Abstract
Phosphoinositide 3-kinases (PI3Ks) are a family of lipid kinases that phosphorylates the 3'OH of the inositol ring of phosphoinositides (PIs). They are responsible for coordinating a diverse range of cellular functions. Class IA PI3K is a heterodimeric protein composed of a regulatory p85 and a catalytic p110 subunit. In this study, we conditionally deleted the p110α-subunit of PI3K in cone photoreceptor cells using the Cre-loxP system. Cone photoreceptors allow for color vision in bright light (daylight vision). Cone-specific deletion of p110α resulted in cone degeneration. Our studies suggest that PI3K signaling is essential for cone photoreceptor functions.
Keywords: Phosphoinositide 3-kinases, Phosphoinositides, Cre-loxP system, Cone photoreceptors, Retina, Vision
1. INTRODUCTION
Growth factor signaling is mediated through Class IA phosphoinositide 3-kinases (PI3Ks). Class IA PI3K is an obligatory heterodimeric complex comprised of regulatory p85 and catalytic p110 subunits [1]. Class IA PI3K is the principal kinase which, when activated, phosphorylates phosphatidylinositol at the D3 position of the inositol ring [2]. This reaction generates the D3 phosphoinositides (PIs) PI-3-P, PI-3,4-P2, PI-3,5-P2, and PI-3,4,5-P3. These lipid products serve as second messengers that recruit specific phospholipid-binding proteins to the membrane, initiating downstream transduction pathways [3–5]. To date, studies have implicated D3-PIs in a variety of cell activities such as vesicular trafficking, cytoskeletal reorganization, cell growth, adhesion, and survival [2; 6], as well as photoreceptor-specific functions, such as neuroprotection (our work), modulation of phototransduction [7], disc biogenesis [8], protein translocation [9], synaptic ribbon formation [10], and glutamate release [10].
Human beings are highly dependent on vision. Rod photoreceptors provide sensitivity in dim light (night vision), while cone photoreceptors allow for color vision and visual acuity in bright light (daylight vision). Cone photoreceptors constitute a small percent (3–5%) of retinal photoreceptors in rodents and humans, and are essential for optimal visual acuity, color vision, and visual perception under moderate to high light intensities in humans. Age-related macular degeneration and diabetic retinopathy are the most common disorders affecting cones in humans [11–15]. Cones are indirectly affected by diseases such as retinitis pigmentosa and directly by cone and cone-rod dystrophies [16–18]. The specific mechanisms of cone cell death may be different in various degenerative diseases, depending on genetic predispositions and environmental factors [12; 19–23]. Studies from our laboratory over the past two decades have shown that both rods and cones have an active PI3K signaling pathway [6; 24–26]. Interestingly, conditional deletion of the regulatory subunit of p85α from cones resulted in age-related cone degeneration [24]. To further confirm that the absence of p85α is the cause of cone degeneration, rather than some pleiotropic effect of gene deletion producing degeneration, we conditionally deleted the catalytic p110α-subunit of PI3K from cones. We found cone, but not rod, degeneration in these mice, similar to what we reported for p85α knockout mice [24].
2. MATERIALS AND METHODS
2.1. Materials
Rabbit polyclonal PI3-kinase p110α (C73F8, Cat # 4249) antibody was purchased from Cell Signaling Technology (Beverly, Massachusetts). Rabbit polyclonal PI3-kinase p110α antibody (H-201, Cat # sc-7174) was procured from Santa Cruz Biotechnology (Dallas, Texas). Rabbit polyclonal anti-cone arrestin antibody was obtained from Millipore (Temecula, California). Mouse monoclonal anti-Cre antibody, suitable for immunohistochemistry, was purchased from Abcam (Cambridge, Massachusetts). Biotinylated peanut agglutinin (PNA) and secondary antibodies were acquired from Vector Laboratories (Burlingame, California). DAPI stain used for nuclear staining was procured from Invitrogen-Molecular Probes (Carlsbad, California). All other reagents used for buffer preparations were of analytical grade and purchased from Sigma (St. Louis, Missouri).
2.2. Animals
The p110α floxed mice [27] were kindly provided by Dr. Jean Zhao (Harvard Medical School). The generation of mice expressing Cre-recombinase under control of the human red/green pigment gene promoter was reported previously [24]. All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the NIH Guide for the Care and Use of Laboratory Animals. All protocols were approved by the IACUC at OUHSC and Dean A. McGee Eye Institute before experiments were initiated. Animals were born and raised in our vivarium and kept under dim cyclic light (40–60 lux, 12 h light/dark cycle). For experiments that required enucleating the eye or removing the retina, mice were killed by asphyxiation with CO2, followed by cervical dislocation.
2.3. Generation of cone photoreceptor-specific p110α KO mice
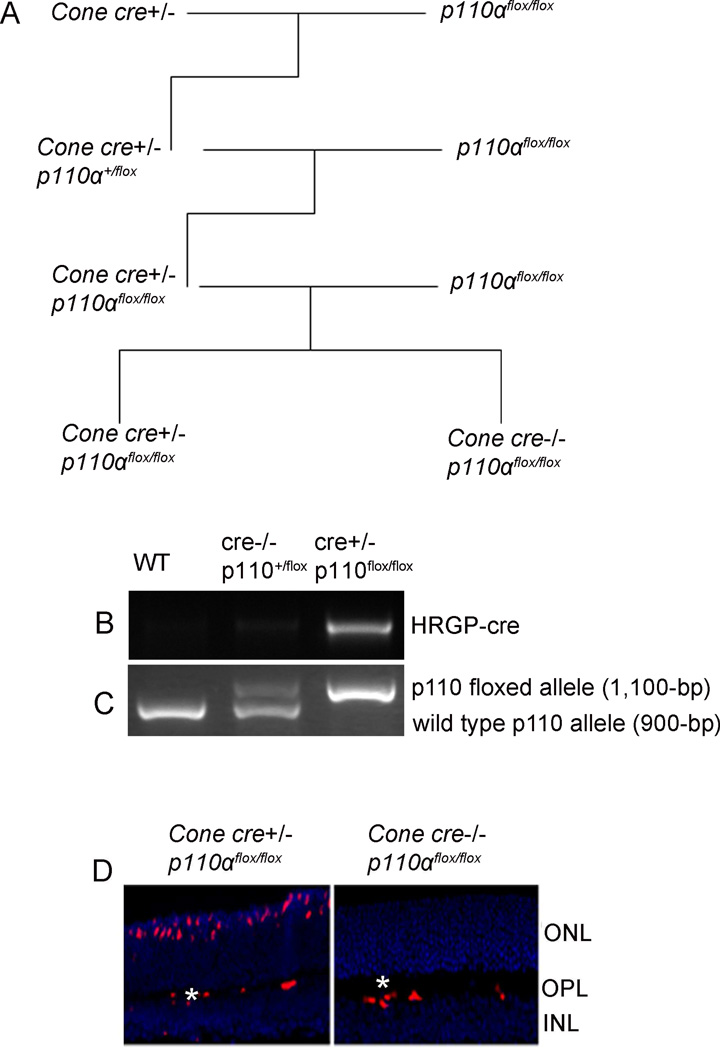
To produce mice with cone-specific KO of p110α, we bred mice expressing Cre recombinase specifically in cones under the control of the human red/green pigment gene promoter with p110α floxed mice, in which the mouse PIK3CA gene containing exon 1 was flanked with loxP sites, which enabled deletion of p110α, as previously described [27]. The desired transgenic mice were generated by back-crossing and identified by genotyping tail DNA for Cre and floxed p110α, using PCR screening. For Cre genotype screening, a forward primer TTG GTT CCC AGC AAA TCC CTC TGA designed within the promoter DNA sequence and a reverse primer GCC GCA TAA CCA GTG AAACAG CAT designed within the Cre sequence were used to amplify PCR product of 411 bp. In order to distinguish the p110α floxed allele from the WT p110α, the allele primer pair CTG TGT AGC CTA GTT TAG AGC AAC CAT CTA and ACA GCC AAG GCT ACA CAG AGA AAC CCT GTC TTG were used to amplify a 900-bp fragment from the wild-type p110α allele and a 1,100-bp fragment from the floxed p110α allele.
2.4. Immunostaining of retinal whole-mounts
Eyes were enucleated and placed in cold Hanks’ balanced salt solution buffered with 25 mM HEPES (pH 7.2). After enucleation, the cornea and lens were removed, and retinas were carefully isolated. Relaxing cuts were made in the retinal margins and the whole retina was flattened onto a black filter membrane. Whole-mounted retinas were fixed in 4% paraformaldehyde in PBS at 4°C for 2 h, rinsed in PBS, and non-specific labeling was blocked using 10% horse serum in PBS. Whole-mounts were incubated in a combination of biotinylated PNA (1:500) and anti-cone arrestin (1:500) overnight at 4°C. Streptavidin conjugated to Texas red (1:250) was used to visualize peanut agglutinin (PNA) labeling. Cone arrestin immunoreactivity was visualized using an FITC-conjugated secondary antibody (1:200). Labeling in retinal whole mounts was imaged using either a Nikon Eclipse E800 (Tokyo, Japan) or an Olympus IX70 (Olympus USA, Center Valley, Pennsylvania) epifluorescence microscope.
2.5. Preparation of tissue for cryosectioning
Mice were euthanized by CO2 asphyxiation. The eyeballs were placed in a dish containing 4% paraformaldehyde (PFA) with 1 mM sodium orthovanadate. A hole was made in the cornea, which we then placed in PFA for 5 min. After 5 min, the eye was placed in 1XPBS in the dish, and we removed the cornea and lens carefully using forceps and scissors. The dissected eye cup was transferred into a 2 ml vial containing 4% PFA and 1mM sodium orthovanadate, and we fixed the eye tissue for additional 15 min. The PFA was replaced with 15% sucrose. We incubated the eye cup at room temperature until the eye cup sank to the bottom of the dish. Then, the 15% sucrose was replaced by 30% sucrose and we further incubated the eye cup at 4°C overnight. The 30% sucrose was replaced with fresh 30% sucrose and the eye cup was incubated at room temperature until the eye cup again sank to the bottom of the dish. The eye cup was embedded in O.C.T compound and frozen rapidly with liquid nitrogen using a Styrofoam box as a container for the liquid nitrogen. We kept the samples at –20°C until processing. Before sectioning, we equilibrated the sample temperature with the temperature inside the cryostat for 20 min to 1 h. The samples were sectioned (12–16 µm) and allowed to freeze inside the cryostat while the O.C.T turned white. The slides were briefly treated with methanol at –20°C in a Joplin jar inside the cryostat. The slides were air-dried completely and stored at –20°C until use. We then rinsed the slides in 1XPBS, set the slides in a sequenza rack, and washed them 3 times with 1% Trion X-100 in 1XPBS. The sections were blocked with 150 µl of 10% normal horse serum (NHS) for 1 h. We then added 120 µl of primary antibody (p110α 1 in 25 and PNA 1 in 1000) diluted in 10% NHS and incubated the sections overnight at 4°C. The sections were washed 3 times in 1XPBS. Then, we added 120 µl of secondary antibody diluted in 10% NHS to sections and incubated the sections at room temperature for 1 h. To stain the nuclei, 100 µl of DAPI diluted in 1XPBS was added and the slides were covered with aluminum foil to prevent bleaching. The slides were then washed 3 times in 1XPBS. We removed the slides from the rack and added a drop of 50% glycerol/1XPBS mounting media and placed a coverslip that did not press on the sections. The slides were kept in a slide box to protect them from light and they were stored at 4°C until imaged. Antibody-labeled complexes were examined on a Nikon Eclipse E800 microscope equipped with a digital camera. Images were captured using Metamorph (Universal Imaging, West Chester, PA) image analysis software. All images were taken using identical microscope and camera settings.
2.6. Other methods
The structure and morphology of p110α cKO and control retinas were examined after tissue fixation and sectioning, as previously described [24; 29]. Retinal function was examined using a Ganzfeld-type ERG recording system (UTAS-E3000), according to the method described earlier [29].
2.7. Statistical analysis
One-way ANOVA and post-hoc statistical analysis using Bonferroni’s pairwise comparisons were used to determine statistical significance (p<0.05).
3. RESULTS
3.1. Generation of cone photoreceptor-specific p110α KO mice
Systemic deletion of the p110α subunit of PI3K results in embryonic or neonatal lethality [30; 31]. To circumvent these difficulties, we used Cre/lox technology to generate a cone-photoreceptor-specific deletion of the PIK3CA gene, which resulted in deletion of the p110α subunit of PI3K only in cone photoreceptors. The breeding strategy to generate bigenic mice is shown in Figure 1A. We mated cone opsin-cre (human red/green pigment (HRGP) gene [32]) to homozygous p110α floxed mice (p110αflox/flox), and screened the resulting offspring with PCR to identify mice that were heterozygous for both cre+/− and p110αflox (Fig. 1B and C). We then crossed the cre+/−/p110αflox mice to homozygous p110αflox/flox mice, and used PCR to identify mice that were cre+/−/p110 αflox/flox. The resultant mice were heterozygous for cone-cre and homozygous for p110αflox. We maintained this genetic combination by mating cre+/−/p110αflox/flox to p110αflox/flox mice. All of these offspring were homozygous for p110αflox/flox. Half were heterozygous for cre (cre+/−) and were our experimental animals, while the other half were wild-type mice (cre−/−) and served as controls. These mice were used for all experiments described in this manuscript.
Figure 1. Generation of the cone-specific p110α KO mouse model.
Cone photoreceptor-specific deletion of PIK3CA, a pan-p110α catalytic subunit of PI3K, was performed by cross-breeding floxed p110α mice to cone-specific Cre mice (A). PCR diagnostic for cone-opsin cre (B), and floxed p110α and WT genes (C) using mouse tail DNA samples. Immunohistochemical analysis of Cre recombinase immunolabeling in p110α KO and WT control retinas harvested from littermates. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. Red indicates Cre-positive cone cells. Labeling of blood vessels (*) is non-specific.
Since mice have rod-dominant retinas, in which rods outnumber cones, it would be challenging to demonstrate a reduction in protein levels of p110α from total retinal extracts in cases where p110α is conditionally lost only in cones. To ensure proper loss of function of p110α in cones by our cone-expressing Cre line, we assessed Cre protein localization in the retinas of WT and p110α KO littermates with immunofluorescence microscopy using an anti-Cre antibody. Cre expression was localized to cone photoreceptor nuclei in p110α KO retinas, but was absent from WT controls (Fig. 1D). The secondary anti-mouse antibody non-specifically labeled endogenous IgGs in the blood vessels of both WT and p110α KO mouse retinas (Fig.1D). Earlier, we used immunohistochemistry to demonstrate that almost 100% of cone cells in the parent Cre-expressing mouse line express Cre [32]. Since successful Cre-mediated recombination only requires four Cre molecules [33] and a protein level that is much lower than the threshold of detection by IHC, and because the locus for PI3K-p110α for an unusually low frequency of Cre-mediated recombination is unknown [27], we believe that deletion of the PI3K–p110α subunit likely occurred in all cone photoreceptors. The parent Cre mouse line that we used here was previously shown to have normal cone distribution, morphology, and function up to 10 months of age [32].
3.2. Reduced expression of p110α in cone photoreceptors of p110α knockout mice
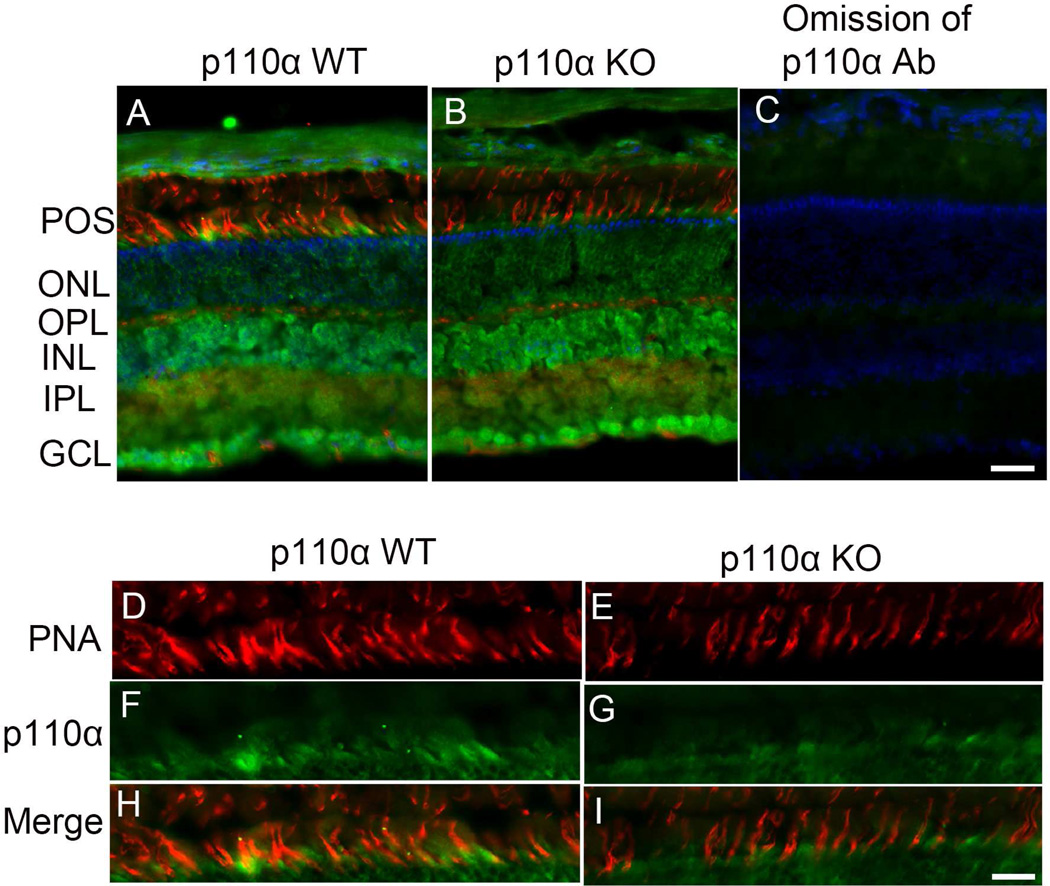
In this study, we characterized two PI3K-p110α antibodies, one from Cell Signaling Technology and the other from Santa Cruz Biotechnology (SCBT), on retinal sections prepared from either prefer-, paraffin-, or cryo-fixation. Neither antibody worked with prefer- or paraffin-fixed sections, even with antigen retrieval (data not shown). However, the antibody from SCBT worked on cryosections. Retinal sections prepared from wild-type mice co-labelled with PNA and anti-p110α antibody suggest that p110α immunoreactivity was predominantly observed in the inner retinal layer (INL, IPL, and GCL), including the photoreceptor layer (Fig. 2A). The PNA labeling of cones (Fig. 2A) was co-localized with p110α (Fig. 2A and H), suggesting the expression of p110α in cones. Co-labelling of retinal sections prepared from p110α knockout co-stained with PNA and p110α indicated that the expression of p110α in cones was reduced in cone-conditional p110α knockout mice compared with control littermates (Fig. 2B and I). The omission of primary antibody indicated the absence of immunoreactivity; this experiment served as a negative control (Fig. 2C). The results also show a reduced number of PNA-positive cones in p110α knockout mice, compared with wild-type mice (Fig. 2D–I).
Figure 2. Reduced expression of p110α in cones of cone-conditional p110α knockout mice.
PNA (red), anti-p110α (green), and DAPI (blue) immunofluorescence staining of retinal sections from two-month-old p110α wild-type littermates (A) and p110α KO mice (B). The images shown are representative of four retinas examined from WT and KO mice. Panel C represents the omission of p110α antibody. A region of the photoreceptor layer was enlarged in both WT and KO mice (H, I). Scale bar = 50 µm for panels A-C, 20 µm for panels H - I.
3.3. Effect of p110α deletion on cone cell viability
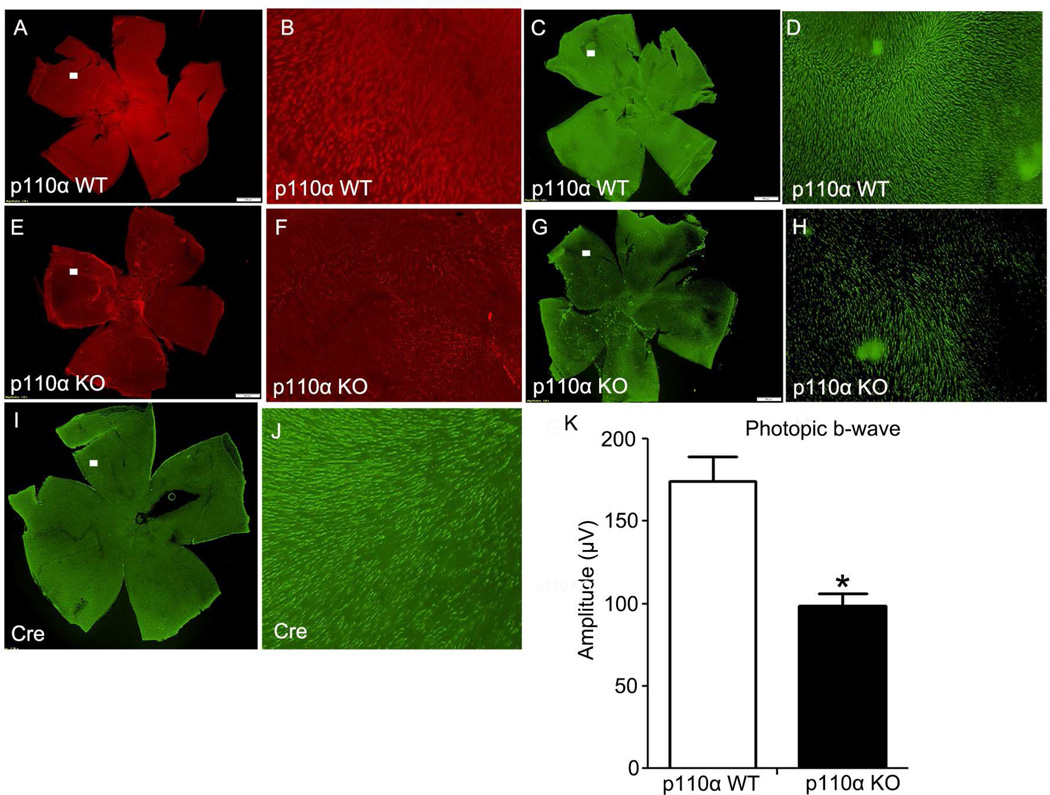
To test if functional loss is caused by cone structural degeneration and cone cell death, we performed lectin cytochemical and immunohistochemical analysis of whole retinal flat-mounts using PNA and anti-cone arrestin-4 (cArr4) to label cone outer and inner segments [34], respectively. Fluorescence microscopic analysis of WT and p110α KO retinal flat mounts indicated that distribution and density of cone photoreceptors was affected at 3 months of age in cone-p110α KO retinas compared with retinas from WT mice (Fig. 3A–H). To further confirm that the degeneration phenotype observed in cone-p110α KO mouse retinas is not due to the expression of Cre-recombinase, we stained Cre-recombinase-expressed mouse retinas with cArr4 antibody. Our results indicate that the presence of cArr4-positive healthy cones and cone density is comparable to that found in wild-type mouse retinas (Fig. 3 I, J).
Figure 3. Flat mounts of cone photoreceptors in p110α KO retinas at 3 months of age.
PNA (red) and anti-cone arrestin (cArr, green) immunofluorescence staining of retinal whole mounts from WT and p110α KO mice (A, C, E, G, I). The images shown are representative of four retinas examined from WT and KO mice. Scale bar = 100 µm for all panels. A region (□) on the flat mount was imaged at a higher magnification (B, D, F, H, J). Photopic b-wave electroretinographic (ERG) analysis of WT and p110α KO mice at 3 months of age (K). Values are mean ± SEM, n= 10. * P<0.05.
3.4. Effect of p110α deletion on retinal function and rod cell viability
The retinal function of cone-p110α KO mice was assessed by electroretinography (ERG). Scotopic ERG recordings of rod photoreceptors (scotopic a- and b-wave) were functionally normal and statistically comparable in WT and p110α KO retinas at 3 months of age (data not shown). Photopic (cone-driven) ERG b-wave recordings indicated that cone-driven signaling to the inner retina was significantly lower in cone-p110α KO mice than in WT mice at 3 months of age (Fig. 3K). The overall morphology and structural integrity of the retinas were indistinguishable between WT and cone-p110α KO mice at 3 months of age (not shown).
3.5. Quantification of cone cell loss in cone p110α KO mice
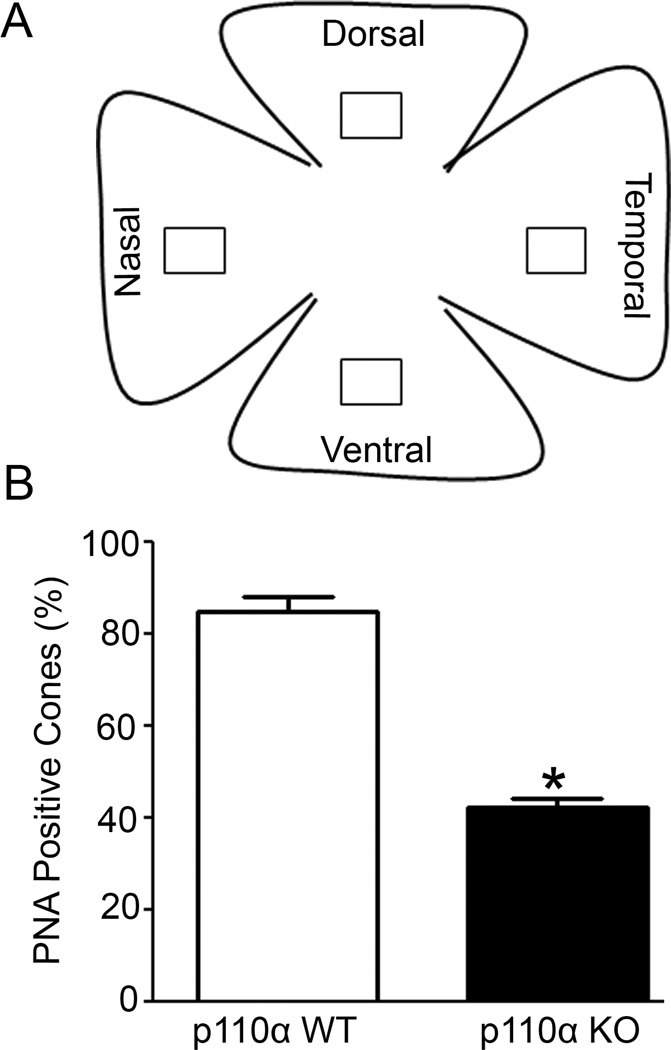
To quantitate the cone cell loss in cone p110α KO mice, we employed ImageJ software (http://rsb.info.nih.gov/nih-image/) to measure cone degeneration on retinal flat mounts. We measured the cone density on four regions of the retina (ventral, dorsal, nasal, and temporal regions) in wild-type and KO mice (Fig. 4A). We combined the results from selected areas of all regions of the flat mount and expressed this as percentage of PNA-positive cones (Fig. 4B). Our analysis suggests that three-month-old p110α KO mice had significantly fewer PNA-labeled cones than their WT littermates (Fig. 4B). Our studies clearly demonstrate that conditional deletion of p110α in cones leads to significant loss of cones, indicating that PI3K signaling is critical to cone survival.
Figure 4. Quantification of cone cell loss in cone photoreceptors in p110α KO retinas.
PNA-labelled retinal whole mounts from WT and p110α KO mice were subjected to imageJ analysis on selected areas (□) of dorsal, ventral, nasal and temporal regions of the retina (A). Combined PNA-labeled positive cones were expressed as percentage and the wild-type was set as 100 percent (B). Data are mean ± SEM, n = 3, *p<0.001.
4. DISCUSSION
Characterization of one-month-old cone-specific p110α KO mice did not reveal any detectable biochemical, morphological, or functional phenotypes, suggesting that expression of PI3K is not required for the maturation of adult retinas (data not shown). However, three-month-old mice showed significantly reduced cone photoreceptor function and cone loss due to cone-specific deletion of p110α. The loss of cone function was not the result of Cre-recombinase expression. Interestingly, the dying cone photoreceptors did not affect the viability or function of the surrounding rod population, i.e., there was no “bystander” effect [35]. It has also been reported that deprivation from a rod-derived cone viability factor, which may be constantly released by healthy rod photoreceptors, could result in secondary cone death [36]. Based on these studies, we speculate that even though cone-p110α KO mice have healthy rods, the cone cell death could not be rescued in the absence of p110α in cones, suggesting that rods may communicate survival signals to cones through the cone PI3K pathway. Further studies are required to establish that cone-PI3K mediates this function.
In humans, daylight vision is primarily mediated by cone photoreceptors. Our earlier studies on cone-conditional deletion of the p85α regulatory subunit of PI3K, and the current study on the cone-specific deletion of p110α catalytic subunit of PI3K, clearly suggest two important aspects of PI3K signaling in cones: 1) that PI3K is essential for cone structure and function, and 2) that cone degeneration in the p110α catalytic subunit of PI3K is not due to pleiotropic effects, since conditional deletion of the regulatory subunit of p85α also exhibited a cone-degeneration phenotype [24]. Transgenic Cre mice are usually created on the standard FVB/n background, which carries a recessive mutation, rd, which causes retinal degeneration. This was not a problem with our mating strategy, as we had already bred the rd mutation out of our Cre-expressing animals. Further, cone-p110α cKO mice were screened for rd1 [19] and rd8 [37] mutations that cause retinal degeneration, and we found no evidence of these mutations in our cone-conditional p110α KO mice.
In humans and rodents, cone photoreceptors constitute a small percent (3–5%) of total retinal photoreceptors [38; 39]. It is technically challenging to study the cone-specific expression of a particular protein that is common to both rod and cone photoreceptors in the rod-dominant retina. Because of this technical limitation, we could not quantify the loss of p110α protein in cones. However, our co-labeling experiments may suggest reduced p110α expression in PNA-positive cones from p110α KO mice. In this study, we also indirectly examined the cellular localization of Cre recombinase protein expression. In our previous study, we examined the cellular localization of Cre recombinase as a measure of p85α deletion in cones [24]. However, when we generated an Nrl−/−/p85α double KO mouse strain, we observed a deletion of >80% of p85α in cones [40]. Mice lacking the transcription factor Nrl experience a block in the differentiation of rod precursor cells, resulting in retinas containing a single class of photoreceptors that are indistinguishable from authentic cones on the basis of a number of criteria [41–44]. Our future experimental plans include the generation and characterization of Nrl−/−/p110α double KO mice.
The molecular mechanism of cone degeneration in cone-conditional p110α KO mice is currently unknown. Studies from our laboratory and others show that PI3K-generated PIs regulates several photoreceptor functions, such as phototransduction [7], protein trafficking [9], synaptic ultrastructure in cone terminals [24], and regulation of mitochondrial hexokinases for the generation of glucose 6-phosphate for generation of NADPH via the pentose phosphate pathway (PPP) [45]. NADPH is essential for a number of vital reactions in rod and cone photoreceptors, including antioxidant metabolism (regeneration of GSH from GSSG), reduction of toxic all-trans-retinal following photobleaching of rod and cone opsins, and synthesis of proteins, RNA, and lipids, all necessary for the massive amount of membrane synthesis that occurs in rods and cones daily [46; 47]. We hypothesize that the absence of PI3K affects these processes and, over time, leads to compromised photoreceptor function and, ultimately, to their demise. These studies are currently undergoing in our laboratory.
In conclusion, our findings show that PI3K is important for the maintenance of cone viability and function. Our study suggests that rods may communicate survival signals to cones via cone PI3K. Age-related macular degeneration, diabetic retinopathy, and retinitis pigmentosa are retinal diseases that result in loss of cone function and, ultimately, cone death, leading to blindness. The generated cone-specific p110α KO mice will be a useful model in which to study the upstream and downstream regulators of PI3K, as well as a model for therapeutic intervention. Activation of the PI3K pathway may have clinical relevance. These findings may have significance for other chronic neurological diseases, such as Parkinson’s, Huntington’s, and Alzheimer’s diseases.
Highlights.
Phosphoinositides regulate neuron survival.
Phosphoinositide 3-kinase catalytic subunit is essential for color vision.
Cone-specific deletion of PI3K leads to cone-photoreceptor neurodegeneration.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institutes of Health (EY016507, EY00871, and EY021725), bridge funding from the Presbyterian Health Foundation (RVR), and by unrestricted departmental grants from Research to Prevent Blindness, Inc. The authors thank Dr. Jean Zhao, Harvard Medical School, for providing floxed p110α mice.
ABBREVIATIONS
- PI3K
phosphoinositide 3-kinase
- PI-3-P
phosphoinositide-3-phosphate
- PI-3,4-P2
phosphoinositide-3,4-diphosphate
- PI-3,4,5-P3
phosphoinositide-3,4,5-triphosphate
- Nrl
neural retina leucine zipper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare that there are no competing interests. RVR and REA designed the study. RVR, MRB, YW, and AR conducted the experiments and carried out the analysis. RVR and REA wrote the manuscript. All authors approved the final version submitted for publication.
REFERENCES
- 1.Guo X, Ghalayini AJ, Chenand H, Anderson RE. Invest Ophthalmol.Vis.Sci. 1997;38:1873–1882. [PubMed] [Google Scholar]
- 2.Cantley LC. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 3.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplanand DR, Greenberg ME. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 4.Fukami K, Endo T, Imamuraand M, Takenawa T. J.Biol.Chem. 1994;269:1518–1522. [PubMed] [Google Scholar]
- 5.Fukami K, Sawada N, Endoand T, Takenawa T. J.Biol.Chem. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]
- 6.Rajala RV. J.Lipid Res. 2010;51:4–22. doi: 10.1194/jlr.R000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He F, Maoand M, Wensel TG. J.Biol.Chem. 2004;279:8986–8990. doi: 10.1074/jbc.M311488200. [DOI] [PubMed] [Google Scholar]
- 8.Chuang JZ, Zhaoand Y, Sung CH. Cell. 2007;130:535–547. doi: 10.1016/j.cell.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Xu H, Kang LW, Amzeland LM, Montell C. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz F, Drenckhahn D. Brain Res. 1993;604:142–148. doi: 10.1016/0006-8993(93)90360-y. [DOI] [PubMed] [Google Scholar]
- 11.Adler R, Curcio C, Hicks D, Priceand D, Wong F. Mol.Vis. 1999;5:31. [PubMed] [Google Scholar]
- 12.Stone EM, Braun TA, Russell SR, Kuehn MH, Lotery AJ, Moore PA, Eastman CG, Casavantand TL, Sheffield VC. N.Engl.J.Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Yang X, Dong A, Petters RM, Peng YW, Wongand F, Campochiaro PA. J.Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 14.Cho NC, Poulsen GL, Ver Hoeveand JN, Nork TM. Arch.Ophthalmol. 2000;118:1393–1400. doi: 10.1001/archopht.118.10.1393. [DOI] [PubMed] [Google Scholar]
- 15.Nork TM. Trans.Am.Ophthalmol.Soc. 2000;98:331–363. [PMC free article] [PubMed] [Google Scholar]
- 16.Hamel CP. Orphanet.J.Rare.Dis. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellner U, Foerster MH. Ger J.Ophthalmol. 1993;2:170–177. [PubMed] [Google Scholar]
- 18.Kohl S. Ophthalmologe. 2009;106:109–115. doi: 10.1007/s00347-008-1864-2. [DOI] [PubMed] [Google Scholar]
- 19.Bowes C, Li T, Danciger M, Baxter LC, Appleburyand ML, Farber DB. Nature. 1990;347:677–680. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 20.Lem J, Krasnoperova NV, Calvert PD, Kosaras B, Cameron DA, Nicolo M, Makinoand CL, Sidman RL. Proc.Natl.Acad.Sci U.S.A. 1999;96:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naash MI, Hollyfield JG, Al Ubaidiand MR, Baehr W. Proc.Natl.Acad.Sci U.S.A. 1993;90:5499–5503. doi: 10.1073/pnas.90.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang SH, Gouras P, Yamashita CK, Kjeldbye H, Fisher J, Farberand DB, Goff SP. Science. 1996;272:1026–1029. doi: 10.1126/science.272.5264.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, Peachey NS, Lem J, Fliesler SJ, Pepperberg DR, Naashand MI, Al Ubaidi MR. Invest Ophthalmol.Vis.Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- 24.Ivanovic I, Anderson RE, Le YZ, Fliesler SJ, Sherryand DM, Rajala RV. Invest Ophthalmol Vis Sci. 2011;52:3775–3783. doi: 10.1167/iovs.10-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanovic I, Allen DT, Dighe R, Le YZ, Andersonand RE, Rajala RV. Invest Ophthalmol Vis Sci. 2011;52:6355–6362. doi: 10.1167/iovs.10-7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajala RV, Anderson RE. Mol.Neurobiol. 2010;42:39–47. doi: 10.1007/s12035-010-8130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao JJ, Cheng H, Jia S, Wang L, Gjoerup OV, Mikamiand A, Roberts TM. Proc.Natl.Acad.Sci U.S.A. 2006;103:16296–16300. doi: 10.1073/pnas.0607899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumoand S, Cantley LC. Mol.Cell Biol. 2005;25:9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Anderson RE, Tomita H, Adler R, Liu X, Zackand DJ, Rajala RV. J.Neurosci. 2007;27:203–211. doi: 10.1523/JNEUROSCI.0445-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bi L, Okabe I, Bernard DJ, Wynshaw-Borisand A, Nussbaum RL. J.Biol.Chem. 1999;274:10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 31.Fruman DA, Mauvais-Jarvis F, Pollard DA, Yballe CM, Brazil D, Bronson RT, Kahnand CR, Cantley LC. Nat.Genet. 2000;26:379–382. doi: 10.1038/81715. [DOI] [PubMed] [Google Scholar]
- 32.Le YZ, Ash JD, Al Ubaidi MR, Chen Y, Maand JX, Anderson RE. Mol.Vis. 2004;10:1011–1018. [PubMed] [Google Scholar]
- 33.Guo F, Gopauland DN, van Duyne GD. Nature. 1997;389:40–46. doi: 10.1038/37925. [DOI] [PubMed] [Google Scholar]
- 34.Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh EN, Jr, Craft CM. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ripps H. Exp.Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Mohand-Said S, Danan A, Simonutti M, Fontaine V, Clerin E, Picaud S, Leveillardand T, Sahel JA. Mol.Ther. 2009;17:787–795. doi: 10.1038/mt.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitzand S, Heckenlively JR. Vision Res. 2002;42:517–525. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 38.Carter-Dawson LD, LaVail MM. J.Comp Neurol. 1979;188:263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- 39.Carter-Dawson LD, LaVail MM. J.Comp Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- 40.Rajala A, Dighe R, Agbaga MP, Andersonand RE, Rajala RV. J.Biol Chem. 2013;288:19503–19515. doi: 10.1074/jbc.M113.469064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sievingand PA, Swaroop A. Nat.Genet. 2001;29:447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Brown B, Li A, Mears AJ, Swaroopand A, Craft CM. J.Neurosci. 2003;23:6152–6160. doi: 10.1523/JNEUROSCI.23-14-06152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nikonov SS, Daniele LL, Zhu X, Craft CM, Swaroopand A, Pugh EN., Jr J.Gen.Physiol. 2005;125:287–304. doi: 10.1085/jgp.200409208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, Swaroop A, Williamsand DS, Pugh EN., Jr Invest Ophthalmol.Vis.Sci. 2005;46:2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajala A, Gupta VK, Andersonand RE, Rajala RV. Mitochondrion. 2013;13:566–576. doi: 10.1016/j.mito.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruning NM, Rinnerthaler M, Bluemlein K, Mulleder M, Wamelink MM, Lehrach H, Jakobs C, Breitenbachand M, Ralser M. Cell Metab. 2011;14:415–427. doi: 10.1016/j.cmet.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iqbal MA, Siddiqui FA, Gupta V, Chattopadhyay S, Gopinath P, Kumar B, Manvati S, Chamanand N, Bamezai RN. Mol.Cancer. 2013;12:72. doi: 10.1186/1476-4598-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]