Abstract
Spontaneous autoimmune polyneuropathy (SAP) in B7-2 knockout non-obese diabetic (NOD) mice mimics the progressive form of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), and is mediated by myelin P0-reactive Th1 cells. In this study, we focused on the effect of B7-2 deletion on the function of dendritic cells (DCs) within the context of SAP. We found that development of SAP was associated with a preponderance or increase of CD11b+ DCs in peripheral lymph nodes and sciatic nerves. B7-2 deletion led to altered immunophenotypic properties that differ between CD11b+ DCs and CD8α+ DCs. Both DC subsets from B7-2 knockout (KO) NOD mice exhibited impaired capacity to capture fluorophore-labeled myelin P0, but diminished antigen-presenting function was observed only in CD11b+ DCs. Clinical assessment, electrophysiologic studies and splenocyte proliferation studies revealed that absence of B7-2 on DCs was sufficient to cause impaired ability to induce tolerance to P0, which could be overcome by pre-conditioning with interleukin-10 (IL-10). Tolerance induction by antigen-pulsed WT NOD DCs was dependent on IL-10 and was associated with increased CD4+ regulatory T cells (Tregs), whereas tolerance induction by IL-10-conditioned B7-2 deficient DCs was associated with increased percentages of both Tregs and B10 cells in the spleen. We conclude that B7-2 deletion has an impact on the distribution of DC subsets in lymphoid organs, and alters the expression of co-stimulatory molecules, but functional consequences are not uniform across DC subsets. Defective tolerance induction in the absence of B7-2 can be restored by preconditioning of DCs with IL-10.
Keywords: Autoimmunity, dendritic cells, CIDP, Guillain Barré, syndrome, co-stimulatory molecules, Tregs
INTRODUCTION
Dendritic cells (DCs) are potent antigen presenting cells and play a crucial role in the orchestration of immune responses, both in terms of T cell immunity and in tolerance induction. There are two major categories of DCs (CD11c+ cells) in mice: 1) plasmacytoid DCs [pDCs (CD11cloCD11b−B220+)], also known as interferon-producing cells, and 2) conventional DCs (cDCs). The latter includes migratory DCs and lymphoid organ resident DCs, which are classified into CD8α+CD11b− and CD8α−C11b+ DC subsets. Though CD8α+ DCs and CD11b+ DCs have been shown to promote polarization of T helper (Th cells) toward Th1 and Th2 cells, respectively, both DC subsets are capable of interleukin-12 (IL-12) production, albeit requiring different stimuli (1–5). The capacity of DCs for initiating immunity or tolerance depends on their maturational and functional state, and on specialized DC subsets. Mature DCs are characterized by upregulation of MHC class II, B7 family molecules and CD40 resulting in enhanced immunogenicity (6). That DCs are required to maintain self-tolerance is supported by DC ablation studies showing the development of myeloid proliferative disease or fatal spontaneous autoimmunity depending on whether pDCs and Langerhans cells are spared or not (7, 8).
The role of DCs in the development of human autoimmune neuropathies such as Guillain Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) or their animal models has not been well-elucidated. Press and colleagues found increased number of CD11b+ DCs in the cerebrospinal fluid (CSF) of GBS and CIDP patients, which correlated positively with the severity of clinical disability in CIDP (9). In experimental autoimmune neuritis (EAN) in Lewis rats, disease severity can be attenuated by atorvastatin-modified DCs, which exhibit lower levels of co-stimulatory molecules B7-1, B7-2, and MHC class II (10).
The B7-1/B7-2:CD28/CTLA4 pathway plays a crucial role in T cell priming, CD4/CD8 homeostasis, as well as homeostasis of regulatory T cells (Tregs) (11, 12). In non-obese diabetic (NOD) mice, B7-2 elimination leads to protection from diabetes that is attributed to defective CD4 T cell priming, but it triggers the development of a spontaneous autoimmune polyneuropathy (SAP) (13, 14). The latter is characterized by electrophysiological findings of demyelinating features and axonal loss as well as presence of inflammatory cells in sections of sciatic nerves and dorsal root ganglia (13, 14). These findings mimic those of human CIDP, though the latter can be relapsing-remitting or progressive. In contrast, SAP mice exhibit progressive weakness to the point of quadriparesis. We and other investigators found that SAP is mediated by myelin P0-reactive Th1 cells and at least two epitopes are involved – P0 (180–199) and P0 (1–25) (13, 15–17). More recently, we have demonstrated that B cell autoreactivity to myelin P0 also contributes to the pathogenesis of SAP (18).
The goal of this study was to delineate the consequences of B7-2 deletion on DC function within the context of SAP, and to investigate whether absence of B7-2 on DCs is sufficient to cause defect in peripheral tolerance to myelin P0 in vivo. There is evidence that B7-2 promotes the survival and function of DCs. B7-2-deficient DCs exhibited enhanced susceptibility to death. In addition, there was reduced proportion of CD8α+ DCs and increased frequency of both B7-H1 and B7-DCs in pancreatic lymph nodes (PLN) of B7-2 knockout (KO) NOD mice (19). Here, we report that the functional consequences of B7-2 deletion differ between CD11b+ DCs and CD8α+ DCs in some aspects. In addition, we provide evidence to support the concept that absence of B7-2 on DCs contributes to the loss of tolerance to myelin P0 in SAP, which could be overcome by preconditioning with IL-10.
MATERIALS AND METHODS
Animals, clinical and electrophysiologic assessment
WT NOD, B7-2 KO NOD, IL-10 KO NOD and Foxp3-eGFP-Cre NOD mice (Jackson Laboratory, Bar Harbor, Maine) were housed and bred in pathogen-free conditions in the Animal Barrier Facility. All animal use procedures were conducted in strict accordance to the National Institutes of Health and University of Chicago institutional guidelines. Female B7-2 KO NOD mice were used in this study unless otherwise stated. For clinical assessment, the following scale was used: 0- normal; 0.5- mild ruffled coat; 1- less active or flaccid tail; 1.5- one leg is curled in when held by tail; 2- mild paraparesis (both legs curled in); 2.5- drags one leg; 3- severe paraparesis (drags both legs); 3.5- severe tetraparesis; 4- death. Grip strength testing consisted of five separate measurements in each of two trials per session using a grip strength meter (Columbus Instruments, OH). Results of two trials were averaged for each mouse per session. After the last grip strength measurement, electrophysiologic studies of sciatic nerves were performed as described in our previous publications (20). Latencies, conduction velocities, and peak to peak amplitudes were measured. Results from stimulation of bilateral sciatic nerves were averaged for each animal, with “n” representing the number of animals in each study group.
Generation and purification of extracellular domain of P0 (P0-ECD)
The P0-ECD construct that contained the 124 amino acid residues (aa 1–124) from the extracellular domain (ECD) of rat P0 was cloned and expressed using the expression vector C5 (GenScript, Piscataway, NJ) or pET23d (+)( EMD Millipore, Billerica MA) as described previously (18). The purity of the His-tagged P0-ECD protein was confirmed by Western blot analysis using HRP-conjugated goat anti-6-His antibody (Ab) (1: 10,000) (Bethyl Lab, Montgomery, TX). The final endotoxin level was reduced to <1 EU/µg using the ToxinEraser Endotoxin Removal kit (Genscript, Piscataway, NJ). Purified P0-ECD was quantified using the Pierce BCA method (Thermo Scientific, Rockford, IL), then labeled with Alexa-Fluor 546 using a protein labeling kit (Invitrogen, Eugene, OR) according to the manufacturer’s instructions.
In vitro co-cultures and cell proliferation studies
Splenocyte proliferation was determined using [3H]thymidine incorporation assay as described previously (16). Cells were stimulated with P0 (180–199) (20 µg/ml) or P0–ECD (20 µg/ml) for 72 hrs. On day 3, cultures were pulsed for 16 h with 1 µCi methyl-[3H]thymidine. The stimulation index was defined by cpm in the presence of antigen divided by cpm in the absence of antigen. P0 peptide (180–199) was purchased from Genscript (Piscataway, NJ).
For DC-CD4+ T cell co-cultures, DCs were purified from splenocytes by incubation with CD11c microbeads (magnetically labeled) for 30 min followed by positive selection through MACS separation columns (Miltenyl Biotech, Auburn, CA). Purified DCs were further separated into CD11b+CD11c+ and CD8α+CD11c+ DC subsets by sequential positive selection steps with CD11b microbeads or with PE-conjugated anti-CD8α Ab followed by PE-microbeads, respectively. CD4+ T cells were isolated using a CD4 T cell negative selection kit (Invitrogen, Carlsbad, CA). Purified CD11b+ DCs and CD8α+ DCs were co-cultured with CD4+ T cells at varying ratios in R10 media (RPMI +10% FBS v/v, 0.1 mM nonessential amino acids, 50 µM mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin) in the presence or absence of antigen for 3 days. Culture medium also contained an inhibitor of inducible nitric oxide synthase (iNOS) aminoguanidine (1 mM), and a nonselective cyclooxygenase (COX) inhibitor indomethacin (20 µM). For CD4+ T cell proliferation, these cultures were pulsed for 16 h with 1 µCi methyl-[3H]thymidine on day 3.
For DC-induced proliferation of Tregs, a BD FACSAria cell sorter was used to sort CD4+Foxp3+ (eGFP+) from splenocytes and peripheral LN cells isolated from Foxp3-eGFP-Cre NOD mice. Total splenic CD11c+ DCs were purified from WT or B7-2 KO NOD mice using a Dynabeads mouse DC negative isolation kit (Invitrogen, Carlsbad, CA). Sorted Tregs (2 × 105) were labeled with 1 µM Dye eFluor 670 (eBioscience, San Diego, CA) and co-cultured with either WT or B7-2 KO DCs at 2:1 ratio in R10 media plus recombinant mouse IL-2 (200 U/ml) (R&D, Minneapolis, MN) with or without P0 (20 µg/ml) for 5 days, followed by harvesting for flow cytometric analysis of dye dilution. For generation of induced Tregs (iTregs), sorted CD4+Foxp3− (eGFP−) were co-cultured with splenic DCs at 2:1 ratio in a U-bottomed 96-well plate pre-coated with 5 µg/ml anti-CD3 Ab (Biolegend, San Diego, CA) in the presence of 5 ng/ml transforming growth factor-beta (TGF-β) (R&D, Minneapolis, MN) with or without 200 U/ml IL-2 or 5 µg/ml anti-CD28 Ab (Biolegend, San Diego, CA).
Flow cytometry and intracellular cytokine staining
Single-cell suspensions from spleen and lymph nodes were stained at 4°C using predetermined optimal concentrations of Abs for 30 min. Cells with the forward and side scatter properties of lymphocytes were analyzed using Fortessa flow cytometer (BD Bioscience, San Jose, CA). Background staining was assessed using isotype matched control (Ctrl) Abs. The following Abs were used: APC-conjugated anti-mouse CD11c, FITC-conjugated anti-mouse CD11b (BioLegend, San Diego, CA) , FITC-conjugated anti-mouse CD8α (BD Biosciences, San Jose, CA), PE-conjugated anti-mouse B7-1, MHC class II (anti-RT1b), CD40, ligand of inducible co-stimulatory molecule (ICOSL) (Biolegend, San Diego, CA). Splenic CD1dhiCD5+ B cells were determined using V450-conjugated anti-mouse CD19, PE-conjugated anti-CD5, Alexa fluor 647-conjugated CD1d (Biolegend, San Diego, CA). For the detection of Tregs, splenocytes were stained with FITC-conjugated anti-mouse CD4 and APC-conjugated anti-mouse CD25 Abs, fixed, permeabilized, and subsequently stained with PE-conjugated anti-mouse Foxp3 Ab (eBioscience, San Diego, CA).
For intracellular cytokine staining, splenocytes (1 ×106/well) in 96-well plates were stimulated at 37°C in a humidified CO2 incubator for 4 hours with Leukocyte Activation Cocktail (BD Pharmingen, San Jose, CA). This was followed by staining for cell surface CD4 and intracellular interferon-γ (IFN-γ), IL-17, IL-10, or tumor necrosis factor-α (TNF-α) using the Intracellular Cytokine Staining Starter Kit (BD Pharmingen, San Diego, CA). The percentage of IFN-γ, IL-17, IL-10, and TNF-α producing CD4+ T cells was analyzed by Fortessa flow cytometer and FlowJo software. With regards to B10 cells, splenocytes were incubated for 4 hours in 96-well plates with lipopolysaccharide (LPS, 10 µg/ml) in addition to Leukocyte Activation Cocktail. Cells were then stained with V450-conjugated anti-mouse CD19 Ab followed by fixation and permeabilization using Cytofix Kit prior to staining with PE-conjugated anti-mouse IL-10 Ab (BD Biosciences, San Jose, CA).
Real-time RT-PCR
The total RNA was isolated using RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcription was performed from 1 µg total RNA and the complementary DNA (cDNA) was used for SYBR green real-time PCR. Amplification was performed with forward and reverse primers for transcripts of interest, which was designed using Primer3 software. The expression of each cytokine gene was normalized by corresponding amount of GAPDH mRNA for each condition. The relative amounts of each product were calculated by the comparative CT (2−ΔΔCT) method as described in the user manual #2 of ABI Prism 7900 Sequence Detection System (Applied Biosystems). Primer sequences used are listed in Supplemental Table 1.
Antigen capture
Alexa546-labeled P0-ECD (200 µg) was injected i.v. into the tail veins of 6 wk old WT NOD and B7-2 KO NOD mice. One hour later, animals were sacrificed, and splenocytes were isolated for flow cytometric analysis of Alexa546 signal in CD11b+ and CD11b− DCs.
Adoptive transfer (AT) studies
DCs (CD11c+ cells) were purified from splenocytes using Dynal Mouse DC negative isolation kit (Invitrogen, Carlsbad, CA), and treated overnight with P0 (180–199) (20 µg/ml) prior to AT. Each recipient mouse (5 mo old female B7-2 KO NOD) was injected i.v. with 6 × 106 WT, B7-2 KO or IL-10 KO DCs from 3 mo old donor animals. In a subset of experiments, purified B7-2 KO DCs were pretreated with recombinant mouse IL-10 (200 ng/ml) for 3 days in R10 media. On day 3, P0 (180–199) (20 µg/ml) was added for 16 hours (overnight) prior to AT.
Data analysis
Results from clinical severity, immunologic studies, grip strength measurements and electrophysiology are expressed as mean ± SEM. Statistical significance for these data was determined by analysis of variance (ANOVA) followed by Student’s t test and the Bonferroni method for multiple group experiments. Significance levels were set at p < 0.05.
RESULTS
DC subsets, immunophenotypic properties, and cytokine expression
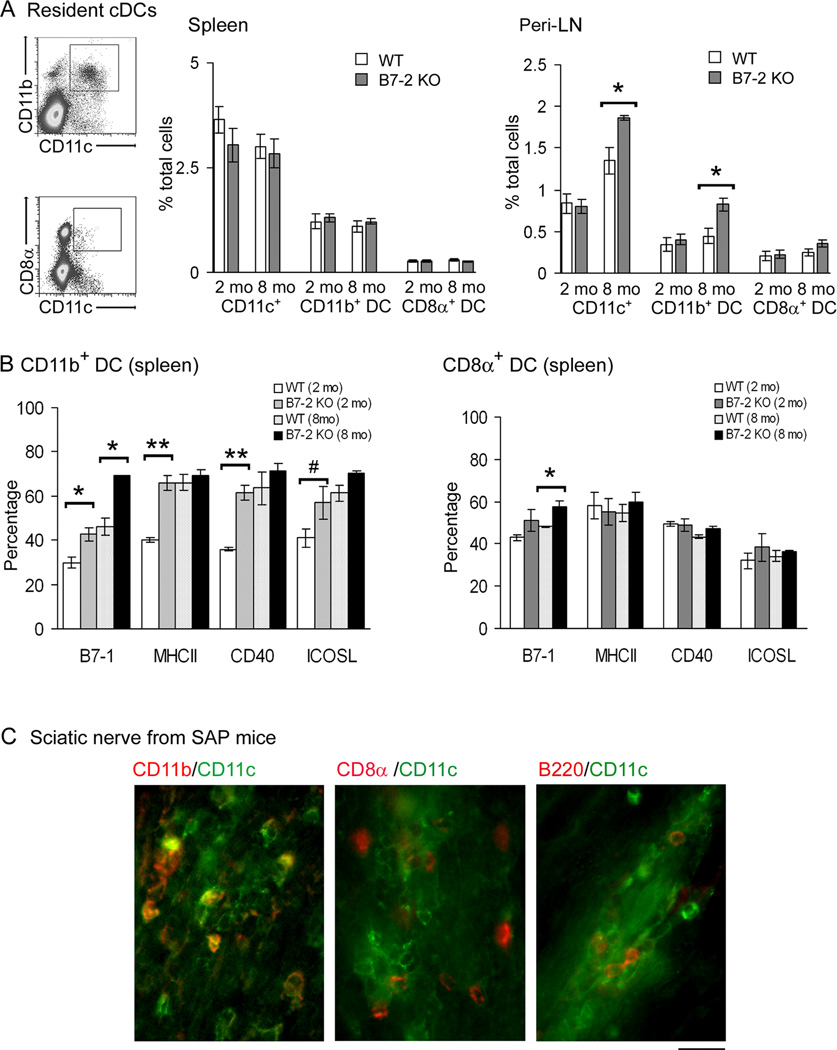
B7-2 KO NOD mice usually start to develop hindlimb weakness ~5–6 mo of age. By 8 mo, 100% of female B7-2 KO NOD mice would exhibit clinical and electrophysiological findings of SAP (13). Flow cytometry was performed to investigate whether the development of SAP is accompanied by changes in the proportion of CD11b+ DCs and CD8α+ DCs in the spleen and peripheral LN (inguinal and axillary). Compared to the WT NOD mice, B7-2 KO NOD mice exhibited an increase in the percentage of total DCs and CD11b+ DCs in the peripheral LN (Peri-LN) at 8 mo but not at 2 mo (Fig. 1A). The proportion of CD11b+ DCs and CD8α+ DCs in the spleen did not differ between WT and B7-2 KO NOD mice. The percentage of pDCs in the spleen was also not affected by B7-2 elimination (n = 3, data not shown). The frequency of pDCs in Peri-LN was too low to permit accurate determination.
Figure 1. Age-dependent changes in conventional DC subsets in B7-2 KO NOD mice.
A. Increase in the percentages of total DCs (CD11c+) and CD11b+ DCs, but not CD8α+ DCs in Peri-LN from 8 mo old B7-2 KO NOD mice compared to age-matched WT NOD mice. Total splenocytes or Peri-LN cells were gated with exclusion of dead cells, followed by analysis of CD11c, CD11b and CD8α expression. *p <0.0005 for Peri-LN at 8 mo, but not at 2 mo. No significant difference in DC subsets was noted in the spleen. Values represent mean ± SEM (n = 5). B. Immunophenotypic analysis of DC subsets (B7-2 KO vs WT). Cells were gated based on CD11c, CD11b and CD8α staining, then analyzed for B7-1, MHC class II, CD40 and ICOSL expression. Comparing B7-2 KO vs WT CD11b+ DCs, *p < 0.01 for B7-1 at 2 mo & 8 mo; **p < 0.001 for MHCII and CD40 at 2 mo; #p < 0.04 for ICOSL at 2 mo. Comparing B7-2 KO vs WT CD8α+ DCs, *p < 0.02 for B7-1 at 8 mo. Values represent mean ± SEM (n = 3). C. Examples of fluorescence micrographs of sciatic nerve sections from SAP mice showing the frequent colocalization of CD11c with CD11b, but only rarely with CD8α or B220. Calibration bar represents 20 µm.
B7-2 deletion resulted in a higher proportion of B7-1+CD11b+ DCs in the spleen at 2 mo and 8 mo, and increased percentages of MHCII+, CD40+ and ICOSL+ CD11b+ DCs at 2 mo. For CD8α+ DCs, the only difference was an increased proportion of B7-1+ cells in the spleen of B7-2 KO mice at 8 mo (Fig. 1B). A higher proportion of B7-1+, MHCII+, and CD40+CD11b+ DCs was also noted in the peri-LN of B7-2 KO NOD mice at 2 mo and 8 mo, whereas only increased percentages of B7-1+, and MHCII+ CD11b+ DCs were noted in PLN of B7-2 KO NOD at 2 mo, but not at 8 mo (Suppl. Fig. 1). Immunophenotypic properties were not assessed for CD8α+ DCs from Peri-LN and PLN due to low frequency. Immunofluorescence studies on sciatic nerve sections from SAP mice revealed frequent co-localization of CD11c with CD11b, but not with CD8α. Rare pDCs (B220+CD11c+) were observed (Fig. 1C). Subsequent studies were focused on splenic CD11b+ DCs, CD8α+ DCs or total DCs.
Effect of B7-2 elimination on DC function
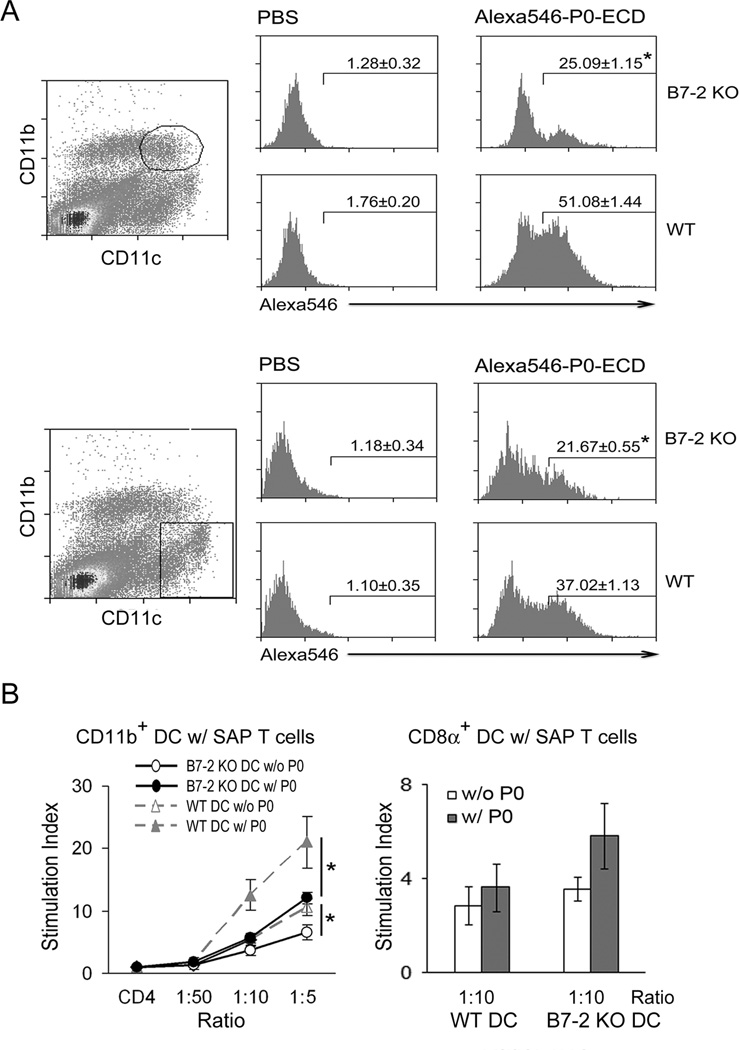
The extracellular domain of P0 (P0-ECD) was purified and labeled with Alexa546, which was used to determine whether B7-2 elimination leads to altered capability of DCs to capture SAP antigen in vivo. At one hour after intravenous injection, both splenic CD11b+ DCs and CD11b− DCs from 6 wk old B7-2 KO mice exhibited a significant reduction in the percentage of labeled P0-ECD+ cells when compared to those from WT NOD mice (Fig. 2A).
Figure 2. Effect of B7-2 deletion on antigen capture and T cell proliferation-induced by DCs.
A. Reduced antigen capture by DCs from B7-2 KO mice compared to those from WT NOD mice. Six weeks old animals were injected intravenously with 200 µg of Alexa546-labelled P0-ECD and sacrificed 1 hr later. Splenocytes were processed for flow cytometric analysis of Alexa546 signal in CD11b+ DCs and CD11b DCs. Values shown in the histogram represent mean percentage ± SEM (n = 3). *p < 0.003 for both CD11b+ DCs and CD11b DCs comparing B7-2 KO vs WT NOD. B. Proliferative responses of CD4+ T cells from SAP mice (8 mo) alone or co-cultured with splenic DCs (3 mo) with or without myelin P0 (180–199) (20 µg/ml). Left panel: CD11b+ DC-CD4 T cell co-cultures. Comparing WT vs B7-2 KO CD11b+ DC at 1:5 ratio, *p < 0.05 with or without P0 (180–199) (n = 3 each). Right panel: CD8α+ DC-CD4 T cell co-cultures. Comparing WT vs B7-2 KO CD8α+ DC, p > 0.05 with or without P0 (180–199) (n = 5).
Next, we examined the ability of CD11b+ DCs to stimulate T cell proliferation at varying DC-T cell ratio (1:50, 1:10, 1:5). For these experiments, CD4+ T cells were isolated from symptomatic 8 mo old B7-2 KO mice, while DC subsets were isolated from 2–3 mo old B7-2 KO or WT NOD mice. As shown in Fig. 2B, B7-2 KO CD11b+ DCs exhibited a diminished capacity to stimulate T cell proliferation at baseline and in response to 20 µg/ml P0 (180–199) when compared to WT CD11b+ DCs. In contrast, there was a trend towards slightly enhanced T cell proliferative response to P0 (180–199) by B7-2 KO CD8α+ DCs compared to WT CD8α+ DCs, but the difference did not reach statistical significance.
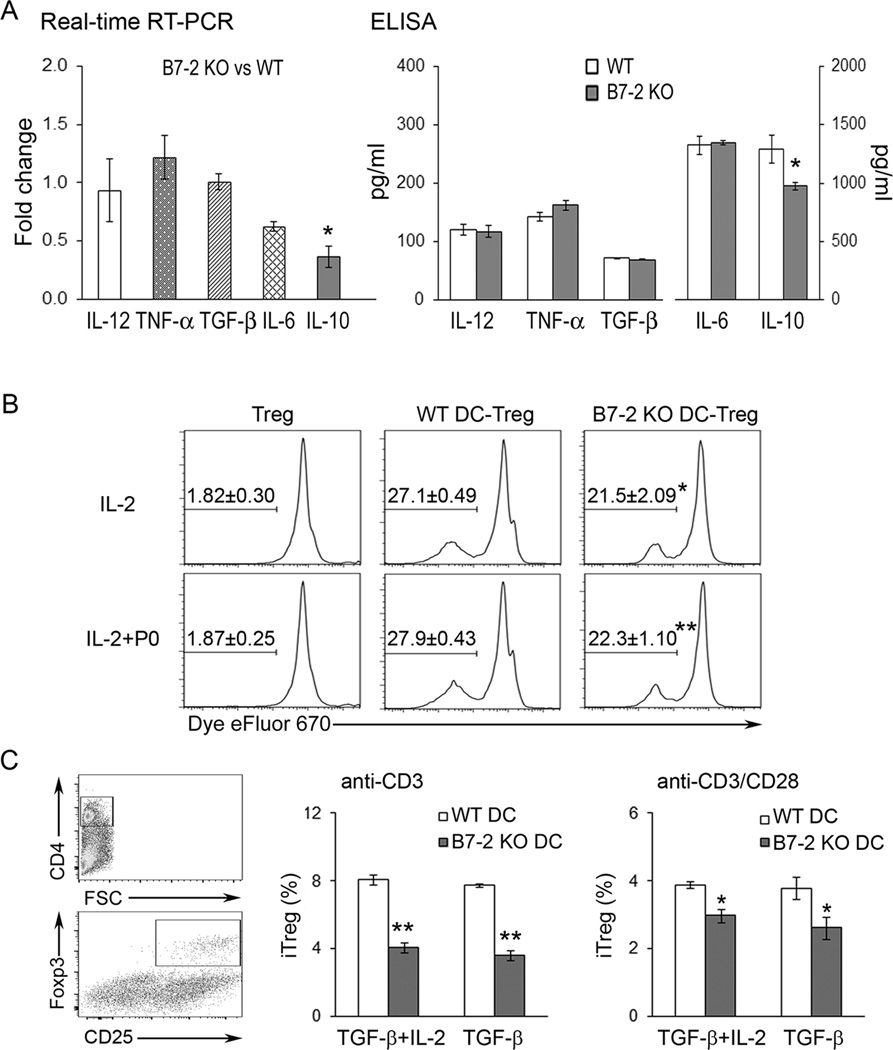
Further studies on the functional consequences of B7-2 deletion were carried out using total splenic DCs. Real-time RT-PCR technique was used to compare the mRNA levels of IL-12 p40, TNF-α, TGF-β, IL-6 and IL-10 in purified, unstimulated WT and B7-2 KO DCs from 2–3 mo old animals. Comparing B7-2 KO DCs vs WT DCs (n = 3 each), there was a significant decrease in the mRNA levels of IL-10 but not of other cytokines (Fig. 3A). Cytokine secretion was also determined by ELISA in DCs cultured in the presence of LPS (10 µg/ml) for 2 days. Lack of B7-2 on DCs led to decreased IL-10 production in response to LPS compared to WT NOD DCs, but did not affect the secretion of the other cytokines tested, including IL-12 p70 (Fig. 3A). Results were similar when DCs were exposed to LPS for one day only (n = 3, data not shown).
Figure 3. Effect of B7-2 deletion on other DC functions.
A. Cytokine expression and secretion. IL-10 mRNA levels were decreased in freshly isolated splenic DCs from B7-2 KO compared to WT NOD mice (*p < 0.05, n = 3). Real-time PCR results are expressed as fold change using the comparative CT (2−ΔΔCT) method. For cytokine secretion in response to LPS (10 µg/ml) for 2 days, levels of IL-10 in supernatants were also reduced in splenic DCs from B7-2 KO compared to those from WT NOD mice (*p < 0.05, n =3). B. DC-induced Treg proliferation in the presence of IL-2 (200 U/ml) with or without P0 (180–199) (20 µg/ml) for 5 days. Treg: sorted CD4+ Foxp3+ (eGFP+) cells from Foxp3-eGFP-Cre NOD mice. Values shown in the histogram represent mean percentage ± SEM (n = 6). Comparing WT DC vs B7-2 KO DC, *p < 0.01 for IL-2 only, and **p <0.001 for IL-2+P0. C. Generation of iTregs from sorted CD4+Foxp3 (eGFP) cells by WT or B7-2 KO DCs upon exposure to plate-bound anti-CD3 Ab in the presence of TGF-β (5 ng/ml) ± IL-2 (200 U/ml). Gating strategy is shown in the scatterplots. Comparing WT DC vs B7-2 KO DC, **p < 0.001 with anti-CD3 Ab only; *p < 0.04 with anti-CD3 plus soluble anti-CD28 Ab (5 µg/ml) (n = 3).
B7/CD28 interactions are required not only for optimal T cell activation, but also for the generation and homeostasis of Tregs (12, 21). To assess the impact of B7-2 deletion on the expansion of splenic Tregs, we utilized Foxp3-eGFP-Cre NOD mice as the source of CD4+Foxp3+ (eGFP+) and CD4+Foxp3− (eGFP−) T cells. Splenic CD4+GFP+ cells were first confirmed to be > 95% Foxp3+ by flow cytometry (data not shown). Dye dilution studies revealed that absence of B7-2 on DCs led to decreased proliferation of Tregs (CD4+eGFP+ cells) (Fig. 3B). In the presence of DCs and IL-2 (200 U/ml), myelin P0 was not required to induce Treg proliferation. The percentage of iTregs generated from CD4+eGFP− cells upon anti-CD3 stimulation was also reduced when B7-2 KO DCs were used in co-cultures instead of WT DCs. Results were similar with TGF-β (5 ng/ml) alone or with TGF-β plus IL-2 (200 U/ml). Paradoxically, addition of anti-CD28 mAb (5 µg/ml) led to decreased frequency of iTregs and did not overcome the effect of B7-2 deficiency on DCs (Fig. 3C).
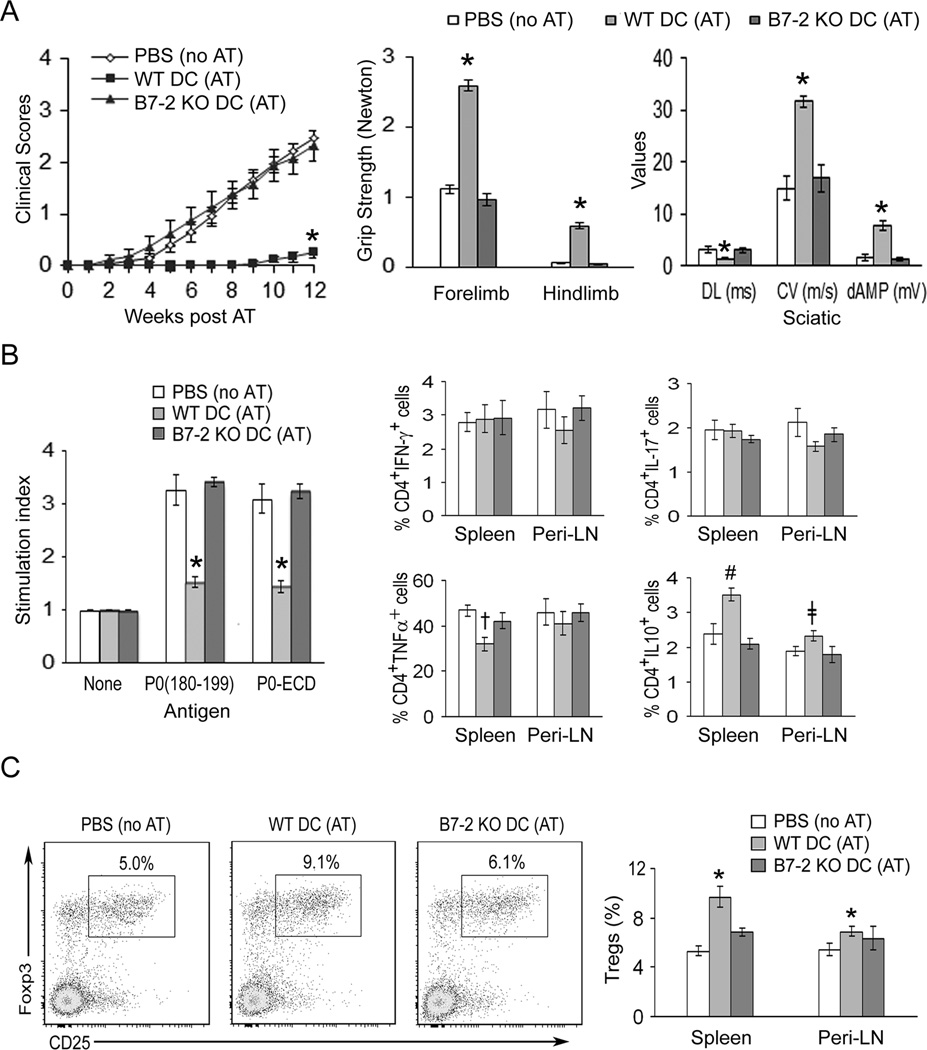
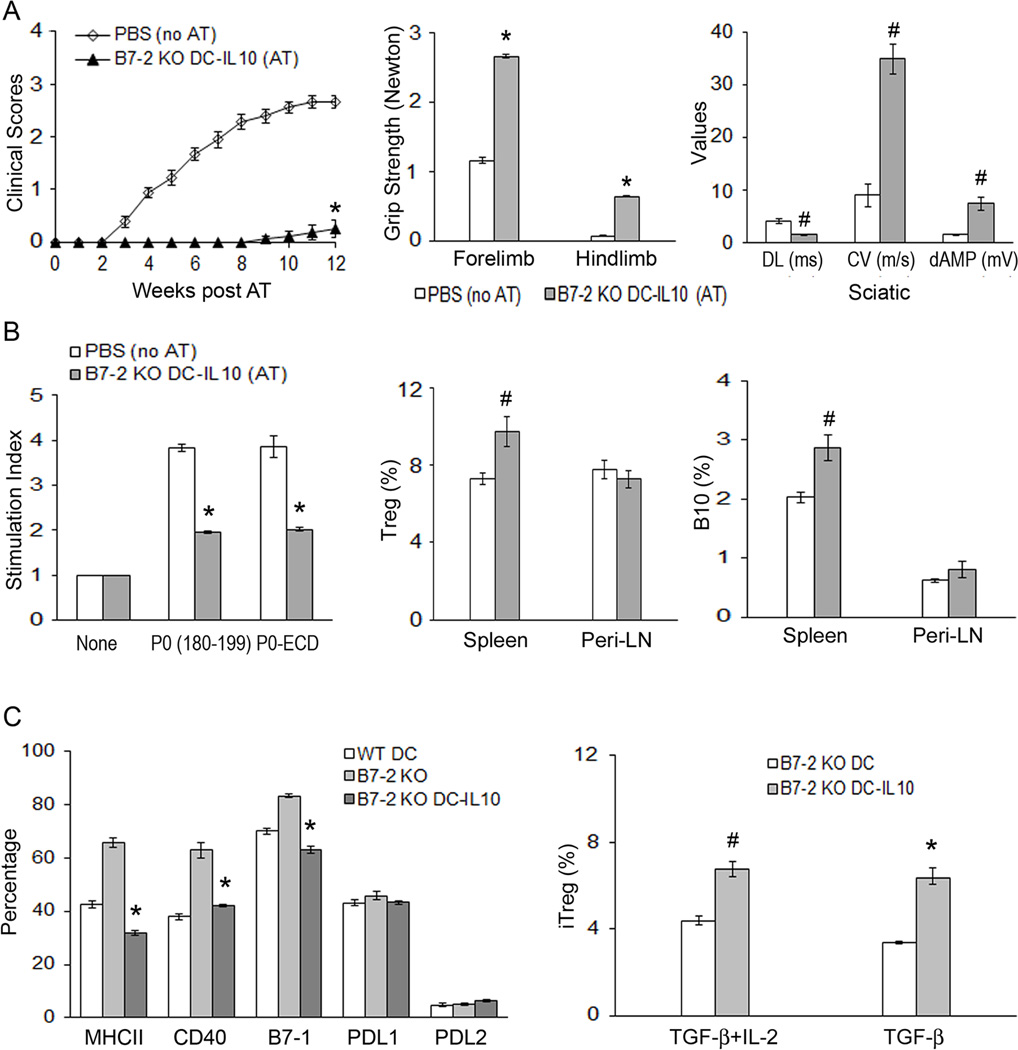
To investigate the consequence of B7-2 deletion on DC function in vivo, we compared the capacity of WT vs B7-2 KO DCs to induce tolerance in adoptive transfer (AT) experiments. Recipient B7-2 KO NOD mice (5 mo old) were divided into 3 groups: Group A received PBS injections (no AT); Group B received P0 (180–199)-pulsed WT DCs, designated as WT DC (AT); Group C received P0 (180–199)-pulsed B7-2 KO DCs, designated as B7-2 KO DC (AT). Clinical assessment (clinical score and grip strength measurements) revealed that the development of SAP was abrogated by adoptive transfer of P0 (180–199)-pulsed WT DCs, but not by P0 (180–199)-pulsed B7-2 KO DCs (Fig. 4A). Induction of tolerance was confirmed by electrophysiological studies showing dramatic improvement in distal latencies, conduction velocities and distal amplitudes of sciatic motor response (Fig. 4A). Of note, WT DCs that were not pulsed with P0 (180–199) did not induce tolerance to the development of SAP (n = 5) (Suppl. Fig. 2).
Figure 4. Induction of tolerance by P0 (180–199)-pulsed DCs from WT mice, but not by DCs from B7-2 KO NOD mice.
A. Attenuation of disease severity. Splenic DCs from 3 mo old mice were exposed to 20 µg/ml P0 (180–199) overnight. Adoptive transfer (AT) of DCs (6 × 106) into B7-2 KO NOD mice was performed at 5 mo. For mean clinical scores, *p < 0.0001 for WT DC (AT) vs B7-2 KO DC (AT) or control mice (PBS, no AT). Grip strength measurements and sciatic nerve electrophysiology were performed at 12 weeks post AT. Comparing WT DC (AT) vs the other two groups, *p < 0.0001 for forelimb and hindlimb, and for all the parameters of sciatic motor responses [Distal latency (DL), conduction velocity (CV), and distal amplitude (dAMP)]. B. Proliferation and cytokine profile at 12 wks post AT. For splenocyte proliferation, results from 3[H]-thymidine incorporation were expressed as stimulation index. [Ag]: 20 µg/ml; treatment duration: 72 hr. *p < 0.0001 for WT DC (AT) vs B7-2 KO DC (AT) or PBS (no AT). Comparing cytokine profile of WT DC (AT) vs the other two groups,†p < 0.001 for TNFα+ splenic CD4+T cells; #p < 0.01 for IL-10+ CD4 T cells in spleen and ǂp < 0.03 for IL-10+ CD4+ T cells in Peri-LN. Data represent mean ± SEM (n = 10 for PBS, n = 12 for WT DCs, and n = 8 for B7-2 KO DCs) for A &B. C. Increased percentage of Tregs (CD25+Foxp3+/CD4+) in tolerized mice. *p < 0.02 for WT DC (AT) vs other two groups in the spleen, and for WT DC (AT) vs PBS (no AT) in LN. Bar graphs in C indicate mean ± SEM (n = 7 for PBS, n = 10 for WT DC, and n = 7 for B7-2 KO DC).
Animals from AT experiments were sacrificed at 12 weeks post transfer (~ 8 mo of age) for immunologic studies. Splenocyte proliferation-induced by 20 µg/ml P0 (180–199) or P0-ECD was decreased in tolerized mice [WT DC (AT) group] compared to B7-2 KO DC (AT) group or PBS (no AT) group. There was no difference in the percentage of CD4+IFNγ+ T cells or CD4+IL17+ T cells in spleens and Peri-LN of animals from these 3 groups. However, there was a reduction in splenic CD4+TNFα+ T cells, and an increase in CD4+IL10+ T cells in spleens and peri-LN of WT DC (AT) group (Fig. 4B).
To delineate the effect of adoptive transfer of antigen-pulsed DCs on regulatory mechanisms, we examined the frequency of CD4+ Tregs in spleens and Peri-LN of animals from 3 study groups. The frequency of CD4+ Tregs (expressed as % CD25+Foxp3+ cells in CD4+ T cells) was significantly increased in the spleen of tolerized mice [WT DC (AT)] group compared to the other two groups. In Peri-LN, a significant difference in the frequency of Tregs was observed only when comparing WT DC (AT) group to PBS (no AT) group, but not when comparing to B7-2 KO DC (AT) group (Fig. 4C). The effect of DCs on splenic B cell subsets with regulatory activity was also investigated. B cells that express IL-10 after 4–5 hr of exposure to phorbol ester and ionomycin are designated as B10 cells, and are found predominantly within CD1dhiCD5+CD19+ subset (22). In contrast to the effect on Tregs, adoptive transfer of antigen-pulsed WT DCs or B7-2 KO DCs had no effect on the frequency of CD1dhiCD5+ subset in B cells (CD19+) or in the percentage of B10 cells in total splenocytes or Peri-LN cells (n = 7–10, data not shown).
Given that B7-2 KO DCs had lower IL-10 expression compared to WT DCs, we investigated whether its defect in tolerance induction can be corrected by treatment with IL-10 (200 ng/ml) for 3 days in vitro prior to AT. P0 (180–199) was added on the 3rd day of preconditioning. As shown in Fig. 5A, adoptive transfer of IL-10-conditioned B7-2 KO DCs led to induction of tolerance to myelin P0 when compared to PBS (no AT) group. Improvement in clinical scores, grip strength and electrophysiologic parameters was demonstrated in tolerized animals, which was accompanied by decreased splenocyte proliferative response to P0 (180–199) or P0-ECD, and by increased frequency of splenic CD4+ Tregs and B10 cells (Fig. 5B).
Figure 5. Preconditioning of B7-2 KO DCs with IL-10 restores their capacity to induce tolerance to myelin P0.
Adoptive transfer of DCs was performed in 5 mo old B7-2 KO NOD mice, as described in Fig. 4. Statistical analysis was made comparing B7-2 KO DC-IL-10 group vs. PBS (no AT) group in A-B. A. Mean clinical scores. *p < 0.0001 (n = 8–9). Grip strength measurements and sciatic nerve electrophysiology were performed at 12 weeks post AT. For grip strength, *p < 0.0001 (n = 8–9); #p < 0.0003 for all electrophysiologic parameters (DL, CV, dAMP) (n = 6–7). B. Immunologic studies at 12 wks post AT. Tolerance induction by B7-2 KO DC (IL-10) was associated with decreased splenocyte proliferation in response to P0 (180–199) or P0-ECD (20 µg/ml), increased percentages of Tregs (CD25+Foxp3+/CD4+) and B10 cells in the spleen but not in the Peri-LN. *p < 0.0002 (n = 4–5) for splenocyte proliferation; #p < 0.003 (n = 5–6) for splenic Tregs, and p < 0.003 (n = 6–7) for splenic B10 cells. C. Characterization of IL-10-preconditioned B7-2 KO DCs. Left panel: IL-10 pretreatment of B7-2 KO DCs led to altered immunophenotypic properties that mimicked those of WT DCs. Cells were gated based on CD11c staining, then analyzed for MHC class II, CD40, B7-1, PDL1 and PDL2 expression. Comparing B7-2 KO vs B7-2 KO DC-10, *p < 0.0002 for MHCII, CD40 and B7-1 (n = 4 each). Right panel: Improved capacity of B7-2 KO DCs to generate iTregs from sorted CD4+Foxp3 (eGFP) cells by IL-10 preconditioning [#p < 0.002 and *p < 0.0002 (n = 3 each)]. Experimental conditions were as described in Fig. 3C.
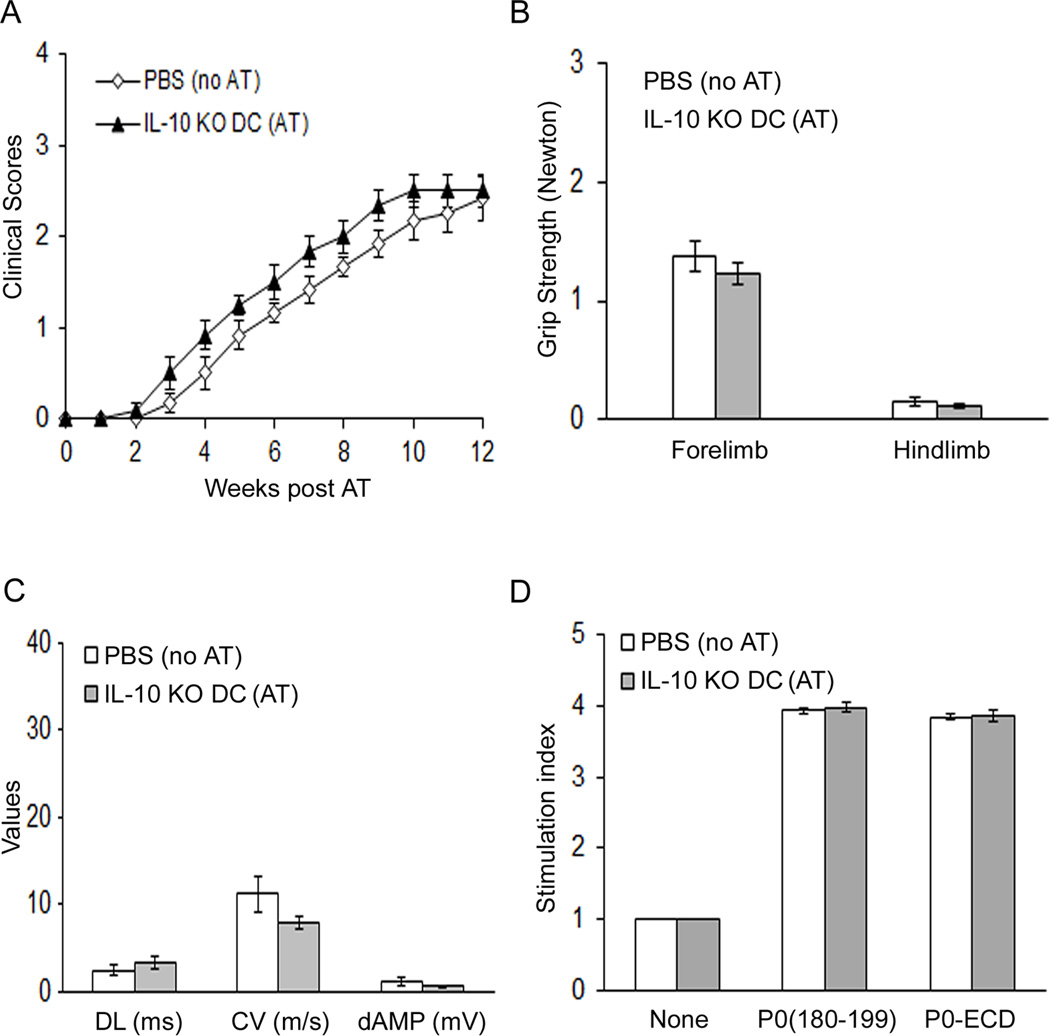
We examined the effect of IL-10 preconditioning in vitro on the proportion of MHCII+, CD4+, B7-1+, PDL1+ and PDL2+ splenic DCs. Pretreatment of splenic DCs from 3 mo old B7-2 KO NOD mice with IL-10 for 3 days led to reversal of immunophenotypic properties to mimic those of WT NOD DCs. Furthermore, IL-10-preconditioned B7-2 KO DCs exhibited improved capacity to generate iTregs from CD4+eGFP− cells upon anti-CD3 stimulation compared to unconditioned B7-2 KO DCs (Fig. 5C). IL-10 pretreatment for 3 days did not have an effect on antigen uptake by WT or B7-2 KO NOD DCs in vitro (Suppl. Fig. 3). Thus, IL-10 preconditioning converted B7-2 KO DCs back to WT NOD phenotype in some but not all aspects. To further confirm the crucial role of IL-10 in the induction of tolerogenic DCs, splenic DCs were purified from 3 mo old IL-10-deficient NOD mice for adoptive transfer into 5 mo old B7-2 KO NOD mice. Deletion of IL-10 led to impaired capacity of WT DCs to induce tolerance to myelin P0, as shown in Fig. 6A–D.
Figure 6. Failure of IL-10-deficient NOD DCs to induce tolerance to myelin P0.
Splenic DCs were purified from 3 mo old IL-10 KO NOD mice for adoptive transfer into 5 mo old B7-2 KO NOD mice, as described in Fig 4. A. Mean clinical scores. B. Grip strength measurements. C. Data from sciatic nerve electrophysiology. D. Splenocyte proliferation. Data represent mean ± SEM (n = 6 for each group).
DISCUSSION
B7-2 plays an important role in the survival and function of DCs. Absence of B7-2 leads to impaired protein kinase C-ε response when stimulated with CD28-Ig (19). In this study, we have demonstrated that deletion of B7-2 leads to age-dependent alterations in immunophenotypic properties that differ between CD11b+ DCs and CD8α+ DCs. We found increased percentages of B7-1+, MHC class II+, CD40+ and ICOSL+ splenic CD11b+ DCs, but no corresponding changes in CD8α+ DCs at 2 mo of age. At 8 mo, increased percentages of B7-1+CD11b+ DCs and B7-1+ CD8α+ DCs were noted in B7-2 KO NOD mice. We observed some tissue heterogeneity in the expression /regulation of co-stimulatory molecules when comparing peri-LN and PLN, though it may not be sufficient to explain the absence of diabetes in SAP mice. For example, the percentage of B7-1+ CD11b+ DCs was increased in peri-LN of B7-2 KO NOD mice at 2 mo and 8 mo when compared to age-matched WT NOD mice, whereas it was increased in PLN at 2 mo, but unchanged at 8 mo. Enhanced expression of MHC class II, B7-1 and CD40 would lead to augmented positive signals to T cells, contributing to enhanced autoimmunity. On the other hand, increased ICOSL-ICOS signaling in mice is associated with induction of Th2 cells or Tregs, though it can also induce Th1 cells and support Th17 cells under certain circumstances (23–26). Studies using bone marrow DCs from NOD mice suggest that there is an inherent bias towards high costimulation and Th1-induction compared to DCs from other mouse strains, though impaired DC maturation in NOD mice has also been reported (27–30).
We found that B7-2 KO NOD mice exhibited an increase of CD11b+ DCs in Peri-LN and sciatic nerves during the symptomatic phase. This finding raises the possibility that the main DC subset involved in the pathogenesis of SAP is CD11b+ DCs, and is consistent with observations in human CIDP where increased number of CD11b+ DCs in the CSF correlates positively with clinical disability (9). Whether a specific DC subset is linked to autoimmunity vs. tolerance has been the subject of intense investigations, and results are not always concordant from one experimental model of autoimmune disease to another (6, 31). Insulitis is attenuated by ablation of CD11b+ DCs, but accelerated by loss of pDCs in NOD mice (32). The protective effect of pDCs is also observed in experimental autoimmune encephalomyelitis (EAE)-induced by myelin oligodendrocyte glycoprotein (MOG) peptide (35–55) (33). On the other hand, CD11b+ DCs in the CNS preferentially induces Th17 cells in EAE-induced by proteolipid protein (PLP) in SJL/J mice, whereas they are associated with induction of Th2 cells and CD4+Tregs in MOG EAE in C57BL/6 or SJL/J mice (3, 34, 35). Therefore, it appears that CD11b+ DCs can be pathogenic or tolerogenic depending on the context, cytokine milieu, and the disease model.
DCs capture, process and present self and exogenous antigens to induce immunity or tolerance. The aberrant autoreactivity to P0 in SAP is not due to enhanced antigen uptake or presentation. We found that B7-2 deletion resulted in reduced uptake of Alexa546-labeled P0-ECD by both splenic CD11b+ DCs and CD8α+ DCs, which confirmed some but not all the findings from the work by other investigators (19). In the latter study, reduced endocytosis of FITC-labeled dextran (polysaccharide) was limited to B7-2 deficient CD8α+ DCs, which correlated with decreased proportion of CD8α+ DCs expressing antigen uptake receptors such as CD205 and CD16/32 (19). As expected, impaired co-stimulation from the absence of B7-2 on CD11b+ DCs led to a decreased proliferative response to P0. That upregulation of B7-1 is insufficient to compensate for the lack of B7-2 in CD11b+ DCs would support the concept that B7-1 and B7-2 differentially regulate immune responses in spite of some overlapping functions. In contrast to CD11b+ DCs, absence of B7-2 on CD8α+ DCs did not result in diminished T cell proliferative response to P0 (180–199). Instead, a trend towards the opposite effect was observed. Hence, the functional consequences of B7-2 deletion are not uniform across DC subsets.
Aside from co-stimulation, the B7-1/B7-2:CD28/CTLA4 pathway also plays a crucial role in the homeostasis of Tregs (11, 12). Conversely, CTLA-4 regulates the function of DCs by either inducing indoleamine 2,3-dioxygenase (IDO), which catabolizes tryptophan and inhibits T cell function, or by removal of B7-1 and B7-2 via trans-endocytosis (36, 37). In B7-2 KO NOD mice which develop neuropathy but not diabetes, CD4+ Tregs are slightly reduced in the spleen and PLN, albeit exhibiting similar efficiency in suppressive function in vitro as Tregs from WT NOD mice (38). In contrast, markedly reduced number of Tregs in B7-1/B7-2 double deficient or CD28 KO NOD mice leads to a rapid progression of insulitis and neuropathy at 8–10 weeks of age (12). There is some evidence that B7-1 and B7-2 contribute equally to the development of Tregs in the thymus, whereas B7-2 appears more important than B7-1 in regulating the peripheral homeostasis of Tregs (39). B7-1 and B7-2 are expressed not only by DCs, but also by macrophages, B cells and activated CD4+ T cells (40–43). However, only DCs exhibit strong capacity to directly expand Tregs (44). Exogenous TGF-β is required for induction of Tregs by CD11b+ DCs, but not when CD8α+CD205+ DCs are used (45). We found that B7-2 deficient DCs were less effective than WT DCs not only in maintaining the proliferation of splenic Tregs, but also in the generation of iTregs de novo in the presence of TGF-β. IL-2 did not exert any additive effect on the generation of iTregs under our experimental conditions.
Findings on our in vitro studies were confirmed by results from our adoptive transfer experiments, which revealed that the lack of B7-2 on DCs is sufficient to cause defect in tolerance induction in our model. Results from clinical, electrophysiological and immunologic studies from B7-2 KO DC (AT) group were similar to those obtained from PBS (no AT) group. Tolerance induction by antigen-pulsed WT DCs correlated with decreased splenocyte proliferative response to P0 (180–199) and P0-ECD, but was not associated with perturbations in Th1/Th17 polarization. Nonetheless, altered T cell cytokine profile was observed in that the percentage of IL10+ CD4+ T cells was increased in the spleen and Peri-LN, while the percentage of TNF+ CD4+ T cells was reduced in the spleen of tolerized animals. IL-10 is secreted not only by Th2 cells, Tregs, Tr1 cells, DCs, but also by Th1 and Th17 cells as part of a negative feedback regulation of CD4+ effector responses (46). There is some evidence that the ratio of IL-10 to the relevant effector cytokine (IFN-γ or IL-17) dictates the outcome of the immune response. Whereas C57BL/6 mice recover rapidly from MOG-induced EAE, IL-10 deficient mice do not recover and develop a progressive form of EAE (47). Proteolipid protein-specific CD4+ T cells that express IL-10 under the control of IL-2 promoter can prevent and suppress EAE (48). In EAN, IL-10 was also suppressive even when administered after the onset of clinical disease (49).
Absence of B7-1 or B7-2 on DCs has been reported to abrogate the suppressive action of IL-10 treated DCs and DC-derived exosomes in the delayed type hypersensitivity (DTH) model (50). In contrast, we found that the defect in tolerance induction by splenic DCs lacking B7-2 in the SAP model could be reversed by pre-conditioning with IL-10, and was associated with increased frequency of both Tregs and B10 cells in the spleen. Conversely, IL-10-deficient DCs exhibited impaired capacity to induce tolerance to myelin P0. Induction of regulatory B cells such as B10 cells by tolerogenic DCs has recently been reported not only in NOD mice, but also in phase 1 clinical trial of tolerogenic autologous DC administration in type 1 diabetes (51, 52). In our current study, increased frequency of Tregs without concomitant increased frequency of B10 cells is sufficient to induce tolerance to myelin P0, as observed in animals tolerized with antigen-pulsed WT DCs.
DC-derived IL-10 can act in a paracrine or autocrine manner. It is plausible that lower expression of IL-10 in B7-2 KO DCs contributes partially to the loss of tolerance to P0. We found that IL-10 preconditioning had no effect on antigen uptake. Our data on altered immunophenotypic properties of B7-2 KO DCs by IL-10 are in agreement with those reported by other investigators in WT DCs (50, 53–57). In addition, IL-10 also upregulates other inhibitory molecules in human DCs such as immunoglobulin-like transcript (ILT3 and ILT4) (50, 53–57). The tolerogenic effect of IL-10-conditioned DCs has been reported in other models such as type 1 diabetes in NOD mice and rheumatoid arthritis models (58, 59). Aside from IL-10, tolerogenic DCs can also be induced in vitro by rapamycin, dexamethasone, TGF-β1, Vitamin D3, retinoic acid, inducers of cyclic AMP or by antisense oligonucleotide targeting CD40, CD80 and CD86 (52, 60). Recently, B7-2 deficient bone marrow DCs transduced with lentiviral vectors expressing vasoactive intestinal peptide (LV-VIP-BCD11b+ DCs) has been shown to delay the onset of disease and attenuate clinical severity in SAP (61). In our study, non-transduced, antigen-pulsed WT and IL-10-preconditioned B7-2 deficient splenic DCs appear more effective in preventing the development of SAP than LV-VIP-BCD11b+ DCs.
In summary, we found that: 1) development of SAP is associated with an increase of CD11b+ DCs in Peri-LN and sciatic nerves, 2) changes in immunophenotypic properties and function induced by B7-2 deletion are not uniform across DC subsets; 3) absence of B7-2 on DCs leads to impaired ability to induce tolerance to P0, which could be overcome by pre-conditioning with IL-10. Our data demonstrate that B7-2 on DCs is critical for protection against the development of SAP in NOD mice, mainly via regulation of peripheral homeostasis of Tregs. The role of B7-2 in autoimmune neuropathy is influenced by the genetic background. In C57BL/6 mice, SAP is not triggered by elimination of B7-2, but by constitutive expression of B7-2 using MHC I promoter and Igµ enhancer, particularly when CD4+ T cells are also depleted (L31/CD4−/− mice) (62). The latter model is characterized by massive infiltration of CD8+ T cells and macrophages, severe demyelination and axonal damage in the sciatic nerves with less extensive involvement in the spinal cords (62). The outcome from perturbations of B7-2 expression or signaling ultimately depends on the balance of autoreactivity vs regulation.
Supplementary Material
Acknowledgement
B7-2 KO NOD mice were generated in the laboratory of Dr. J.A. Bluestone (UCSF).
Grant support: This work was supported by National Institute of Health Grant R01 NS064059 to B. Soliven, and by a pilot grant from the GBS/CIDP Foundation International.
Nonstandard abbreviations
- Bregs
regulatory B cells
- CIDP
chronic inflammatory demyelinating polyradiculoneuropathy
- DC
dendritic cells
- EAE
Experimental autoimmune encephalomyelitis
- EAN
experimental autoimmune neuritis
- GBS
Guillain Barré syndrome
- P0
myelin protein zero
- SAP
spontaneous autoimmune polyneuropathy
- Tregs
regulatory T cells.
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legge KL, Gregg RK, Maldonado-Lopez R, Li L, Caprio JC, Moser M, Zaghouani H. On the role of dendritic cells in peripheral T cell tolerance and modulation of autoimmunity. J Exp Med. 2002;196:217–227. doi: 10.1084/jem.20011061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.v98.5.1512. [DOI] [PubMed] [Google Scholar]
- 6.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 7.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. The Journal of experimental medicine. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Press R, Nennesmo I, Kouwenhoven M, Huang YM, Link H, Pashenkov M. Dendritic cells in the cerebrospinal fluid and peripheral nerves in Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neuroimmunol. 2005;159:165–176. doi: 10.1016/j.jneuroim.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Li XL, Yue LT, Li H, Zhang M, Wang S, Wang CC, Duan RS. Therapeutic potential of atorvastatin-modified dendritic cells in experimental autoimmune neuritis by decreased Th1/Th17 cytokines and up-regulated T regulatory cells and NKR-P1(+) cells. Journal of neuroimmunology. 2014;269:28–37. doi: 10.1016/j.jneuroim.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 12.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 13.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J Exp Med. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav D, Judkowski V, Flodstrom-Tullberg M, Sterling L, Redmond WL, Sherman L, Sarvetnick N. B7-2 (CD86) controls the priming of autoreactive CD4 T cell response against pancreatic islets. J Immunol. 2004;173:3631–3639. doi: 10.4049/jimmunol.173.6.3631. [DOI] [PubMed] [Google Scholar]
- 15.Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Jung CG, Jensen MA, Dukala D, Soliven B. Targeting of myelin protein zero in a spontaneous autoimmune polyneuropathy. J Immunol. 2008;181:8753–8760. doi: 10.4049/jimmunol.181.12.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louvet C, Kabre BG, Davini DW, Martinier N, Su MA, DeVoss JJ, Rosenthal WL, Anderson MS, Bour-Jordan H, Bluestone JA. A novel myelin P0-specific T cell receptor transgenic mouse develops a fulminant autoimmune peripheral neuropathy. J Exp Med. 2009;206:507–514. doi: 10.1084/jem.20082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abraham PM, Quan S, Dukala D, Soliven B. CD19 as a therapeutic target in a spontaneous autoimmune polyneuropathy. Clin Exp Immunol. 2014;175:181–191. doi: 10.1111/cei.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yadav D, Sarvetnick N. B7-2 regulates survival, phenotype, and function of APCs. J Immunol. 2007;178:6236–6241. doi: 10.4049/jimmunol.178.10.6236. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Jung CG, Dukala D, Bae H, Kakazu R, Wollmann R, Soliven B. Fingolimod and related compounds in a spontaneous autoimmune polyneuropathy. J Neuroimmunol. 2009;214:93–100. doi: 10.1016/j.jneuroim.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25- cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. The Journal of experimental medicine. 2005;201:127–137. doi: 10.1084/jem.20041201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Nurieva RI, Duong J, Kishikawa H, Dianzani U, Rojo JM, Ho I, Flavell RA, Dong C. Transcriptional regulation of th2 differentiation by inducible costimulator. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson EH, Zaph C, Mohrs M, Welcher A, Siu J, Artis D, Hunter CA. B7RP-1-ICOS interactions are required for optimal infection-induced expansion of CD4+ Th1 and Th2 responses. Journal of immunology. 2006;177:2365–2372. doi: 10.4049/jimmunol.177.4.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nature immunology. 2009;10:167–175. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marleau AM, Singh B. Myeloid dendritic cells in non-obese diabetic mice have elevated costimulatory and T helper-1-inducing abilities. J Autoimmun. 2002;19:23–35. doi: 10.1006/jaut.2002.0597. [DOI] [PubMed] [Google Scholar]
- 28.Weaver DJ, Jr., Poligone B, Bui T, Abdel-Motal UM, Baldwin AS, Jr, Tisch R. Dendritic cells from nonobese diabetic mice exhibit a defect in NF-kappa B regulation due to a hyperactive I kappa B kinase. J Immunol. 2001;167:1461–1468. doi: 10.4049/jimmunol.167.3.1461. [DOI] [PubMed] [Google Scholar]
- 29.Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–2543. [PubMed] [Google Scholar]
- 30.Dahlen E, Hedlund G, Dawe K. Low CD86 expression in the nonobese diabetic mouse results in the impairment of both T cell activation and CTLA-4 up-regulation. J Immunol. 2000;164:2444–2456. doi: 10.4049/jimmunol.164.5.2444. [DOI] [PubMed] [Google Scholar]
- 31.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 33.Irla M, Kupfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. The Journal of experimental medicine. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides 'preferentially' polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Zhang GX, Chen Y, Xu H, Fitzgerald DC, Zhao Z, Rostami A. CD11c+CD11b+ dendritic cells play an important role in intravenous tolerance and the suppression of experimental autoimmune encephalomyelitis. J Immunol. 2008;181:2483–2493. doi: 10.4049/jimmunol.181.4.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, Puccetti P. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z, Hou TZ, Futter CE, Anderson G, Walker LS, Sansom DM. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng M, Guinet E, Nouri-Shirazi M. B7-1 and B7-2 differentially control peripheral homeostasis of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Transpl Immunol. 2009;20:171–179. doi: 10.1016/j.trim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 40.Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 41.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BL, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 42.Cross AH, Lyons JA, San M, Keeling RM, Ku G, Racke MK. T cells are the main cell type expressing B7-1 and B7-2 in the central nervous system during acute, relapsing and chronic experimental autoimmune encephalomyelitis. European journal of immunology. 1999;29:3140–3147. doi: 10.1002/(SICI)1521-4141(199910)29:10<3140::AID-IMMU3140>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 43.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. Journal of immunology. 2006;177:2948–2958. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 44.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. The Journal of experimental medicine. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. Journal of immunology. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ng TH, Britton GJ, Hill EV, Verhagen J, Burton BR, Wraith DC. Regulation of adaptive immunity; the role of interleukin-10. Front Immunol. 2013;4:129. doi: 10.3389/fimmu.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 48.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O'Garra A. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. The Journal of experimental medicine. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai XF, Zhu J, Zhang GX, Kaponides G, Hojeberg B, van der Meide PH, Link H. IL-10 suppresses experimental autoimmune neuritis and down-regulates TH1-type immune responses. Clin Immunol Immunopathol. 1997;83:117–126. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- 50.Ruffner MA, Kim SH, Bianco NR, Francisco LM, Sharpe AH, Robbins PD. B7-1/2, but not PD-L1/2 molecules, are required on IL-10-treated tolerogenic DC and DC-derived exosomes for in vivo function. European journal of immunology. 2009;39:3084–3090. doi: 10.1002/eji.200939407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giannoukakis N, Phillips B, Finegold D, Harnaha J, Trucco M. Phase I (safety) study of autologous tolerogenic dendritic cells in type 1 diabetic patients. Diabetes Care. 2011;34:2026–2032. doi: 10.2337/dc11-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Caro V, Phillips B, Engman C, Harnaha J, Trucco M, Giannoukakis N. Involvement of suppressive B-lymphocytes in the mechanism of tolerogenic dendritic cell reversal of type 1 diabetes in NOD mice. PLoS ONE. 2014;9:e83575. doi: 10.1371/journal.pone.0083575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. Journal of immunology. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 54.McBride JM, Jung T, de Vries JE, Aversa G. IL-10 alters DC function via modulation of cell surface molecules resulting in impaired T-cell responses. Cellular immunology. 2002;215:162–172. doi: 10.1016/s0008-8749(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 55.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transplant immunology. 2003;11:245–258. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 56.Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--a comparative study of human clinical-applicable DC. Clinical immunology. 2012;142:332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 57.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo MG. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116:935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 58.Tai N, Yasuda H, Xiang Y, Zhang L, Rodriguez-Pinto D, Yokono K, Sherwin R, Wong FS, Nagata M, Wen L. IL-10-conditioned dendritic cells prevent autoimmune diabetes in NOD and humanized HLA-DQ8/RIP-B7.1 mice. Clinical immunology. 2011;139:336–349. doi: 10.1016/j.clim.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Kim SH, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. Journal of immunology. 2005;174:6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 60.Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory Dendritic Cells for Immunotherapy in Immunologic Diseases. Front Immunol. 2014;5:7. doi: 10.3389/fimmu.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yalvac ME, Arnold WD, Hussain SR, Braganza C, Shontz KM, Clark KR, Walker CM, Ubogu EE, Mendell JR, Sahenk Z. VIP-expressing Dendritic Cells Protect Against Spontaneous Autoimmune Peripheral Polyneuropathy. Mol Ther. 2014;22:1353–1363. doi: 10.1038/mt.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang M, Rainone A, Shi XQ, Fournier S, Zhang J. A new animal model of spontaneous autoimmune peripheral polyneuropathy: implications for Guillain-Barre syndrome. Acta Neuropathol Commun. 2014;2:5. doi: 10.1186/2051-5960-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.