Abstract
Experimental autoimmune encephalitis (EAE), the animal model of multiple sclerosis (MS), has provided significant insight into the mechanisms that initiate and drive autoimmunity. Several central nervous system proteins and peptides have been used to induce disease, in a number of different mouse strains, to model the diverse clinical presentations of MS. In this chapter, we detail the materials and methods used to induce active and adoptive EAE. We focus on disease induction in the SJL/J, C57BL/6, and BALB/c mouse strains, using peptides derived from proteolipid protein, myelin basic protein, and myelin oligodendrocyte glycoprotein. We also include a protocol for the isolation of leukocytes from the spinal cord and brain for flow cytometric analysis.
Keywords: Experimental autoimmune encephalomyelitis, Multiple sclerosis, Autoimmune disease, Mouse model, CD4+ T cells
1 Introduction
Multiple sclerosis (MS) is a chronic and debilitating autoimmune disease of the central nervous system (CNS), characterized by lesion formation in the white matter of the brain, spinal cord, and optic nerve (1, 2). The precise mechanisms that trigger and drive MS are not completely understood. However, it is clear that myelin antigens are key targets (3). Autoreactive T cells and other immune cells, particularly macrophages, infiltrate the CNS and cause significant damage to the myelin sheath and underlying axons, resulting in neuronal dysfunction and death (4, 5). Depending on the severity and location of the immune insult, patients may present with a range of neurological symptoms that impact physical functioning (e.g., muscle weakness, numbness or spasms, impaired balance and coordination, fatigue, incontinence) or mental capacity (e.g., memory loss, depression, cognitive difficulties) (6).
The frequency and severity in which MS symptoms occur differs greatly between individuals. Four main categories are used to classify the clinical course of MS in patients (7). Relapsing-remitting MS is the most common clinical pattern observed, and is characterized by recurrent attacks (relapses) followed by periods of remission in which little or no permanent neurological sequelae are evident. Secondary progressive MS describes the clinical course in which patients initially present with relapsing-remitting disease, but develop significant deficits that increase over time. Primary progressive MS describes the clinical course in which patients show progressive worsening of symptoms without relapse or remission phases. Progressive-relapsing MS is characterized by a steadily worsening disease state, in which patients undergo relapses without complete remission (7).
The animal model of MS, Experimental Autoimmune Encephalomyelitis (EAE), aims to replicate the clinical symptoms of disease in vivo, and has been induced in a range of species, including mice, rats, and hamsters (8). Two different methods of EAE induction have been described. Subcutaneous immunization of mice with an emulsion of myelin protein/peptide and complete Freund’s adjuvant (CFA) is referred to as “active EAE,” and models the induction and effector stages of disease (9). This process results in the direct priming of myelin epitope-specific CD4+ T cells in vivo, which migrate to the CNS and mediate autoimmune responses. In comparison, “adoptive EAE” models the effector phase of disease only. Activated CD4+ T cells are isolated from the draining lymph nodes of immunized mice, restimulated with the initiating myelin protein/peptide in vitro for several days, and then injected into naïve recipients. Disease is typically accelerated, more severe and uniform in comparison to active EAE, with higher incidence (9).
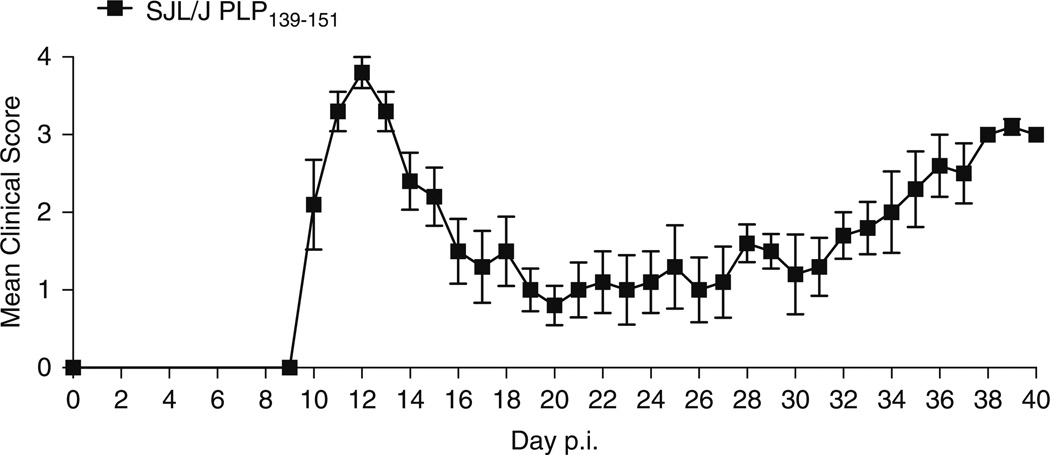
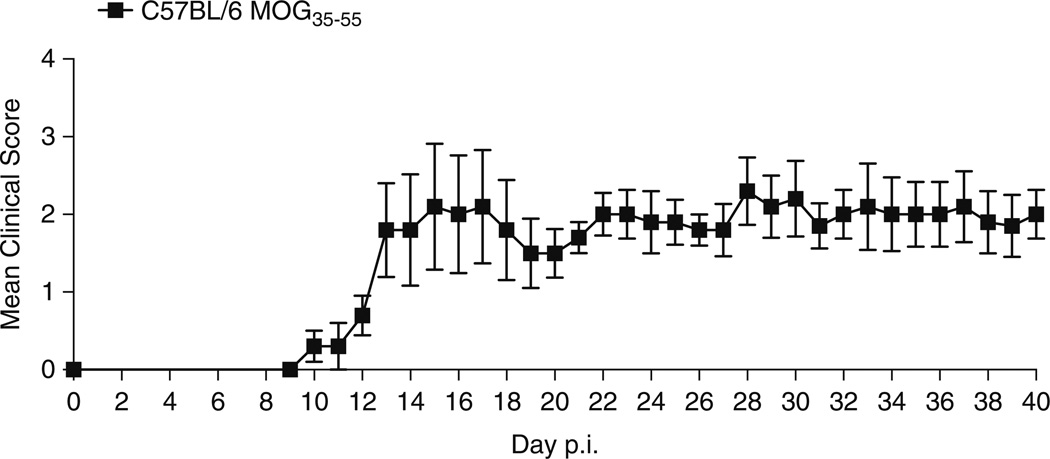
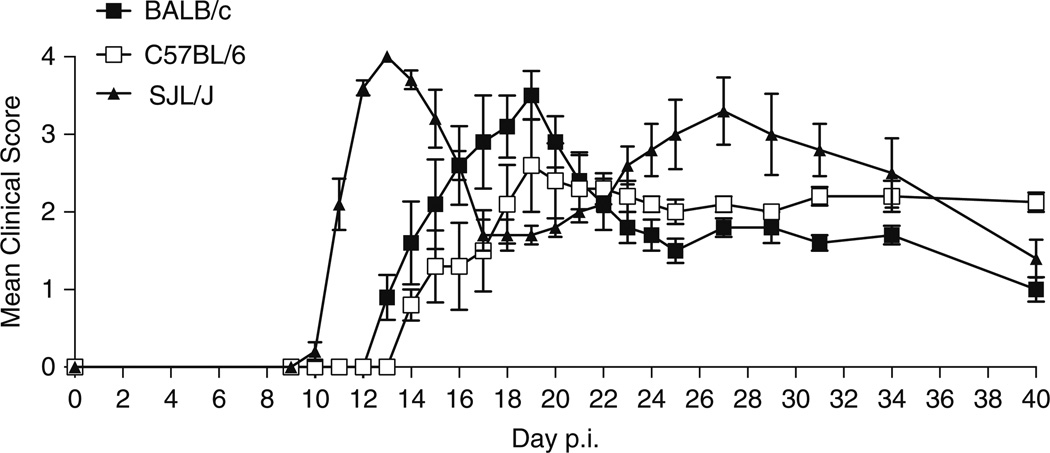
Depending on the initiating protein/peptide, animal species and strain used for the induction of EAE, different clinical etiologies may be mimicked (Table 1). For example, induction of disease in the SJL/J mouse strain using the proteolipid protein (PLP) 139–155 peptide induces relapsing remitting disease (Fig. 1). In comparison, immunization of C57BL/6 mice with the myelin oligodendrocyte glycoprotein (MOG) 35–55 peptide results in a chronic disease course (Fig. 2). Immunization of BALB/c and C57BL/6 mice with the PLP 180–199 peptide also induces chronic disease, but causes relapsing-remitting disease in the SJL/J strain (Fig. 3). The development of transgenic and humanized mice strains, outlined in Table 2, have also proved to be invaluable tools in understanding the processes that drive autoimmunity. This chapter aims to provide a detailed protocol for inducing EAE in the mouse. Here, we focus on whole proteins and peptides derived from PLP, MOG, and myelin basic protein (MBP).We include the protocols to induce both active and adoptive disease, and the methodology used to isolate single-cell suspensions of leukocytes from brain tissue.
Table 1.
Induction of active experimental autoimmune encephalomyelitis in mice
| Mouse strain | Myelin peptide/protein | Sequence | Peptide/protein dose (µg) | Pertussis toxin |
Clinical course |
|---|---|---|---|---|---|
| BALB/c | Whole PLP (10) | From : bovine | 200 | Yes | Chronic |
| PLP180–199 (10) | WTTCQSIAFPSKTSASIGSL | 200 | Yes | ||
| C57BL/6 | MOG35–55 (11) | MEVGWYRSPFSRVVHLYRNGK | 200 | Yes | Chronic |
| PLP178–191 (12) | NTWTTCQSIAFPSK | 200 | Yes | ||
| PLP180–199a | WTTCQSIAFPSKTSASIGSL | 200 | Yes | ||
| SJL/J | Whole MBP (13) | From: mouse, rat, or bovine | 200–400 | Yes | Relapsing-remitting |
| MBP84–104 (14) | VHFFKNIVTPRTPPPSQGKGR | 200b | Yes | ||
| MBP89–101 (15) | VHFFKNIVTPRTP | 200 | Yes | ||
| MOG92–106 (16, 17) | DEGGYTCFFRDHSYQ | 200b | Yes | ||
| Whole PLP (18) | From: rat or bovine | 200 | Yes | ||
| PLP57–70 (19) | YEYLINVIHAFQYV | 100 | Yes | ||
| PLP104–117 (20, 21) | KTTICGKGLSATVT | 50 | Yes | ||
| PLP139–151 (22) | HSLGKWLGHPDKF | 50 | No | ||
| PLP178–191 (23) | NTWTTCQSIAFPSK | 200 | No | ||
| PLP180–199a | WTTCQSIAFPSKTSASIGSL | 200 | Yes | ||
| ABH | Whole MOG (16) | From: rat | 200b | Yes | Chronic-relapsing |
| MOG8–22 (16, 24) | PGYPIRALVGDEQED | 200b | Yes | ||
| PLP56–70 (24) | DYEYLINVIHAFQYV | 100b | Yes | ||
| PL/J, B10.PL | Whole MBP (25) | From: rat or guinea pig | 200 | Yes | Chronic/acute monophasic |
| MBPAc1-11 (26, 25) | Ac-ASQKRPSQRSK | 100 | Yes | ||
| MBP35–47 (27) | TGILDSIGRFFSG | 200 | Yes | ||
| MOG35–55 (28) | MEVGWYRSPFSRVVHLYRNGK | 200 | Yes | ||
| PLP43–64 (29) | EKLIETYFSKNYQDYEYLINVI | 150 | Yes | ||
| C3H/HeJ | Whole PLP (18) | From: rat or bovine | 200 | Yes | Chronic/atypical |
| PLP190–209 (19, 30) | SKTSASIGSLCADARMYGVL | 100 | Yes | ||
| PLP215–232 (31, 19) | PGKVCGSNLLSICKTAEFQ | 100 | Yes | ||
Terry, Harp, and Miller, unpublished data, Fig. 2
Mice require a second immunization on day 7
Fig. 1.
Induction of EAE in the SJL/J mouse using PLP139–151. Immunization of SJL/J mice with 50 µg of the PLP139–151 peptide results in a relapsing-remitting course of EAE
Fig. 2.
Induction of EAE in the C57BL/6 mouse using MOG35–55. Immunization of C57BL/6 mice with 200 µg of the MOG35–55 peptide results in a chronic course of EAE
Fig. 3.
Induction of EAE in the BALB/c, C57BL/6, and SJL/J mouse using PLP180–199. Immunization of BALB/c and C57BL/6 mice with 200 µg of the PLP180–199 peptide results in a chronic course of EAE, but causes relapsing-remitting disease in SJL/J mice
Table 2.
Transgenic mouse models of experimental autoimmune encephalomyelitis
| Background | TCR specificity | Cell-inducing disease |
% of spontaneous disease |
Age of onset (in weeks) |
Clinical course |
|---|---|---|---|---|---|
| B10.PL/TCR tg B10.PL/TCR tg × RAG-1−/− |
MBPAc1–11 (32, 33) | CD4 | 14–44 % 100 % |
5–20 6–20 |
Chronic/AM Chronic |
| C57BL/6 HLA-DR2/TCR tg DR2/TCR tg × RAG-2−/− |
huMBP84–102 (34) | CD4 | 4 % 100 % |
ND 7–15 |
Variable |
| C57BL/6 HLA-DR15/TCR tg DR15/TCR tg × RAG-2−/− |
huMBP85–99 (35) | CD4 | 60 % 80–100 % |
16–24 5–16 |
Chronic |
| SJL/J 5B6 | PLP139–151 (36) | CD4 | 40–60 % | 6 and older | Chronic |
| C57BL/6 2D2 | MOG35–55 (37) | CD4 | 4–15 % 30–40 % |
10–20 10–52 |
Chronic Optic neuritis |
| C57BL/6 2D2 × IgHMOG | MOG35–55 (38, 39) | CD4 B cells |
50–60 % | 4–10 | Chronic with lesions only in spinal cord and optic nerve |
| SJL/J TCR1640 | MOG92–106 (40) | CD4 B cells |
60–90 % | 8–23 | RR on female PP on male |
| C57BL/6 B7.2 expressed on microglia and T cells | ND (41, 42) | CD8 | 100 % | 12–30 | Chronic |
| C57BL/6 ODC-OVA × OT-I | OVA257–264 (43) | CD8 | 90–100 % | 1–3 | Chronic/lethal |
| C57BL/6 HLA-A3/TCR tg | huPLP45–53 (44) | CD8 | 4 % | ND | Motor deficit |
| NOD 1C6 × IgHMOG | MOG35–55 (45) | CD4 CD8 B cells |
45–80 % | 12–18 | RR to chronic |
AM acute monophasic, RR relapsing-remitting, PP primary progressive, tg transgenic, ODC oligodendrocytes, ND not defined
2 Materials
2.1 Active Induction of EAE
Female SJL/J, C57BL/6, or BALB/c mice at 6–8 weeks old (Jackson Laboratories).
Small animal clippers (e.g., model A5, blade size 50; Oster).
Incomplete Freund’s Adjuvant (IFA; Difco).
Mycobacterium Tuberculosis H37Ra, inactivated and desiccated (Difco).
Desired myelin protein or peptide (see Table 1).
Phosphate-buffered saline (PBS).
Pertussis Toxin, if required (see Table 1; List Biologicals).
15 mL polystyrene test tubes.
18 and 25 G needles.
1 mL glass tuberculin syringes with Luer-Lok (VWR).
2.2 Adoptive Induction of EAE
Materials listed in Section 2.1.
Dissection forceps and scissors.
100 µm sieves (Becton Dickinson).
1 mL syringes.
50 mL conical polystyrene tubes.
Recombinant mouse IL-12, if required (R&D Systems).
175 cm2 culture flasks.
37 °C, 6 % CO2 tissue culture incubator.
30.5 G needles.
Complete Roswell Park Memorial Institute (cRPMI): 440 mL of calcium-free, l-glutamine-free RPMI medium, 5 mL of 100× l-glutamine, 5 mL of 100× Penicillin-Streptomycin, 500 µL of 55 µM 2-mercaptoethanol, and 50 mL of Fetal Bovine Serum (FBS).
2.3 Isolation of CNS Infiltrating Leukocytes
Institution-approved anesthetic.
Dissection forceps and scissors.
30 mL syringes with Luer-Lok.
21.5 G needles.
PBS.
Non-treated plastic petri dishes, 60 mm.
5 mL syringes.
18 G needle.
Stainless steel wire mesh, 200–300 µm.
15 mL polystyrene tubes.
50 mL conical polystyrene tubes.
Liberase, low Thermolysin concentration (Roche).
DNase I (Sigma).
Percoll (GE Healthcare).
10× no calcium, no magnesium, no phenol red Hank’s Balanced Salt Solution (HBSS).
10× no calcium, no magnesium HBSS.
0.5 M EDTA, pH 8.
FBS.
3 Methods
3.1 Active Induction of EAE
Shave the back of the mice using the small animal clippers (see Note 1).
Prepare complete Freund’s adjuvant (CFA) by combining 50 mL IFA and 200 mg M. tuberculosis H37Ra, resulting in a final concentration 4 mg/mL M. tuberculosis (see Note 2).
Prepare an emulsion of CFA and desired protein/peptide (Table 1) by mixing 1 mL of CFA with 1 mL of desired peptide/protein diluted in PBS. Repeatedly draw up and expel the liquid from a 1 mL glass syringe into a 15 mL polystyrene test tube, using an 18 G needle (see Notes 3–5).
Slowly draw up the emulsion into a new 1 mL glass syringe using an 18 G needle, taking care not to introduce air bubbles. Replace the 18 G needle with a 25 G needle.
Inject 100 µL of emulsion subcutaneously into the shaved backs of the mice, distributing evenly over three injection sites. One injection should be placed on the midline of the back just below the shoulders, and two on either side of the midline on the lower back.
Refer to Table 1 to determine if pertussis toxin is needed for the mouse strain and initiating protein/peptide you are using. If required, dilute pertussis toxin to 1 µg/mL in sterile PBS (see Note 6). Inject 200 µL of diluted pertussis toxin (200 ng per mouse) into the peritoneal cavity or intravenously on the day of disease induction, and again 48 h after induction (see Note 7).
Monitor mice every day to observe the development of clinical symptoms, which usually occur between day 10 and 28 post-induction (see Figs. 1, 2, and 3). Symptoms are measured using the scoring system shown in Table 3.
Table 3.
Clinical scoring of mice with experimental autoimmune encephalomyelitis
| Clinical score |
Clinical symptoms |
|---|---|
| 0 | Mouse shows no symptoms of disease (asymptomatic) |
| 1 | Mouse has a limp tail (complete flaccidity, absence of curling at the tip) or hind limb weakness (waddling gait, mouse’s hind limbs fall through the top of a wire cage), not both |
| 2 | Mouse has both a limp tail and shows hind limb weakness |
| 3 | Mouse has partial paralysis of the hind limbs (can no longer maintain posture of the rump, but can still move one or both limbs to an extent) |
| 4 | Mouse shows complete hind limb paralysis (complete loss of movement of the hind limbs, all movement is the result of the mouse dragging on the forelimbs)a |
| 5 | Moribund (death caused by EAE), mice are euthanized for humane reasons |
Mice at this stage of disease are given soft gel food on the cage floor, long sipper tubes, and daily saline injections to prevent dehydration
3.2 Adoptive Induction of EAE
Immunize female donor SJL/J, C57BL/6, or BALB/c mice with the desired myelin protein/peptide as detailed in Section 3.1.
Prepare cRPMI.
Between day 7 and day 14 post-induction (see Table 4), euthanize the mice and remove the draining lymph nodes (inguinal, brachial, and axillary). Place the lymph nodes into cRPMI.
Place a 70 µm filter into a non-treated culture plate. Generate a single-cell suspension by pressing the lymph nodes in cRPMI through the filter with the plunger from a 1 mL syringe.
Transfer the cRPMI solution containing the cells into a 50 mL polystyrene tube and centrifuge for 10 min at 300 × g.
Remove the supernatant and raise the pellet in 1 mL of cRPMI and gently resuspend using a pipette. Add another 19 mL of cRPMI to increase the total volume to 20 mL.
Count the number of viable white blood cells using trypan blue exclusion of dead cells on a hemocytometer slide.
Culture the cells at a concentration of 8 × 106 cells per mL in cRPMI in 175 cm2 flasks (up to 30 mL total volume per flask). Add in the required amount of initiating protein/peptide to restimulate the cells (see Table 4; Notes 8–9). Add in 10 ng/mL IL-12 if necessary (see Table 4; Notes 10–11). Incubate for 72 h in a 37 °C, 6 % CO2 tissue culture incubator (see Note 12).
Harvest the cells by transferring into 50 mL conical tubes and centrifuging at 300 × g for 15 min. Count the number of live blasts by using trypan blue exclusion. Blasts will appear larger than other leukocytes in the culture and should comprise at least 10–30 % of the culture.
Wash the cells twice in PBS and raise to the required concentration in PBS. Inject the recommended number of blasts in a final volume of 200 µL (see Table 4; Note 13) into recipient mice using a 1 mL syringe and 30.5 G needle via intravenous tail vein. Alternatively, blasts may be injected into the peritoneum.
Inject 200 µL of diluted pertussis toxin (200 ng per mouse) into the peritoneal cavity or intravenously on the day of disease induction, and again 48 h after induction if necessary (refer to Table 4).
Table 4.
Induction of adoptive experimental autoimmune encephalomyelitisa
| Mouse strain |
Myelin peptide/ protein |
Donor immunization period (days) |
In vitro peptide/ protein dose (µg/mL) |
No. of blasts transferred (×106) |
Disease type and severity |
Pertussis |
|---|---|---|---|---|---|---|
| BALB/c | PLP180–199 | 10–14 | 20b | 5–10 | Chronic, Moderate | Yes |
| C57BL/6 | Whole MOG | 10–14 | 50b | 20 | Chronic, Moderate | Yes |
| MOG35–55 | 10–14 | 10b | 20 | Chronic, Moderate | Yes | |
| PLP178–191 | 10–14 | 20 | 10–20 | Chronic, Moderate | Yes | |
| PLP180–199 | 10–14 | 20 | 5–10 | Chronic, Moderate | Yes | |
| SJL/J | Whole MBP | 7–14 | 50–100 | 40–60 | RR, Moderate | Yes |
| MBP84–104 | 7–14 | 50 | 10–20 | RR, Moderate | Yes | |
| Whole PLP | 7–14 | 50–100 | 5–10 | RR, Severe | Yes | |
| PLP139–151 | 7–14 | 20 | 1–5 | RR, Severe | No | |
| PLP178–191 | 7–14 | 20 | 10–20 | RR, Severe | No | |
| PLP180–199 | 10–14 | 20b | 5–10 | RR, Severe | Yes | |
3.3 Isolation of CNS Infiltrating Leukocytes
Induce deep anesthesia by injecting mice with approved anesthetic (e.g., Nembutal). Ensure deep anesthesia is achieved by testing reflex responses of the footpads.
Draw up PBS into a 30 mL syringe with a 21.5 G needle.
Using surgical scissors and forceps, expose the chest cavity by making an incision at the diaphragm and cutting upwards through the rib cage.
Using the forceps, carefully hold the heart in place. Make a small incision in the right atrium of the heart.
Immediately insert the 30 mL syringe with 21.5 G needle into the left atrium of the heart. To ensure correct placement, put a small amount of pressure on the syringe plunger. An efflux of dark red blood should immediately flow from the right atrium (see Note 14). Slowly perfuse the animal by continuing to apply pressure on the plunger (see Notes 15, 16).
Remove the head of the mouse by carefully cutting under the skull, through the neck. Using scissors or a scalpel, cut the skin and cutaneous muscle by making a single incision along the midline length of the head, from the posterior aspect of the skull to the nose of the mouse. Using the forceps, fold back the two flaps of skin to expose the skull.
Carefully cut through the top of the skull along the midline, from the posterior aspect of the skull to the nose. Make two incisions from the posterior aspect of the skull to the eye sockets, and use the forceps to remove the pieces of skull from the top of the head. Carefully remove the brain from the cranial cavity and place in a 50 mL tube containing 20 mL of PBS.
Using a scalpel or scissors, make a long incision through the midline of the mouse (from the neck to the base of the tail). Peel the skin back to expose the spinal column. Identify where the base of the spinal column attaches to the pelvis, and make a perpendicular cut through the spine at this point. Cut along each side of the column to the neck to remove the column.
Attach an 18 G needle to a 5 mL syringe that has been filled with PBS. Hold the column in a petri dish with forceps, and insert the needle into the spinal column at the caudal end. Push the plunger of the syringe to expel PBS into the spinal column. Initial resistance should be felt, followed by release, in which the spinal cord will be flushed from the column. Transfer spinal cord into a 50 mL tube containing 20 mL PBS.
Carefully pass the brain and spinal cord through the stainless steel wire mesh in the 60 mm petri dishes using the plungers taken from 10 mL syringes. Rinse all tissue off the mesh with additional PBS. Transfer back into 50 mL tubes and centrifuge for 20 min at 300 × g at 4 °C. Remove the supernatant.
Prepare digestion enzyme mix by adding 1 g DNase I (50 µg/mL final concentration) and 800 U Liberase (40 U/mL final concentration) to 20 mL PBS (see Note 17). Raise cells in 2 mL of digestion enzyme mix and incubate for 30 min at 37 °C.
Prepare 500 mL FACS buffer by adding 10 mL FCS (2 % final concentration) and 2 mL 0.5M EDTA (2 mM final concentration) to 488 mL PBS.
Add 30 mL FACS buffer to samples and pass through a 70 µM sieve into a new 50 mL tube. Centrifuge for 10 min at 300 × g at 4 °C. Wash cells again twice in FACS buffer.
Prepare 30 % Percoll by adding 5 mL 10 % HBSS (with phenol red) and 15 mL Percoll to 35 mL of water. Prepare 70 % Percoll by adding 5 mL 10 % HBSS (without phenol red) and 35 mL Percoll to 15 mL of water (see Note 18).
Raise the CNS samples in 5mL of 30 % Percoll and transfer to a 15 mL tube. Underlay samples with 5 mL of 70 % Percoll. Centrifuge the samples at 1,000 × g for 25 min at room temperature with no brake.
Using a pipette, remove the myelin layer from the top of the gradient. Using a new pipette tip, collect the mononuclear cells at the interface between the 30 and 70 % Percoll interface and transfer into a new 15 mL tube containing 5 mL FACS buffer. Centrifuge for 10 min at 300 × g at 4 °C. Wash cells again twice in FACS buffer.
Count live cells using trypan blue exclusion.
Single cells can now be further processed for the desired assay, e.g., flow cytometric analysis.
Abbreviations
- CNS
Central nervous system
- EAE
Experimental autoimmune encephalomyelitis
- IFA
Incomplete Freund’s adjuvant
- MBP
Myelin basic protein
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- PLP
Proteolipid protein
- TCR
T cell receptor
Footnotes
It is recommended to shave the back of the mice at least 24 h before inducing disease. The animals will then be easier to handle on the day of induction.
It is critical to purchase IFA and M. tuberculosis separately and prepare CFA at a final concentration of 4 mg/mL. Commercially available CFA only contains 1 mg/mL M. tuberculosis.
The lowest concentration of a given myelin protein/peptide that can be used to reliably induce EAE may vary by source and batch number. The source and age of the mice used may also alter the disease course from that expected. Thus, the concentrations of protein/peptide indicated in Table 1 should be used as a guide only and should be confirmed by the individual investigator before conducting large-scale experiments.
CFA can be prepared up to 24 h in advance and stored it in polystyrene tubes or glass syringes at 4 °C until use.
To test the consistency of the emulsion, a small droplet should be expelled onto the surface of water in beaker. If the emulsion is stable, the droplet will remain in a bead on the water surface. If the droplet disperses across surface, further emulsification is required.
It is recommended to dissolve the lyophilized pertussis toxin in sterile PBS at least 24 h before injection and store at 4 °C until use.
We inject 200 ng of pertussis per mouse on day 0 and day 2 post-immunization where indicated on Table 2. However, some papers report injection of up to 500 ng of pertussis per mouse. The individual investigator should determine the optimal dose for the protein/peptide and mouse strain used.
The amount of peptide needed to effectively restimulate T cells in vitro may vary by protein/peptide source and batch number. The source and age of the mice used may also affect the amount of protein/peptide needed for effective restimulation. Thus, the concentrations of protein/peptide indicated in Table 4 should be used as a guide only and should be confirmed by the individual investigator before conducting large-scale experiments.
Con A may be used at a dose of 1 µg/mL to stimulate T cells for 48 h in vitro in place of specific myelin protein/peptide for the induction of adoptive EAE. Con A activation, however, will reduce the frequency of myelin epitope-specific T cells in the culture, thus an increased number of total cells will need to be injected into recipient mice. The individual investigator will need to titrate these numbers in vivo to determine the lowest number of cells required to achieve severe and reliable EAE.
In addition to IL-12, IL-2 may also be added into the media at a concentration of 10 ng/mL to enhance T cell activation and proliferation. The individual investigator should confirm the optimum concentration of these cytokines before conducting large-scale experiments.
IL-23 may be added to the media at a concentration of 10 ng/mL to induce a Th17-skewed T cell phenotype. The individual investigator should confirm the optimum concentration of IL-23 before conducting large-scale experiments.
We typically incubate cells in vitro for 3 days before transferring into recipients. Most papers report incubation periods of 3–4 days. The individual investigator should determine the optimal incubation time for the protein/peptide and mouse strain used.
The lowest number of cells that are needed to reliably induce EAE may vary by myelin protein/peptide source and batch number. The source and age of the mice used may also alter the disease course from that expected. The numbers listed in Table 4 should be used as a guide only. The individual investigator will need to titrate these numbers in vivo to determine the lowest number of cells required to achieve severe and reliable EAE.
If the needle is not placed correctly, dark red blood will not flow from the right atrium and the lungs may inflate. Loss of red coloration of the liver is a good indicator of correct perfusion. The authors recommend practicing this technique before conducting large-scale experiments.
Perfusions should be conducted slowly (over a period of at least 3–5 min per mouse) to avoid tissue damage.
If the mouse is not well perfused after the initial procedure, the syringe may be refilled with 30 mL of PBS and perfusion repeated.
The protocol listed here using Liberase and DNase is optimized for the isolation of total leukocytes. Different enzymatic digestion may be performed on the CNS tissues to isolate different target cell populations. For example, we have found that digesting the CNS using Accutase (Millipore) in place of Liberase and DNase is optimal for the isolation of oligodendrocyte progenitor cell isolation.
The use of HBSS with and without phenol red for the 30 % Percoll and 70 % Percoll solutions, respectively, will increase the ease of which to see the interface between the two gradients and identify the mononuclear cell layer here.
References
- 1.Bruck W, Stadelmann C. The spectrum of multiple sclerosis: new lessons from pathology. Curr Opin Neurol. 2005;18:221–224. doi: 10.1097/01.wco.0000169736.60922.20. [DOI] [PubMed] [Google Scholar]
- 2.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stinissen P, Hellings N. Activation of myelin reactive T cells in multiple sclerosis: a possible role for T cell degeneracy? Eur J Immunol. 2008;38:1190–1193. doi: 10.1002/eji.200838371. [DOI] [PubMed] [Google Scholar]
- 4.Al-Omaishi J, Bashir R, Gendelman HE. The cellular immunology of multiple sclerosis. J Leukoc Biol. 1999;65:444–452. doi: 10.1002/jlb.65.4.444. [DOI] [PubMed] [Google Scholar]
- 5.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feinstein A. The neuropsychiatry of multiple sclerosis. Can J Psychiatry. 2004;49:157–163. doi: 10.1177/070674370404900302. [DOI] [PubMed] [Google Scholar]
- 7.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 8.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 9.Miller SD, Karpus WJ, Davidson TS. Experimental autoimmune encephalomyelitis in the mouse. Curr Protoc Immunol. 2010;Chapter 15(Unit 15):11. doi: 10.1002/0471142735.im1501s88. [DOI] [PubMed] [Google Scholar]
- 10.Lyons JA, Ramsbottom MJ, Trotter JL, Cross AH. Identification of the encephalitogenic epitopes of CNS proteolipid protein in BALB/c mice. J Autoimmun. 2002;19:195–201. doi: 10.1006/jaut.2002.0619. [DOI] [PubMed] [Google Scholar]
- 11.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 12.Tompkins SM, Padilla J, Dal Canto MC, Ting JP, Van Kaer L, Miller SD. De novo central nervous system processing of myelin antigen is required for the initiation of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4173–4183. doi: 10.4049/jimmunol.168.8.4173. [DOI] [PubMed] [Google Scholar]
- 13.Fritz RB, Chou CH, McFarlin DE. Induction of experimental allergic encephalomyelitis in PL/J and (SJL/J × PL/J)F1 mice by myelin basic protein and its peptides: localization of a second encephalitogenic determinant. J Immunol. 1983;130:191–194. [PubMed] [Google Scholar]
- 14.Tan LJ, Kennedy MK, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. II. Fine specificity of effector T cell inhibition. J Immunol. 1992;148:2748–2755. [PubMed] [Google Scholar]
- 15.Sakai K, Zamvil SS, Mitchell DJ, Lim M, Rothbard JB, Steinman L. Characterization of a major encephalitogenic T cell epitope in SJL/J mice with synthetic oligopeptides of myelin basic protein. J Neuroimmunol. 1988;19:21–32. doi: 10.1016/0165-5728(88)90032-x. [DOI] [PubMed] [Google Scholar]
- 16.Amor S, Groome N, Linington C, Morris MM, Dornmair K, Gardinier MV, et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994;153:4349–4356. [PubMed] [Google Scholar]
- 17.Tsunoda I, Kuang LQ, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000;10:402–418. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greer JM, Klinguer C, Trifilieff E, Sobel RA, Lees MB. Encephalitogenicity of murine, but not bovine, DM20 in SJL mice is due to a single amino acid difference in the immunodominant encephalitogenic epitope. Neurochem Res. 1997;22:541–547. doi: 10.1023/a:1027336516785. [DOI] [PubMed] [Google Scholar]
- 19.Greer JM, Sobel RA, Sette A, Southwood S, Lees MB, Kuchroo VK. Immunogenic and encephalitogenic epitope clusters of myelin proteolipid protein. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 20.Tuohy VK, Thomas DM. Sequence 104–117 of myelin proteolipid protein is a cryptic encephalitogenic T cell determinant for SJL/J mice. J Neuroimmunol. 1995;56:161–170. doi: 10.1016/0165-5728(94)00143-c. [DOI] [PubMed] [Google Scholar]
- 21.Greer JM, Denis B, Sobel RA, Trifilieff E. Thiopalmitoylation of myelin proteolipid protein epitopes enhances immunogenicity and encephalitogenicity. J Immunol. 2001;166:6907–6913. doi: 10.4049/jimmunol.166.11.6907. [DOI] [PubMed] [Google Scholar]
- 22.Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- 23.Greer JM, Kuchroo VK, Sobel RA, Lees MB. Identification and characterization of a second encephalitogenic determinant of myelin proteolipid protein (residues 178–191) for SJL mice. J Immunol. 1992;149:783–788. [PubMed] [Google Scholar]
- 24.Amor S, O’Neill JK, Morris MM, Smith RM, Wraith DC, Groome N, et al. Encephalitogenic epitopes of myelin basic protein, proteolipid protein, myelin oligodendrocyte glycoprotein for experimental allergic encephalomyelitis induction in Biozzi ABH (H-2Ag7) mice share an amino acid motif. J Immunol. 1996;156:3000–3008. [PubMed] [Google Scholar]
- 25.Ando DG, Clayton J, Kono D, Urban JL, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 26.Zamvil SS, Mitchell DJ, Moore AC, Kitamura K, Steinman L, Rothbard JB. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986;324:258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- 27.Zamvil SS, Mitchell DJ, Powell MB, Sakai K, Rothbard JB, Steinman L. Multiple discrete encephalitogenic epitopes of the autoantigen myelin basic protein include a determinant for I-E class II-restricted T cells. J Exp Med. 1988;168:1181–1186. doi: 10.1084/jem.168.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerlero de Rosbo N, Mendel I, Ben-Nun A. Chronic relapsing experimental autoimmune encephalomyelitis with a delayed onset and an atypical clinical course, induced in PL/J mice by myelin oligodendrocyte glycoprotein (MOG)-derived peptide: preliminary analysis of MOG T cell epitopes. Eur J Immunol. 1995;25:985–993. doi: 10.1002/eji.1830250419. [DOI] [PubMed] [Google Scholar]
- 29.Whitham RH, Jones RE, Hashim GA, Hoy CM, Wang RY, Vandenbark AA, et al. Location of a new encephalitogenic epitope (residues 43 to 64) in proteolipid protein that induces relapsing experimental autoimmune encephalomyelitis in PL/J and (SJL × PL)F1 mice. J Immunol. 1991;147:3803–3808. [PubMed] [Google Scholar]
- 30.Muller DM, Pender MP, Greer JM. A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol. 2000;100:174–182. doi: 10.1007/s004019900163. [DOI] [PubMed] [Google Scholar]
- 31.Endoh M, Kunishita T, Nihei J, Nishizawa M, Tabira T. Susceptibility to proteolipid apoprotein and its encephalitogenic determinants in mice. Int Arch Allergy Appl Immunol. 1990;92:433–438. doi: 10.1159/000235176. [DOI] [PubMed] [Google Scholar]
- 32.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 33.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 34.Madsen LS, Andersson EC, Jansson L, krogsgaard M, Andersen CB, Engberg J, et al. A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet. 1999;23:343–347. doi: 10.1038/15525. [DOI] [PubMed] [Google Scholar]
- 35.Ellmerich S, Mycko M, Takacs K, Waldner H, Wahid FN, Boyton RJ, et al. High incidence of spontaneous disease in an HLA-DR15 and TCR transgenic multiple sclerosis model. J Immunol. 2005;174:1938–1946. doi: 10.4049/jimmunol.174.4.1938. [DOI] [PubMed] [Google Scholar]
- 36.Waldner H, Whitters MJ, Sobel RA, Collins M, Kuchroo VK. Fulminant spontaneous autoimmunity of the central nervous system in mice transgenic for the myelin proteolipid protein-specific T cell receptor. Proc Natl Acad Sci U S A. 2000;97:3412–3417. doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zehntner SP, Brisebois M, Tran E, Owens T, Fournier S. Constitutive expression of a costimulatory ligand on antigen-presenting cells in the nervous system drives demyelinating disease. FASEB J. 2003;17:1910–1912. doi: 10.1096/fj.03-0199fje. [DOI] [PubMed] [Google Scholar]
- 42.Brisebois M, Zehntner SP, Estrada J, Owens T, Fournier S. A pathogenic role for CD8+ T cells in a spontaneous model of demyelinating disease. J Immunol. 2006;177:2403–2411. doi: 10.4049/jimmunol.177.4.2403. [DOI] [PubMed] [Google Scholar]
- 43.Na SY, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, et al. Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system. Brain. 2008;131:2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- 44.Friese MA, Jakobsen KB, Friis L, Etzensperger R, Craner MJ, McMahon RM, et al. Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis. Nat Med. 2008;14:1227–1235. doi: 10.1038/nm.1881. [DOI] [PubMed] [Google Scholar]
- 45.Anderson AC, Chandwaskar R, Lee DH, Sullivan JM, Solomon A, Rodriguez-Manzanet R, et al. A transgenic model of central nervous system autoimmunity mediated by CD4+ and CD8+ T and B cells. J Immunol. 2012;188:2084–2092. doi: 10.4049/jimmunol.1102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nath N, Prasad R, Giri S, Singh AK, Singh I. T-bet is essential for the progression of experimental autoimmune encephalomyelitis. Immunology. 2006;118:384–391. doi: 10.1111/j.1365-2567.2006.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw MK, Kim C, Hao HW, Chen F, Tse HY. Induction of myelin basic protein-specific experimental autoimmune encephalomyelitis in C57BL/6 mice: mapping of T cell epitopes and T cell receptor V beta gene segment usage. J Neurosci Res. 1996;45:690–699. doi: 10.1002/(SICI)1097-4547(19960915)45:6<690::AID-JNR5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 48.Clark RB, Grunnet M, Lingenheld EG. Adoptively transferred EAE in mice bearing the lpr mutation. Clin Immunol Immunopathol. 1997;85:315–319. doi: 10.1006/clin.1997.4450. [DOI] [PubMed] [Google Scholar]
- 49.Segal BM, Dwyer BK, Shevach EM. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J Exp Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendel I, Shevach EM. Differentiated Th1 autoreactive effector cells can induce experimental autoimmune encephalomyelitis in the absence of IL-12 and CD40/CD40L interactions. J Neuroimmunol. 2002;122:65–73. doi: 10.1016/s0165-5728(01)00465-9. [DOI] [PubMed] [Google Scholar]
- 51.Pettinelli CB, McFarlin DE. Adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice after in vitro activation of lymph node cells by myelin basic protein: requirement for Lyt 1+ 2- T lymphocytes. J Immunol. 1981;127:1420–1423. [PubMed] [Google Scholar]
- 52.Pettinelli CB, Fritz RB, Chou CH, McFarlin DE. Encephalitogenic activity of guinea pig myelin basic protein in the SJL mouse. J Immunol. 1982;129:1209–1211. [PubMed] [Google Scholar]
- 53.Miller SD, Tan LJ, Kennedy MK, Dal Canto MC. Specific immunoregulation of the induction and effector stages of relapsing EAE via neuroantigen-specific tolerance induction. Ann N Y Acad Sci. 1991;636:79–94. doi: 10.1111/j.1749-6632.1991.tb33440.x. [DOI] [PubMed] [Google Scholar]
- 54.McRae BL, Kennedy MK, Tan LJ, Dal Canto MC, Picha KS, Miller SD. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. J Neuroimmunol. 1992;38:229–240. doi: 10.1016/0165-5728(92)90016-e. [DOI] [PubMed] [Google Scholar]
- 55.Kim C, Tse HY. Adoptive transfer of murine experimental autoimmune encephalomyelitis in SJL.Thy-1 congenic mouse strains. J Neuroimmunol. 1993;46:129–136. doi: 10.1016/0165-5728(93)90242-q. [DOI] [PubMed] [Google Scholar]
- 56.Skundric DS, Kim C, Tse HY, Raine CS. Homing of T cells to the central nervous system throughout the course of relapsing experimental autoimmune encephalomyelitis in Thy-1 congenic mice. J Neuroimmunol. 1993;46:113–121. doi: 10.1016/0165-5728(93)90240-y. [DOI] [PubMed] [Google Scholar]
- 57.Fritz RB, Zhao ML. Encephalitogenicity of myelin basic protein exon-2 peptide in mice. J Neuroimmunol. 1994;51:1–6. doi: 10.1016/0165-5728(94)90122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segal BM, Raine CS, McFarlin DE, Voskuhl RR, McFarland HF. Experimental allergic encephalomyelitis induced by the peptide encoded by exon 2 of the MBP gene, a peptide implicated in remyelination. J Neuroimmunol. 1994;51:7–19. doi: 10.1016/0165-5728(94)90123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]