Treg:Tconv ratios are shown (middle). (E) The division index of Tconvs at Tconv:Treg ratios of 1:2 and 1:1 were normalized to the baseline Tconv division (no Tregs) for each experiment and represented as mean % suppression ± SEM (n = 3 mice total).
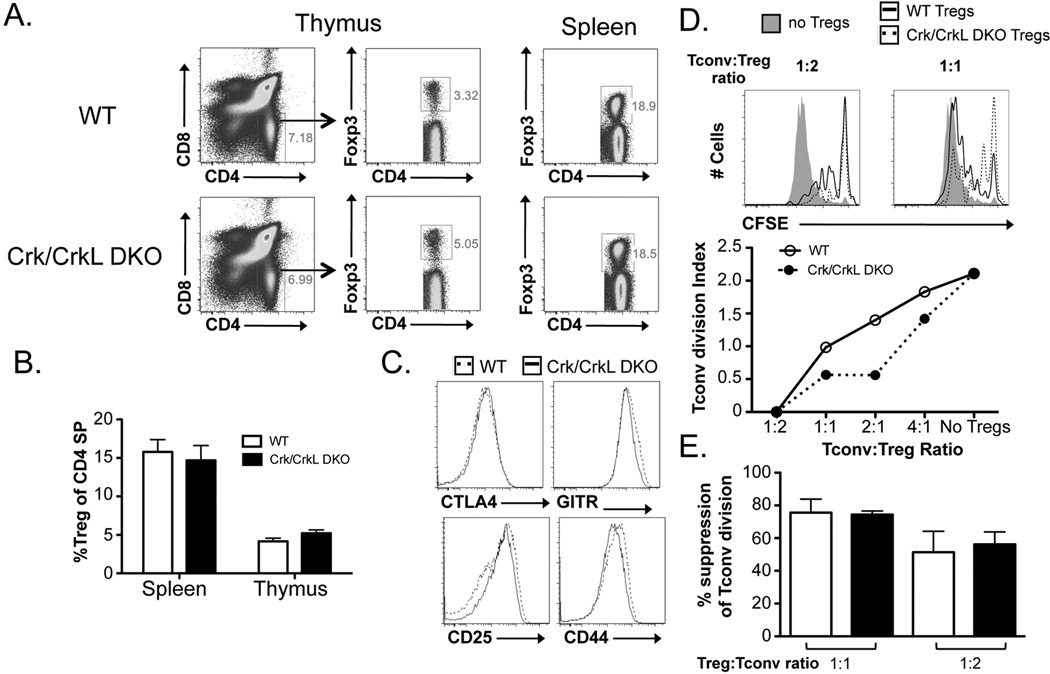
As a complementary approach, we additionally tested the role of the Crk family of proteins in Treg function. Crk and CrkL proteins associate with the guanine nucleotide exchange factor (GEF) C3G to promote activation of the GTPase Rap1 downstream of TCR signaling (44–46). Since Rap1 activation is critical for TCR-mediated inside-out activation of integrins such as LFA-1, Crk/CrkL double knockout (DKO) CD4+ T cells exhibit a partial defect in cellular adhesion upon TCR-induced activation (J. Burkhardt and Y. Huang, unpublished results). Flow cytometric analyses revealed a mild increase in Treg frequencies within the Crk/CrkL DKO thymus but not in the periphery (Fig. 5A). As observed for ADAP KO Tregs, the expression of CTLA-4, CD25, and GITR were similar between Crk/CrkL DKO Tregs and WT littermate control Tregs (Fig. 5B). Functional assessment of splenic Tregs (CD25+CD4+) revealed Crk/CrkL DKO Tregs suppress Tconv division at least as well as their WT counterparts (Fig. 5C). Together, these findings suggest that ADAP- and Crk/CrkL-dependent integrin activation may not be absolutely required for the suppressive function of Tregs.
Figure 5. Crk/CrkL DKO Tregs do not show decreased suppressive function compared to WT Tregs.
(A) Representative flow cytometric plots and (B) the fraction of Tregs (out of CD4+ T cells) from the thymus and spleen of WT and Crk/CrkL DKO Tregs are shown. (C) The expression of CTLA-4, CD25, GITR, and CD44 on Tregs from WT and Crk/CrkL DKO Tregs is shown. (D) CFSE-labeled splenic CD4+Foxp3− Tconvs were cultured with either WT or Crk/CrkL DKO Tregs for four days in the presence of irradiated T cell-depleted splenocytes and anti-CD3. Representative CFSE plots from four-day cultures lacking Tregs or containing a 1:2 or 1:1 Tconv to Treg ratio of either WT or Crk/CrkL DKO Tregs are shown (top). A plot of the division index of Tconvs at various Treg:Tconv ratios are shown (middle). (E) The division index of Tconvs at Tconv:Treg ratios of 1:2 and 1:1 were normalized to the baseline Tconv division (no Tregs) for each experiment and represented as mean % suppression ± SEM (n = 4 mice total).
Discussion
The requirement of TCR stimulation and the downstream signaling pathways in the suppressive function of Tregs has been somewhat controversial. Here, using Tregs with genetically altered TCR signaling capacities, we probed the requirement of TCR-mediated signaling in the ability of Tregs to suppress Tconv division. Our data demonstrate that phosphorylation of SLP-76 tyrosine 145 is critical for Treg suppressive function, suggesting that PLCγ1 activation is important for Treg function. This notion was further supported by the augmented suppressive function observed by DGKζ KO Tregs, which exhibit selectively enhanced TCR-mediated DAG production. Surprisingly, however, Tregs lacking molecules involved in TCR-mediated integrin activation displayed intact suppressive function. Together, these data suggest that TCR-mediated PLCγ activation but not ADAP- and Crk/CrkL-dependent integrin activation is required for Tregs to inhibit Tconv proliferation in vitro.
A requirement for continuous TCR expression in the homeostasis and function of Tregs was recently demonstrated (37, 38). Our data are consistent with these findings and provide insight as to the signaling pathways required for these processes. While Tregs lacking DGKζ maintained a cell surface phenotype similar to that of WT mice, our data demonstrate that SLP-76 is required to maintain CTLA4, KLRG1, CD103, CD69 and CXCR3 expression, suggesting that continued expression of these cell surface markers requires SLP-76-mediated signals. These changes in cell surface phenotype between SLP-76 cKO are similar to those observed in mice lacking TCR signaling in mature Tregs. However, SLP-76 cKO Tregs were also skewed toward a “naïve” CD62Llo phenotype which differed from mice lacking TCR expression in Tregs (37). These differences may represent SLP-76 requirements in non-TCR mediated signaling or may simply be the result of the different approaches to timed deletion – Treg specific (37), inflammatory (38), or ubiquitous (our data). Direct comparison using identical Cre deleter lines will be needed to differentiate between these possibilities.
Our finding that TCR-mediated PLCγ1 activation and DAG production is important for Treg function is consistent with a previous report showing that ablation of PLCγ1 in the T cell lineage results in a paucity of Tregs with reduced suppressive function (47). However, the underlying mechanism of DAG involvement in the suppressive ability of Tregs is unclear. DAG typically activates multiple molecules downstream of the TCR stimulus, including Ras-GRP and PKCθ, leading to induction of the MAPK and NF-κB signaling pathways, respectively. It is possible that transcriptional alterations driven by these pathways promote upregulation of surface molecules or soluble factors associated with suppression. Little has been published, however, about how Treg-intrinsic transcription is altered post-TCR stimulation to achieve active suppression. In addition to activation of these signal transduction pathways, DAG has been reported to activate the guanine-nucleotide exchange factor CALDAG-GEF, which along with C3G, is required for efficient Rap1 activation and inside-out signaling to integrins such as LFA-1 (48, 49). Indeed, T cells lacking PLCγ1, and thus DAG production, fail to adhere to ICAM-coated surfaces (50). Since Treg-intrinsic integrin activation is thought to facilitate interactions between Tregs and DCs that are required for suppression (26, 27), we reasoned that it was possible that DAG-mediated signaling could contribute to Treg function by promoting integrin activation.
To assess the importance of TCR-induced inside-out integrin activation during Treg-mediated suppression, we evaluated the suppressive abilities of Tregs lacking molecules critical to this process. Surprisingly, Tregs deficient in either ADAP or Crk/CrkL proteins exhibited no appreciable functional defects in vitro. Since Crk/CrkL family members drive efficient TCR-driven Rap1 activation, and ADAP is necessary for TCR-driven plasma membrane localization of Rap1 (43–46), our results suggest that TCR-driven inside-out signaling leading to integrin activation may not be required for the suppressive function of Tregs in vitro. These findings are in contrast to recent evidence suggesting TCR-driven integrin activation is sufficient to induce suppression in vitro (25). Additionally, other reports have shown that disruption of surface LFA-1 molecules renders Tregs unable to suppress Tconv division (26, 27). Tregs have been shown to out-compete Tconvs to form aggregates around DCs, and in doing so, they downregulate CD80/CD86 costimulatory molecules on the surface of DCs to prevent Tconvs from being fully stimulated (26). The integrin LFA-1 was shown to be required for this process, as LFA-1 deficient Tregs were unable to form these aggregates and unable to suppress Tconv proliferation (26, 27). Since ADAP and Crk proteins are required for optimal TCR-driven inside-out activation of LFA-1, it is peculiar that ADAP or Crk/CrkL KO Tregs have no defect in in vitro suppressive function. It is possible that compensatory pathways can induce integrin activation in Tregs apart from those directly mediated by the TCR. For example, CTLA-4 engagement can mediate inside-out signaling to integrins as well, and has been found to augment TCR-induced LFA-1 activation (51, 52). Since Tregs constitutively express CTLA-4 and require this molecule to mediate suppression (53, 54), perhaps CTLA-4 is sufficient to induce cellular adhesion in the ADAP KO and/or Crk/CrkL DKO setting. Indeed, C3G can promote Rap1-driven integrin activation downstream of CTLA-4 engagement in a manner that may not involve ADAP (55). Alternatively, the defect in integrin activation by ADAP or Crk/CrkL deficiency may not be complete. In response to TCR stimulation, ~50% of integrin activation is lost in Crk/CrkL KO T cells (Huang and Burkhardt, unpublished results); a level that may still be sufficient for Tregs to interact with DCs. However, alterations of peptide/MHC quantity or affinity of TCR to peptide/MHC complexes could potentially bring out defects in suppression mediated by Tregs lacking ADAP or Crk/CrkL.
In one of the aforementioned studies, Tregs were able to exert suppression even when the catalytic function of ZAP-70 was inhibited. This was proposed to be because ZAP-70 phosphorylation sites necessary for scaffolding interactions between ZAP-70 and Crk proteins remained intact and could mediate inside-out signaling to integrins (25). In support of this notion, mutation of these phosphorylation sites abrogated Treg suppressive ability; although it should be noted that other aspects of TCR signal transduction, including PLCγ1 activation, were also disrupted in the absence of these phosphorylation sites (25, 56). Since our data suggest that Crk proteins are not required for Treg suppressive function, it is possible that TCR-driven integrin activation represents a process that is sufficient to induce suppression, but is not required under all circumstances. Furthermore, the ability of phosphorylated ZAP-70 to promote integrin activation by serving as a scaffold might have been augmented by the overexpression of ZAP-70 in these studies (25). It is also possible that the level of inhibitor used to abrogate the catalytic function of ZAP-70 was sufficient to prevent TCR-driven responses in Tconvs, but not potent enough to fully prevent Treg suppressive ability as Tregs have a much lower TCR activation threshold than Tconvs (57). It is clear that integrins play a role in Treg function, but further work is required to dissect the signaling pathway that support this function.
In summary, using T cells with targeted quantitative and qualitative signaling deficits, we have demonstrated the importance of TCR-mediated PLCγ activation and downstream DAG signaling for the suppressive function of Tregs. Although integrins are important for the suppressive function of Tregs, TCR-mediated inside-out signaling through ADAP or Crk/CrkL to activate integrins was not required by Tregs to achieve suppression. Further studies will be required to reveal how these signaling pathways drive Treg-mediated suppression, and will provide additional insight into the mechanisms by which Tregs suppress Tconv division.
Supplementary Material
Acknowledgements
We thank the labs of Drs. Gary Koretzky, Avinash Bhandoola, Edward Behrens, and Paula Oliver for thoughtful discussions and advice. We thank Drs. Gary Koretzky, Xiao-ping Zhong, and Craig Thompson for providing us with valuable reagents.
This project was supported by grants from the National Institutes of Health (R01HL107589, R01HL111501, and R01AI085160, K01AR057577). Amanda Schmidt was supported by a predoctoral fellowship from the American Heart Association.
Abbreviations used
- ADAP
adhesion and degranulation promoting adapter protein
- B6
C57BL/6
- BM
bone marrow
- cHet
conditional heterozygous
- CFSE
Carboxyfluorescein succinimidyl ester (CFSE)
- cKO
conditional knockout
- Crk
CT10 regulator of kinase
- CrkL
Crk-like
- DAG
diacylglycerol
- DC
dendritic cell
- DKO
double knockout
- floxed
loxp-flanked
- GITR
glucocorticoid-induced TNFR family related gene
- PLC
phospholipase C
- IS
immunological synapse
- KO
knockout
- LAT
linker for activated T cells
- SLP-76
Src homology 2 (SH2) domain containing leukocyte protein of 76 kDa
- Tconv
conventional CD4+ T cell
- Treg
regulatory T cell
- WT
wildtype
Footnotes
Taku Kambayashi will communicate with the Editorial and Production offices
Conflict of Interest
The authors declare no conflicts of interest.
References
- 1.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature immunology. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 3.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. The Journal of experimental medicine. 2007;204:57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of immunology. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature immunology. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 6.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowe JH, Ertelt JM, Way SS. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology. 2012;136:1–10. doi: 10.1111/j.1365-2567.2011.03551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens LA, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunological reviews. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- 9.Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Current opinion in immunology. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harbor perspectives in biology. 2010;2:a002279. doi: 10.1101/cshperspect.a002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nature immunology. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 13.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 14.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. The Journal of experimental medicine. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JK, Klinger M, Benjamin J, Xiao Y, Erle DJ, Littman DR, Killeen N. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PloS one. 2009;4:e6580. doi: 10.1371/journal.pone.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. Journal of immunology. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nature immunology. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 22.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O'Garra A, Stockinger B. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. International immunology. 2005;17:279–288. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 23.de la Rosa M, Rutz S, Dorninger H, Scheffold A. Interleukin-2 is essential for CD4+CD25+ regulatory T cell function. European journal of immunology. 2004;34:2480–2488. doi: 10.1002/eji.200425274. [DOI] [PubMed] [Google Scholar]
- 24.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunology. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 25.Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nature immunology. 2010;11:1085–1092. doi: 10.1038/ni.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onishi Y, Fehervari Z, Yamaguchi T, Sakaguchi S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc Natl Acad Sci U S A. 2008;105:10113–10118. doi: 10.1073/pnas.0711106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran DQ, Glass DD, Uzel G, Darnell DA, Spalding C, Holland SM, Shevach EM. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell stem cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, Koretzky GA. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28:359–369. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu GF, Corbo E, Schmidt M, Smith-Garvin JE, Riese MJ, Jordan MS, Laufer TM, Brown EJ, Maltzman JS. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. European journal of immunology. 2011;41:2064–2073. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. The Journal of experimental medicine. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase zeta deficiency. Nature immunology. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 33.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, Shimizu Y, Koretzky GA. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 34.Yablonski D, Kuhne MR, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 35.Bushar ND, Corbo E, Schmidt M, Maltzman JS, Farber DL. Ablation of SLP-76 signaling after T cell priming generates memory CD4 T cells impaired in steady-state and cytokine-driven homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:827–831. doi: 10.1073/pnas.0908126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nature immunology. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S, Riess D, Hein MY, Buch T, Polic B, Schonle A, Zeiser R, Schmitt-Graff A, Kretschmer K, Klein L, Korn T, Sakaguchi S, Schmidt-Supprian M. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 39.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 40.Zhong XP, Hainey EA, Olenchock BA, Zhao H, Topham MK, Koretzky GA. Regulation of T cell receptor-induced activation of the Ras-ERK pathway by diacylglycerol kinase zeta. The Journal of biological chemistry. 2002;277:31089–31098. doi: 10.1074/jbc.M203818200. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt AM, Zou T, Joshi RP, Leichner TM, Pimentel MA, Sommers CL, Kambayashi T. Diacylglycerol kinase zeta limits the generation of natural regulatory T cells. Science signaling. 2013;6:ra101. doi: 10.1126/scisignal.2004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menasche G, Kliche S, Bezman N, Schraven B. Regulation of T-cell antigen receptor-mediated inside-out signaling by cytosolic adapter proteins and Rap1 effector molecules. Immunological reviews. 2007;218:82–91. doi: 10.1111/j.1600-065X.2007.00543.x. [DOI] [PubMed] [Google Scholar]
- 43.Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B. The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol. 2006;26:7130–7144. doi: 10.1128/MCB.00331-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D. The adaptor protein Crk in immune response. Immunology and cell biology. 2014;92:80–89. doi: 10.1038/icb.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolz JC, Nacusi LP, Segovis CM, Medeiros RB, Mitchell JS, Shimizu Y, Billadeau DD. The WAVE2 complex regulates T cell receptor signaling to integrins via Abl- and CrkL-C3G-mediated activation of Rap1. The Journal of cell biology. 2008;182:1231–1244. doi: 10.1083/jcb.200801121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Shao Y, Fang D, Huang J, Jeon MS, Liu YC. Negative regulation of T cell antigen receptor-mediated Crk-L-C3G signaling and cell adhesion by Cbl-b. The Journal of biological chemistry. 2003;278:23978–23983. doi: 10.1074/jbc.M212671200. [DOI] [PubMed] [Google Scholar]
- 47.Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE, Gorski J, Williams CB, Wang D, Wen R. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. The Journal of experimental medicine. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nature medicine. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 49.Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN. Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood. 2007;110:3682–3690. doi: 10.1182/blood-2007-03-077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katagiri K, Shimonaka M, Kinashi T. Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. The Journal of biological chemistry. 2004;279:11875–11881. doi: 10.1074/jbc.M310717200. [DOI] [PubMed] [Google Scholar]
- 51.Dillon TJ, Carey KD, Wetzel SA, Parker DC, Stork PJ. Regulation of the small GTPase Rap1 and extracellular signal-regulated kinases by the costimulatory molecule CTLA-4. Molecular and cellular biology. 2005;25:4117–4128. doi: 10.1128/MCB.25.10.4117-4128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12861–12866. doi: 10.1073/pnas.0505802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 54.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends in immunology. 2011;32:428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Kloog Y, Mor A. Cytotoxic-T-lymphocyte antigen 4 receptor signaling for lymphocyte adhesion is mediated by C3G and Rap1. Molecular and cellular biology. 2014;34:978–988. doi: 10.1128/MCB.01024-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. The Journal of experimental medicine. 2009;206:2527–2541. doi: 10.1084/jem.20082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. International immunology. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.