Persistent tear deficiency was sufficient to cause sensitization of neurons at multiple regions of the trigeminal brainstem and enhanced orbicularis oculi muscle activity.
Keywords: Central sensitization, Dry eye, Electromyography, Ocular pain, Orbicularis oculi, Synaptic blockade, Trigeminal brainstem
Abstract
Chronic dry eye disease (DE) is associated with an unstable tear film and symptoms of ocular discomfort. The characteristics of symptoms suggest a key role for central neural processing; however, little is known about central neuroplasticity and DE. We used a model for tear deficient DE and assessed effects on eye blink behavior, orbicularis oculi muscle activity (OOemg), and trigeminal brainstem neural activity in male rats. Ocular-responsive neurons were recorded at the interpolaris/caudalis transition (Vi/Vc) and Vc/upper cervical cord (Vc/C1) regions under isoflurane, whereas OOemg activity was recorded under urethane. Spontaneous tear volume was reduced by ∼50% at 14 days after exorbital gland removal. Hypertonic saline–evoked eye blink behavior in awake rats was enhanced throughout the 14 days after surgery. Saline-evoked neural activity at the Vi/Vc transition and in superficial and deep laminae at the Vc/C1 region was greatly enhanced in DE rats. Neurons from DE rats classified as wide dynamic range displayed enlarged convergent periorbital receptive fields consistent with central sensitization. Saline-evoked OOemg activity was markedly enhanced in DE rats compared with controls. Synaptic blockade at the Vi/Vc transition or the Vc/C1 region greatly reduced hypertonic saline–evoked OOemg activity in DE and sham rats. These results indicated that persistent tear deficiency caused sensitization of ocular-responsive neurons at multiple regions of the caudal trigeminal brainstem and enhanced OOemg activity. Central sensitization of ocular-related brainstem circuits is a significant factor in DE and likely contributes to the apparent weak correlation between peripheral signs of tear dysfunction and symptoms of irritation.
1. Introduction
Dry eye disease (DE) is a common condition affecting up to 35% of the general population1 and is defined on the basis of signs of an altered tear film and patient-reported symptoms of ocular discomfort.47 Relief from symptoms of ocular irritation is the main reason patients seek medical attention38,53; however, the neural mechanisms underlying sensations of irritation or pain in DE are not well defined. Several lines of evidence suggest that central neural mechanisms play a significant role in symptom development and maintenance in DE. First, peripheral signs of tear film instability or ocular inflammation are poorly correlated with symptoms in DE.23,47,48 Second, although most forms of DE are associated with ocular inflammation,12,45 treatments that effectively reduce ocular surface inflammation2,6 have often proved inadequate to manage symptoms of irritation in moderate and more severe cases of DE.3 Third, reports of comorbid pain located around the eye44 and increased sensitivity and lower tolerance to noxious stimuli applied to the limbs of DE patients54 are consistent with secondary hyperalgesia and central neural mechanisms.56 Fourth, altered sensorimotor integration by central neurons may be sufficient to cause DE-like symptoms. For example, blepharospasm, a focal dystonia of the eyelid muscles, often presents with DE-like symptoms and photophobia.10,16 Furthermore, in an animal model of light-evoked nociception and lacrimation, we determined that altered central nervous system integration of light signals through accessory visual pathways resulting in increased parasympathetic outflow to the eye was required to excite intraocular trigeminal nociceptors and trigeminal brainstem neurons.33,34
Trigeminal sensory fibers innervate the eye and project to trigeminal subnucleus interpolaris/caudalis (Vi/Vc) transition and Vc/upper cervical cord junction (Vc/C1) regions of the trigeminal brainstem (TBNC).29,30,35,51 The significance of eye representations at multiple regions of the TBNC is not certain; however, considerable evidence suggests that neurons in different brainstem regions serve different aspects of ocular function. For example, only neurons at the Vi/Vc transition were responsive to changes in moisture status of the ocular surface and may be critical for reflex lacrimation,20 whereas only neurons at the Vc/C1 region became sensitized after ocular inflammation by systemic endotoxin9 or after local injury by UV irradiation52 and may be necessary for sensory-discriminative aspects of persistent sensitization after injury. This study used exorbital gland removal, a model for aqueous tear deficient DE, to determine the effects of persistent reduced tear volume on the properties of ocular-responsive neurons at the Vi/Vc transition and Vc/C1 regions and evoked eye blink behavior.
2. Methods
2.1. Animal preparation and exorbital gland removal
The animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Minnesota (USA) and conformed to the established guidelines set by The National Institutes of Health guide for the care and the use of laboratory animals (PHS Law 99-158, revised 2002). All efforts were made to minimize the number of the animals used for experiments. Animals were housed in pairs with free access to food and water. Cages remained in climate- and light-controlled environment (25°C ± 2°C, 12:12-hour light/dark cycle with light on at 7:00 am).
Male rats (240-370 g, n = 144, Sprague-Dawley; Harlan, Indianapolis, IN) were anesthetized with isoflurane (3%-5%), and under aseptic conditions, the fur anterior to the ear was shaved, and a small incision was made to expose the masseter muscle and the left exorbital gland. The gland was gently teased off the muscle and excised. The wound margins were treated with 2% xylocaine gel, and the incision was closed with absorbable suture. A single dose of ketoprofen (25 mg/kg, intraperitoneally [i.p.]) was given postsurgically for pain management. Sham controls were treated similarly except that the gland was not removed. Rats survived up to 14 days after surgery.
2.2. Tear volume measurements
Spontaneous tear volume was estimated by the increase in wet length of phenol red thread (Zone-Quick; Menicon Inc, San Mateo, CA). The thread was placed in contact with the cornea/conjunctiva at its inferior-lateral edge, and tear volume was measured over 2 minutes. Tear volume was measured under isoflurane (neural recording) and urethane anesthesia (OOemg activity). Choice of anesthetic (ie, urethane vs isoflurane) did not affect spontaneous tear volume, and data from these 2 conditions were combined for statistical analyses.
2.2.1. Eye blink behavior
The eye blink behavior was measured in awake rats (n = 4) in response to ocular instillation of NaCl solutions (0.15, 1.0, and 2.5 M; 10 μL). Behavior was measured in a plexiglass chamber, and rats were allowed to habituate for 1 hour before testing. Test stimuli were applied to the left eye from a micropipette in ascending order of concentration at 30-minute intervals. The number of eye blinks was counted over 5 minutes before surgery and 2, 5, 7, and 14 days after exorbital gland removal. In separate rats (sham, n = 3; 14d DE, n = 4), 1% fluorescein was applied to the ocular surface and observed through a cobalt blue filter to assess ocular damage.
2.2.2. Eye muscle electromyography
Rats (240-270 g, n = 64) were anesthetized with urethane (1.2-1.5 g/kg, i.p.), and the left femoral artery was catheterized to monitor arterial blood pressure that was maintained at 90 to 110 mm Hg. Rats were allowed to breathe spontaneously. The wound margins were infiltrated with 2% lidocaine, and body temperature was kept at 38°C with a heating blanket. The rat was positioned in a stereotaxic frame, and a small portion of the C1 vertebra was removed to expose the dorsal brainstem surface in experiments that involved microinjections of synaptic blocker into the Vi/Vc transition or Vc/C1 region. A pair of Teflon-coated copper wires (0.12 mm diameter, 5 mm interpolar distance) was implanted by a 26-gauge needle in parallel with muscle fiber at the lateral margin of the left orbicularis oculi (OO) muscle for electromyography (OOemg).40
2.2.3. Experimental design
Saline solutions (0.15, 1.0, 2.5, and 5 M NaCl, pH: 7.2; 10 μL) were applied to the ocular surface by a microsyringe40,52 in a cumulative dose design at 20-minute interstimulus intervals. Test solutions remained on the eye during the 3-minute sampling period and were washed out with artificial tears after each stimulus (total exposure time to each NaCl solution = 3-4 minutes) to prevent desensitization or possible damage to the ocular surface.
Microinjections of CoCl2 (100 mM, 0.3 μL) were used to block the synaptic activity at the Vi/Vc transition and Vc/C1 regions to assess the effect on NaCl-evoked OOemg activity.19,40 A glass micropipette (40-80 μm tip diameter) was filled with CoCl2 or vehicle (phosphate-buffered saline) (PBS) and directed at either the Vi/Vc transition (angle of 28° off vertical and 45° off midline, and 1.5-2.0 mm below the brainstem surface) or the Vc/C1 region (43° off vertical, 60° off midline, within 300 μm of the dorsal brainstem surface). OOemg responses to 2.5 M NaCl were measured before and 10, 30, and 50 minutes after microinjection of either CoCl2 (DE, n = 5, sham, n = 4) or vehicle (DE, n = 4, sham = 5) into the Vi/Vc transition or injection of CoCl2 (DE, n = 5, sham, n = 5) or vehicle (DE, n = 4, sham, n = 4) into the Vc/C1 region. Microinjection sites were confirmed by histology.
2.2.4. Data analysis
OOemg activity was sampled at 1000 Hz, amplified (×10k), filtered (bandwidth 300-3000 Hz), displayed and stored online for later analyses (AD Instruments, Colorado Springs, Co). Muscle activity was sampled continuously for 6 minutes beginning 3 minutes before and until 3 minutes after each stimulus. Muscle activity was rectified and stored as 1-second bins for off-line analyses. Baseline activity was defined as an integrated area under the curve (AUC) for the 3-minute epoch (μV-s per 3 minutes) sampled immediately before each stimulus. Ocular-evoked OOemg activity was calculated as AUC poststimulus minus baseline AUC. The response latency (onset) was defined as the first bin at which OOemg exceeded the average baseline AUC.
The AUC and latency of OOemg were assessed by analysis of variance (ANOVA) corrected for repeated measures. Significant treatment effects were assessed by Newman–Keuls test after ANOVA. The data were presented as mean ± SEM and the significant level set at P < 0.05.
2.2.5. Neural recording procedures
Rats were anesthetized initially with pentobarbital sodium (50 mg/kg, i.p., n = 69), and catheters were positioned in the right femoral artery for monitoring blood pressure and jugular vein for drug infusion (gallamine triethiodide, 25 mg·kg−1·h−1, at the time of recording). After tracheotomy, animals were respired artificially with oxygen-enriched room air and anesthesia was maintained with isoflurane (1.2%-2.0%). Expiratory end-tidal CO2 (3.5%-4.5%), mean arterial pressure (MAP, 90-120 mm Hg), and body temperature (37°C) were monitored continuously and kept within normal range. Animals were placed in a stereotaxic frame and portions of the C1 vertebra were removed to expose the lower brainstem and upper cervical dorsal horn. The exposed brainstem surface was bathed in warm mineral oil. Neurons recorded at the ventrolateral trigeminal subnucleus interpolaris/caudalis (Vi/Vc) transition were approached at an angle of 28° off vertical and 45° off midline and 1.5 to 2.0 mm below the brainstem surface (Fig. 2D). Single neurons were recorded from superficial (I-II) or deep (V) laminae at the Vc/C1 region (Figs. 3D and 4D) by directing the electrode at an angle of 47° off vertical, 60° off midline. For units recorded in superficial laminae, the depth of recording was within 250 μm of the dorsal brainstem surface. Recording sites were confirmed from the location of an electrolytic lesion (5 μA, 10 seconds) marked at the end of the experiment. Extracellular unit activity was recorded using tungsten microelectrodes (5-9 MΩ; Frederic Haer Inc, Bowdoinham, ME) and amplified, discriminated, stored, and analyzed off-line using a PowerLab interface and LabChart software (AD Instruments).
Figure 2.
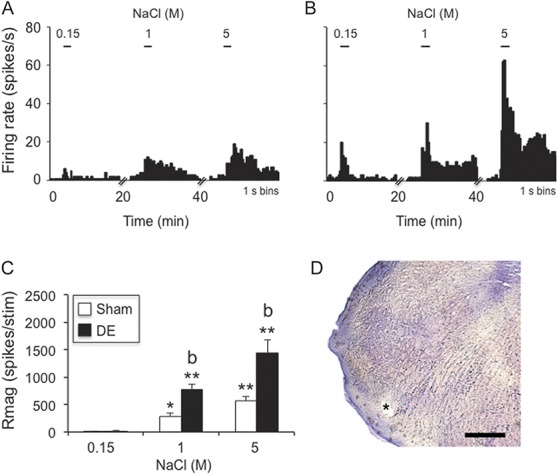
Hypertonic saline–evoked responses of neurons at the Vi/Vc transition were increased in dry eye disease (DE) rats 14 days after surgery. (A) Example of NaCl concentration–evoked neural activity in a wide dynamic range (WDR) cell in a sham rat. (B) Example of NaCl concentration–evoked neural activity in a WDR cell in a DE rat. Scale bars above each histogram in (A) and (B) indicate stimulus period (30 seconds). Bin size = 1 second. (C) Summary of NaCl-evoked Rmag values for sham and 14d DE rats. *P < 0.05, **P < 0.01 vs 0.15 M; b = P < 0.01 vs sham. Sample sizes: sham, n = 11; DE, n = 14. (D) Recording site (*); scale = 100 μm.
Figure 3.
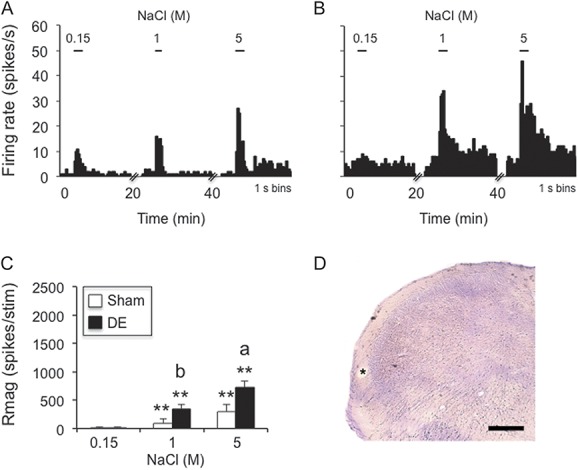
Hypertonic saline–evoked responses of neurons in superficial laminae at the Vc/C1 region were increased in dry eye disease (DE) rats 14 days after surgery. (A) Example of NaCl concentration–evoked neural activity in an NS cell in a sham rat. (B) Example of NaCl concentration–evoked neural activity in an NS cell in a DE rat. Scale bars above each histogram in (A) and (B) indicate stimulus period (30 seconds). Bin size = 1 second. (C) Summary of NaCl-evoked Rmag values for sham and 14d DE rats. **P < 0.01 vs 0.15 M; a = P < 0.05, b = P < 0.01 vs sham. Sample sizes: sham, n = 11; DE, n = 11. (D) *Recording site; scale = 100 μm.
Figure 4.
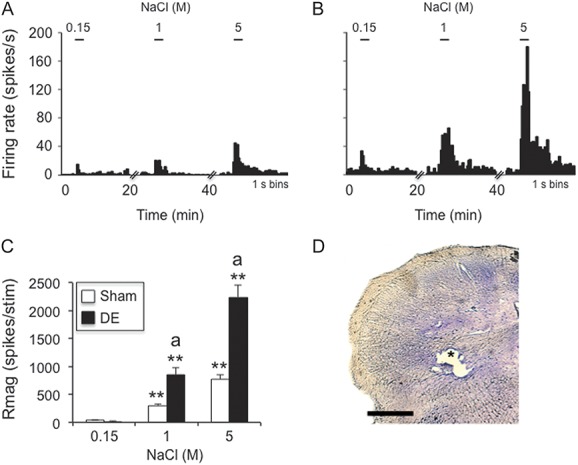
Hypertonic saline–evoked responses of neurons in deep laminae at the Vc/C1 region were increased in dry eye disease (DE) rats 14 days after surgery. (A) Example of NaCl concentration–evoked neural activity in a wide dynamic range (WDR) cell in a sham rat. (B) Example of NaCl concentration–evoked neural activity in a WDR cell in a DE rat. Scale bars above each histogram in (A) and (B) indicate stimulus period (30 seconds). Bin size = 1 second. (C) Summary of NaCl-evoked Rmag values for sham and 14d DE rats. **P < 0.01 vs 0.15 M; a = P < 0.05 vs sham. Sample sizes: sham, n = 11; DE, n = 11. (D) *Recording site; scale = 100 μm.
2.2.6. Characterization of ocular neurons and experimental design
The search stimulus consisted of gently swiping a fine camelhair brush across the ocular surface (ie, cornea and conjunctiva). A single neuron was recorded in each preparation, and all units included for further analyses were activated by mechanical stimulation of the ocular surface. Units with a convergent cutaneous receptive field (RF) were further classified as wide dynamic range (WDR) or nociceptive specific (NS) based on the responses to a low-force von Frey filament (1.2 g) and pinch with blunt forceps as described previously.17 Neurons with no apparent cutaneous RF were classified as cornea only (CO). The ocular surface was kept moist with artificial tears throughout the experiment. We did not specifically test for responses to drying the ocular surface.
Saline solutions (0.15, 1, and 5 M NaCl, pH 7.2; 10 μL) were applied to the ocular surface from a microsyringe40,52 in a cumulative dose design at 20-minute interstimulus intervals. Test solutions remained on the eye during the 3-minute sampling period and were washed out with artificial tears after each stimulus (total exposure time to each NaCl solution = 3-4 minutes) to prevent desensitization or possible damage to the ocular surface. Neural activity was recorded 14 days after surgery (14d DE) and in sham controls.
2.2.7. Data analysis
Neural recording data were acquired and displayed as peristimulus time histograms of spikes per 1-second bins, exported to a spreadsheet and analyzed off-line. Neural responses were quantified as a response magnitude (Rmag) for each stimulus period defined as the cumulative sum of spikes for contiguous bins in which the spike count exceeded the mean + 2 SD of the background activity.17 The total Rmag was calculated for each stimulus period and can be thought of as the “area under the curve.” Neurons were defined as NaCl-responsive if the total Rmag exceeded a value of 10 after stimulation. Response latency was defined as the earliest time after stimulus onset at which 3 consecutive bins exceeded the mean + 2 SD of background activity. Response duration was defined as the time interval after stimulus onset until 3 consecutive bins with a positive spike count occurred above background (initial latency) and until the value of 3 consecutive bins no longer exceeded the mean + 2 SD above background activity. The high-threshold convergent cutaneous RF area was mapped with a small blunt forceps and transferred onto standardized drawings of the rat face, digitized, and quantified by a planimetric method using NIH software (ImageJ). The RF area was mapped before NaCl stimulation of the ocular surface. Neural activity (ie, Rmag, duration, latency) and cutaneous RF areas were assessed by ANOVA corrected for repeated measures. Treatment effects were assessed further by Newman–Keuls test after ANOVA. Comparisons of the frequency of occurrence across neuronal cell classes or recording location were made by χ2 or Fisher exact probability tests. The data were presented as mean ± SEM and significance level set at P < 0.05.
3. Results
3.1. General effects of exorbital gland removal
During the 14 days after surgery DE and sham rats gained weight normally and displayed no obvious differences in overall general appearance. Although we did not quantify this measurement, 14d DE rats displayed little or no fluorescein staining (<5% of ocular surface), ipsilateral to gland removal, compared with the contralateral eye or to sham controls. There were no overt signs of ocular hyperemia or inflammation for 14d DE or sham rats. As seen in Figure 1A, spontaneous tear volume was reduced significantly in the ipsilateral eye of 14d DE rats (9.3 ± 0.2 mm per 2 minutes) compared with sham (18.8 ± 0.4 mm per 2 minutes; F1,87 = 191, P < 0.001). Spontaneous tear volume in the contralateral eye was similar for 14d DE and sham rats (19.8 ± 0.3 and 19.3 ± 0.3 mm per 2 minutes, respectively).
Figure 1.
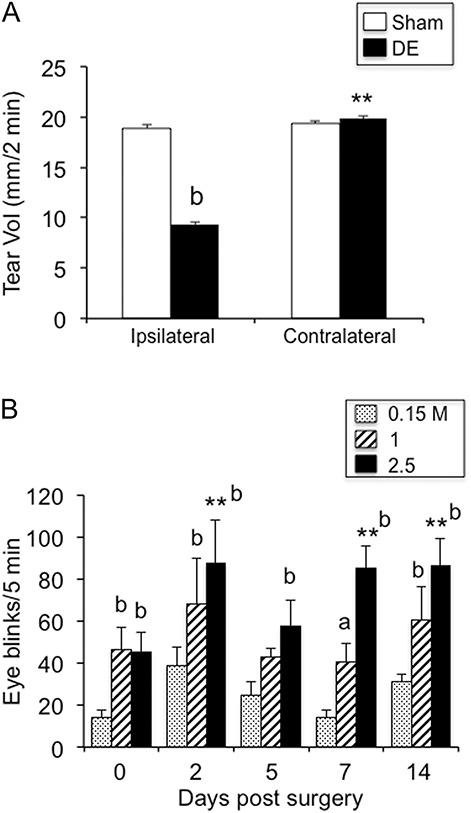
(A) Spontaneous tear volume was reduced ipsilateral to exorbital gland removal at 14 days after surgery. Tear volume measured over 2 minutes under isoflurane or urethane anesthesia. Sample sizes (rats per group): sham, n = 48; 14d DE, n = 41. **P < 0.01 vs ipsilateral; b = P < 0.01 vs sham. (B) Eye blink behavior was evoked by 0.15, 1, and 2.5 M NaCl before surgery (day 0) and at 2, 5, 7, and 14 days after exorbital gland removal. **P < 0.01 vs presurgery; a = P < 0.05, b = P < 0.01 vs response to 0.15 M saline; n = 4.
3.1.1. Eye blink behavior
Ocular application of NaCl solutions increased eye blink behavior in a concentration- and time-dependent manner after gland removal (Fig. 1B). At 1.0 M NaCl concentrations, eye blink behavior displayed a bimodal pattern with increases at 2, 7, and 14 days, whereas after 2.5 M NaCl, significant increases were seen at each day of testing after gland removal compared with before surgery (F2,6 = 54.2, P < 0.001, n = 4). In pilot studies, eye blink responses to NaCl in naive and sham animals were similar (data not shown).
3.2. Neurophysiology
3.2.1. Vi/Vc transition neurons
A total of 25 neurons were recorded at the Vi/Vc transition (sham, n = 11; DE, n = 14). Neurons were further classified based on periorbital cutaneous RF properties and were found in similar proportions for sham and DE rats: sham, WDR, n = 5; NS, n = 4; cornea only, n = 2; and 14d DE rats, WDR, n = 5; NS, n = 5; cornea only, n = 4 (χ2 = 0.23, P > 0.1). Ocular instillation of NaCl solutions caused concentration-dependent increases in neural activity in sham rats and DE rats (Fig. 2A, B). These data are summarized in Figure 2C and reveal significantly greater responses to NaCl by units from DE than sham rats (F1,23 = 15.9, P < 0.001). Response latencies decreased significantly (F2,45 = 32.1, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 58.5 ± 13 seconds, 5 M = 1.9 ± 1 seconds) and sham rats (0.15 M = 58.2 ± 14.6, 5 M = 3.8 ± 1.2 seconds). Response duration of Vi/Vc transition units increased significantly (F2,45 = 111, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 5.9 ± 2 seconds, 5 M = 83.4 ± 6 seconds) and sham rats (0.15 M = 2.3 ± 1 seconds, 5 M = 67.8 ± 10 seconds). Although the sample sizes for subclasses of units were small (n = 2-5 units per class), we found no differences in Rmag, latency, or response duration between WDR, NS, and cornea only units (data not shown).
The spontaneous firing rate of Vi/Vc units in DE rats was elevated compared with sham controls (6.4 ± 1.9 vs 2.9 ± 1.4 Hz, respectively, F1,23 = 5.5, P < 0.05). The convergent periocular cutaneous RF areas of Vi/Vc units classified as WDR were enlarged in DE rats compared with sham controls (Fig. 5B, F1,8 = 7.9, P < 0.025), whereas the RF areas of NS units were not different.
Figure 5.
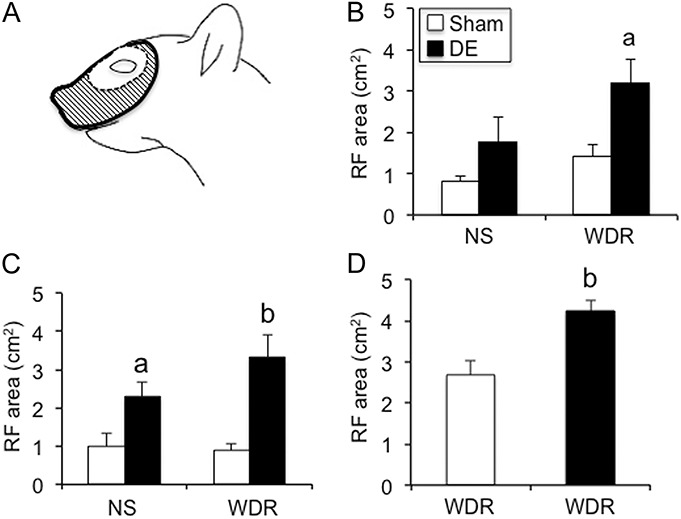
Convergent receptive field (RF) areas from periorbital skin were enlarged in ocular neurons of dry eye disease (DE) rats 14 days after surgery. (A) Example of RF area of a wide dynamic range (WDR) unit recorded at the Vi/Vc transition. The center white region indicates average field size for WDR units in sham rats; hashed area indicates the RF area for this WDR unit from a DE rat. (B) Average RF area for neurons at the Vi/Vc transition of: sham rats, NS (n = 4) and WDR (n = 5); DE rats, NS (n = 5) and WDR (n = 5). (C) Average RF area for neurons in superficial laminae at the Vc/C1 region of: sham rats, NS (n = 6) and WDR (n = 5) units; DE rats, NS (n = 5) and WDR (n = 6) units. (D) Average RF area for WDR units in deep laminae at the Vc/C1 region. Sham, n = 11, DE, n = 11. a = P < 0.05, b = P < 0.01 vs sham.
3.2.2. Vc/C1 region superficial laminae
Twenty-two neurons were recorded in superficial laminae (I-II) at the Vc/C1 region (sham, n = 11; DE, n = 11). Neurons classified further on the basis of periorbital cutaneous RF properties were sampled in similar proportions from sham and DE rats: sham, WDR, n = 5; NS, n = 6; and DE rats, WDR, n = 6; NS, n = 5 (χ2 = 0.18, P > 0.1). Ocular instillation of NaCl solutions caused concentration-dependent increases in neural activity in sham rats and DE rats (Fig. 3A, B). These data are summarized in Figure 3C and indicated markedly greater responses to NaCl from units in DE than sham rats (F1,20 = 13.6, P < 0.005). Response latencies decreased significantly (F2,40 = 10.6, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 40.3 ± 14 seconds, 5 M = 1.2 ± 1 seconds) and sham rats (0.15 M = 24.5 ± 11.6 seconds, 5 M = 3.5 ± 2.2 seconds). Response duration increased significantly (F2,40 = 32.7, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 5.7 ± 2 seconds, 5 M = 49.8 ± 8 seconds) and sham rats (0.15 M = 10.1 ± 3 seconds, 5 M = 52.9 ± 8 seconds).
The spontaneous firing rate of units in superficial laminae at the Vc/C1 region from DE (3.6 ± 1.5 Hz) and sham rats (0.6 ± 0.2 Hz) was low and not different between groups (F1,20 = 3.2, P < 0.1). The convergent periocular cutaneous RF areas of superficial laminae units classified as WDR were enlarged in DE rats compared with sham controls (Fig. 5C, F1,18 = 17.6, P < 0.001). Also, the RF areas of NS units were marginally increased compared with sham rats (Fig. 5C, F1,18 = 5.1, P < 0.05).
3.2.3. Vc/C1 region deep laminae
A total of 22 neurons were recorded in deep laminae (V) at the Vc/C1 region (sham, n = 11; DE, n = 11). All neurons recorded from deep lamina were classified as WDR units. Ocular instillation of NaCl solutions caused concentration-dependent increases in neural activity in sham rats and DE rats (Fig. 4A, B). As summarized in Figure 4C, the responses to NaCl of DE rats were greatly enhanced compared with those of sham rats (F1,20 = 36.2, P < 0.001). Response latencies decreased significantly (F2,40 = 26.3, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 66.5 ± 14 seconds, 5 M = 1.5 ± 1 seconds) and sham rats (0.15 M = 40.1 ± 14 seconds, 5 M = 1.7 ± 1.4 seconds). Response duration of Vc/C1 units in deep laminae increased significantly (F2,40 = 58.2, P < 0.001) and similarly with increasing NaCl concentration in DE (0.15 M = 2.8 ± 1 seconds, 5 M = 82.1 ± 8 seconds) and sham rats (0.15 M = 13 ± 5 seconds, 5 M = 66.3 ± 10 seconds).
The spontaneous firing rate of units in deep laminae at Vc/C1 was similar for DE and sham rats (9.6 ± 2.7 vs 7.3 ± 2.6 Hz, respectively, F1,20 = 0.5, P > 0.1). The convergent periocular cutaneous RF areas of WDR units were greatly enlarged in DE rats compared with sham controls (Fig. 5D, F1,20 = 12.6, P < 0.005).
3.3. Electromyography
3.3.1. OOemg responses to NaCl solutions
Ocular surface application of NaCl solutions increased the OOemg responses in DE and sham rats (Fig. 6A). The AUC response to NaCl occurred in a concentration-related manner (Fig. 6B, F3,59 = 41.8, P < 0.001) and comparison across animal groups revealed significantly greater responses by DE than sham rats (F1,26 = 11.8, P < 0.005).
Figure 6.
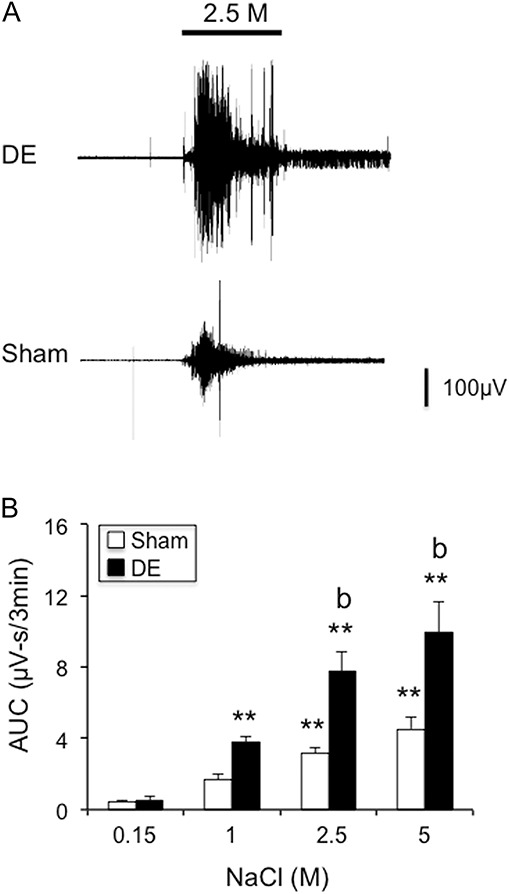
Hypertonic saline–evoked OOemg responses were increased in dry eye disease (DE) rats 14 days after surgery. (A) Examples of hypertonic saline–evoked OOemg activity in a DE rat (upper trace) and sham rat (lower trace). (B) Summary of integrated OOemg responses to hypertonic saline in sham and 14d DE rats. **P < 0.01 vs 0.15 M; b = P < 0.01 vs sham. Sample sizes: sham, n = 14; DE, n = 14.
3.3.2. OOemg activity—effect of synaptic blockade of Vi/Vc or Vc/C1 region
Microinjection of the nonselective synaptic blocking agent, CoCl2 (100 mM, 0.3 μL), into the Vi/Vc transition (Fig. 7A, upper trace) or the Vc/C1 region (Fig. 7A, lower trace) significantly reduced the OOemg response to repeated application of 2.5M NaCl to the ocular surface. As summarized in Figure 7B (left panel), blockade at the Vi/Vc transition caused a marked inhibition of the NaCl-evoked OOemg response at 10 minutes in both DE and sham rats with only minor recovery by 50 minutes compared with vehicle injection (F3,40 = 54.5, P < 0.001). CoCl2 blockade at the Vc/C1 region also reduced the NaCl-evoked OOemg responses compared with vehicle injections in DE and sham rats (Fig. 7B, right panel, F3,42 = 17.1, P < 0.001). However, in contrast to Vi/Vc injections, blockade at Vc/C1 caused only a transient decrease in evoked OOemg response in sham rats at 10 minutes (F3,42 = 6.4, P < 0.005) and a trend towards preinjection values by 50 minutes in DE rats (F3,42 = 27.1, P < 0.001).
Figure 7.
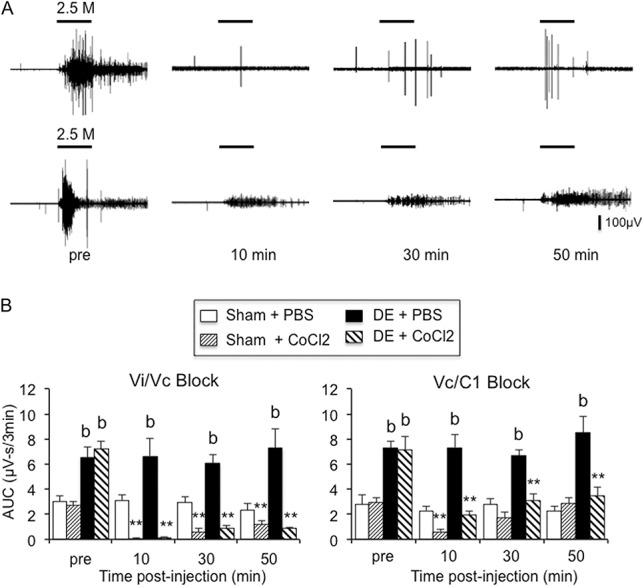
Inhibition of hypertonic saline–evoked OOemg responses after synaptic blockade at the Vi/Vc transition or the Vc/C1 region. (A) Examples of inhibition of hypertonic saline–evoked OOemg activity in dry eye disease (DE) rats after CoCl2 (0.3 μL, 100 mM) injection into the Vi/Vc transition (upper trace) or into the Vc/C1 region (lower trace). Scale bar = 30 seconds. (B) Summary of integrated OOemg responses to hypertonic saline in sham and 14d DE rats after synaptic blockade at the Vi/Vc transition (left panel) or at the Vc/C1 region (right panel). **P < 0.01 vs preinjection value; b = P < 0.01 vs sham. Sample sizes for Vi/Vc transition injections: sham-PBS, n = 5; sham-CoCl2, n = 4; DE-PBS, n = 4, DE-CoCl2, n = 5. Sample sizes for Vc/C1 region injections: sham-PBS, n = 4; sham-CoCl2, n = 5; DE-PBS, n = 4, DE-CoCl2, n = 5.
4. Discussion
This study found that exposure to reduced tear volume for 2 weeks increased the excitability of ocular-responsive neurons at multiple regions of the TBNC. Enhanced excitability was characterized by greater responses to hypertonic saline applied to the ocular surface and by enlarged convergent RFs from periorbital skin. A second key finding was a marked increase in saline-evoked OOemg activity in DE rats that was matched by a corresponding increase in eye blink behavior in awake animals. The enhanced OOemg activity depended on a relay in both the Vi/Vc transition and the Vc/C1 regions and provided further evidence of widespread effects of reduced tear volume on trigeminal brainstem circuits that mediate ocular nociception.
At 2 weeks after exorbital gland removal, there were no signs of ocular injury or inflammation such as fluorescein staining suggesting minimal damage to ocular surface in agreement with previous studies.24 Other studies in the rat have shown that only at 8 weeks after exorbital gland removal does fluorescein staining become prominent.15,26 Spontaneous tear volume was maintained at nearly 50% of normal; however, this was not sufficient to prevent sensitization of ocular-responsive trigeminal brainstem neurons or enhanced evoked OOemg activity.
Tear hyperosmolarity is a key factor in the development of DE and has the highest correlation with disease severity among the signs commonly used to diagnose tear film dysfunction.48–50 Although the value of resting tear osmolarity in the diagnosis of DE remains controversial,32,47 considerable evidence suggests that acute changes in tear hyperosmolarity are sufficient to evoke symptoms of irritation similar to those seen in DE.28 Although the estimated osmotic concentrations (295-9000 mOsm/kg) used here were higher than those measured from lower meniscus samples in DE patients (315-360 mOsm),5 other studies also have noted that much higher osmotic concentrations were required to activate nociceptive neurons and cause pain sensations. In the mouse-excised cornea preparation, polymodal nociceptive nerve terminals responded to osmotic stimuli only at or above 600 mOsm, whereas at 500 mOsm cold fiber terminal activity became unstable and often was lost.36 In healthy subjects, ocular application of saline solutions of nearly 900 mOsm was required to match the pain score reported during a staring tear breakup protocol in which the eye was held open.28 Also, tear osmotic concentrations in other rodent models of DE have been reported as high as 600 mOsm,46 suggesting that species differences cannot be excluded when comparing absolute tear osmotic concentrations in animals and humans. It is not known how many corneal afferent fibers must be excited to evoke a sensation from the eye; however, these studies would suggest that spatial summation of ocular surface afferent fibers is necessary to evoke pain sensation by hyperosmolar tears. We did not specifically test whether exorbital gland removal affected the osmotic concentration needed to evoke neural or behavioral responses; however, general anesthesia would be expected to increase the threshold for polysynaptic activation of trigeminal brainstem neurons55 and to increase the threshold required to evoke OOemg activity.
A significant finding in this study was that ocular-responsive neurons at multiple regions of the caudal TBNC displayed signs of sensitization. Sensitization was indicated by 2 distinct measures. First, hypertonic saline evoked dose-related increases in the responses of neurons at the Vi/Vc transition and in superficial and deep laminae at the Vc/C1 region in DE rats that were significantly greater than for neurons in sham animals. Sensitization of peripheral neurons cannot be excluded, because the spontaneous firing rate of Vi/Vc transition neurons was elevated in DE rats. However, in the mouse in vitro cornea preparation, acute exposure to inflammatory soup did not enhance polymodal nerve terminal responses to hyperosmotic stimuli.36 Contributions from cold fibers to evoked responses to hyperosmotic stimuli also cannot be excluded. Although cornea cold fibers respond to hyperosmotic saline solutions in naive rodents,18 their role in irritation and pain sensation in DE remains uncertain. Cornea application of menthol, a TRPM8 agonist, at concentrations sufficient to activate cold fibers36 had no effect on nocifensive behavior.42 Sensitization of ocular neurons at the Vi/Vc transition after persistent tear reduction differed significantly from previous studies in which endotoxin9 or UV irradiation–induced52 ocular inflammation did not enhance the responsiveness of Vi/Vc transition neurons to chemical or osmotic stimulation, whereas the responses of neurons in superficial laminae at the Vc/C1 were greatly enhanced. The reason for this difference was not certain; however, in this study, neural responses were tested 2 weeks after surgery, whereas only 1 week elapsed after endotoxin- or UV-induced ocular injury before testing. Alternatively, sustained reduction in tear volume may have preferentially altered the behavior of cool-sensitive afferent fibers, the class of fibers that is critical for spontaneous tear production.31,37 Cool-sensitive corneal afferents normally respond poorly to noxious heat; however, after exorbital gland removal, heat-sensitive cool fibers were encountered more often and their responses to noxious heat were greatly enhanced compared with controls.26 Neurons that encode coolness and moisture status of the ocular surface were found preferentially at the Vi/Vc transition.20,25
A second line of evidence in support of central sensitization after reduced tear volume was the finding of enlarged convergent cutaneous RF areas of WDR neurons at the Vi/Vc transition and Vc/C1 region. Although sensitization of primary sensory neurons is a common feature of inflammatory pain states,4,57 expansion of cutaneous RF areas of dorsal horn neurons after peripheral inflammation is most likely due to central mechanisms. Chronic inflammation did not affect the RF area of single dorsal root ganglion cells, whereas the RF areas of spinal dorsal horn neurons were markedly expanded.21 Injury or inflammation-induced expanded RF areas of dorsal horn neurons requires NMDA receptor activation,22,41 a receptor pathway thought to act mainly by postsynaptic mechanisms. Because the peripheral RF of corneal afferent fibers typically includes only a portion of the cornea, although some terminals may extend into the limbus and conjunctiva,7,27 it seems unlikely that a change in the RF area of corneal afferents could account for expanded RF areas of TBNC neurons in DE rats. Blepharospasm10 and chronic DE patients often display symptoms of increased sensitivity around the eyes44; however, the characteristics of periorbital skin sensitivity in DE patients have not yet been determined by quantitative methods.
Ocular neurons in both superficial and deep laminae at Vc/C1 displayed evidence of sensitization. Furthermore, when the average NaCl-evoked Rmag values were compared across all regions in DE rats, we found that neurons at the Vi/Vc transition and deep laminae at Vc/C1 had the greatest average response magnitude and each was significantly greater (P < 0.01) than that of neurons in superficial laminae at Vc/C1. The expansion of periorbital RFs by WDR neurons at each region was similar. The role of superficial and deep laminae neurons in spinal dorsal horn in nociceptive processing remains uncertain and likely depends on several factors such the modality of the applied stimulus and the efferent projection targets of second-order neurons.11,13,14,39 Corneal and conjunctival afferents projected to superficial laminae at Vc/C1 with sparser label seen in deep laminae of the rat.35 Thus, neurons in superficial and deep laminae at Vc/C1 as well as the Vi/Vc transition likely receive direct input from trigeminal ganglion neurons that supply the eye, and coupled with the response characteristics, suggested that multiple groups of second-order ocular neurons in the lower TBNC become sensitized after persistent reduction in tear volume. The basis for central sensitization during persistent inflammation could involve facilitatory input from supraspinal brain regions.43 Alternatively, the extensive longitudinal fiber system that connects the different subnuclei of the TBNC also could contribute.8 Previously, we reported that synaptic blockade at the Vc/C1 region inhibited cornea-evoked responses of Vi/Vc neurons by CO2 gas, whereas blockade at the Vi/Vc transition enhanced the responses of superficial laminae neurons at the Vc/C1 region.19 In this study, we found that synaptic blockade at the Vi/Vc transition or the Vc/C1 region significantly reduced the evoked OOemg response in DE and sham rats consistent with a distributed circuit for eye blink responses and agreed well with our previous results in naive rats.40 Collectively, these results suggest that persistent exposure to reduced tear volume sensitizes neurons at multiple regions of the TBNC. Central neuroplasticity may be a critical factor in DE and may help explain why efforts to manage symptoms by topical ocular treatments alone often prove inadequate.
Conflict of interest statement
The authors have no conflicts of interest to declare.
This study was supported by a grant from NIH (EY021447).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5:93–107. [DOI] [PubMed] [Google Scholar]
- [2].Alves M, Fonseca EC, Alves MF, Malki LT, Arruda GV, Reinach PS, Rocha EM. Dry eye disease treatment: a systematic review of published trials and a critical appraisal of therapeutic strategies. Ocul Surf 2013;11:181–92. [DOI] [PubMed] [Google Scholar]
- [3].Asbell PA, Spiegel S. Ophthalmologist perceptions regarding treatment of moderate-to-severe dry eye: results of a physician survey. Eye Contact Lens 2010;36:33–8. [DOI] [PubMed] [Google Scholar]
- [4].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009;139:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, Boboridis KG, Akova YA, Geerling G, Labetoulle M, Rolando M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf 2013;11:246–58. [DOI] [PubMed] [Google Scholar]
- [6].Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O'Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 2006;25:900–7. [DOI] [PubMed] [Google Scholar]
- [7].Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol 1981;321:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: peripheral and brainstem organization. In: Basbaum A, Bushnell MC, editors. Science of pain, Vol. 5 New York: Elsevier, 2009. p. 435–460. [Google Scholar]
- [9].Bereiter DA, Okamoto K, Tashiro A, Hirata H. Endotoxin-induced uveitis causes long-term changes in trigeminal subnucleus caudalis neurons. J Neurophysiol 2005;94:3815–25. [DOI] [PubMed] [Google Scholar]
- [10].Borsook D, Rosenthal P. Chronic (neuropathic) corneal pain and blepharospasm: five case reports. PAIN 2011;152:2427–31. [DOI] [PubMed] [Google Scholar]
- [11].Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron 2005;47:787–93. [DOI] [PubMed] [Google Scholar]
- [12].Calonge M, Enriquez-de-Salamanca A, Diebold Y, Gonzalez-Garcia MJ, Reinoso R, Herreras JM, Corell A. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm 2010;18:244–53. [DOI] [PubMed] [Google Scholar]
- [13].Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci 2003;26:1–30. [DOI] [PubMed] [Google Scholar]
- [14].Eckert WA, III, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. PAIN 2006;126:184–97. [DOI] [PubMed] [Google Scholar]
- [15].Fujihara T, Murakami T, Fujita H, Nakamura M, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci 2001;42:96–100. [PubMed] [Google Scholar]
- [16].Hallett M, Evinger C, Jankovic J, Stacy M. Update on blepharospasm: report from the BEBRF International Workshop. Neurology 2008;71:1275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirata H, Hu JW, Bereiter DA. Responses of medullary dorsal horn neurons to corneal stimulation by CO2 pulses in the rat. J Neurophysiol 1999;82:2092–107. [DOI] [PubMed] [Google Scholar]
- [18].Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci 2010;51:3969–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hirata H, Okamoto K, Bereiter DA. GABAA receptor activation modulates corneal unit activity in rostral and caudal portions of trigeminal subnucleus caudalis. J Neurophysiol 2003;90:2837–49. [DOI] [PubMed] [Google Scholar]
- [20].Hirata H, Okamoto K, Tashiro A, Bereiter DA. A novel class of neurons at the trigeminal subnucleus interpolaris/caudalis transition region monitors ocular surface fluid status and modulates tear production. J Neurosci 2004;24:4224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hylden JL, Nahin RL, Traub RJ, Dubner R. Expansion of receptive fields of spinal lamina I projection neurons in rats with unilateral adjuvant-induced inflammation: the contribution of dorsal horn mechanisms. PAIN 1989;37:229–43. [DOI] [PubMed] [Google Scholar]
- [22].Jinks SL, Carstens E. Spinal NMDA receptor involvement in expansion of dorsal horn neuronal receptive field area produced by intracutaneous histamine. J Neurophysiol 1998;79:1613–18. [DOI] [PubMed] [Google Scholar]
- [23].Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocul Surf 2009;7:199–211. [DOI] [PubMed] [Google Scholar]
- [24].Kaminer J, Powers AS, Horn KG, Hui C, Evinger C. Characterizing the spontaneous blink generator: an animal model. J Neurosci 2011;31:11256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kurose M, Meng ID. Corneal dry-responsive neurons in the spinal trigeminal nucleus respond to innocuous cooling in the rat. J Neurophysiol 2013;109:2517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kurose M, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol 2013;110:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lele PP, Weddell G. Sensory nerves of the cornea and cutaneous sensibility. Exp Neurol 1959;1:334–59. [DOI] [PubMed] [Google Scholar]
- [28].Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci 2009;50:3671–9. [DOI] [PubMed] [Google Scholar]
- [29].Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol 1987;261:450–9. [DOI] [PubMed] [Google Scholar]
- [30].Marfurt CF, Echtenkamp SF. Central projections and trigeminal ganglion location of corneal afferent neurons in the monkey, macaca fascicularis. J Comp Neurol 1988;272:370–82. [DOI] [PubMed] [Google Scholar]
- [31].Meng ID, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res 2013;117:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Messmer EM, Bulgen M, Kampik A. Hyperosmolarity of the tear film in dry eye syndrome. Dev Ophthalmol 2010;45:129–38. [DOI] [PubMed] [Google Scholar]
- [33].Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. PAIN 2010;149:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Okamoto K, Tashiro A, Thompson R, Nishida Y, Bereiter DA. Trigeminal interpolaris/caudalis transition neurons mediate reflex lacrimation evoked by bright light in the rat. Eur J Neurosci 2012;36:3492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Panneton WM, Hsu H, Gan Q. Distinct central representations for sensory fibers innervating either the conjunctiva or cornea of the rat. Exp Eye Res 2010;90:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. PAIN 2014;155:1481–91. [DOI] [PubMed] [Google Scholar]
- [37].Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 2010;16:1396–9. [DOI] [PubMed] [Google Scholar]
- [38].Pflugfelder SC. Tear dysfunction and the cornea: LXVIII Edward Jackson Memorial Lecture. Am J Ophthalmol 2011;152:900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Price DD, Greenspan JD, Dubner R. Neurons involved in the exteroceptive function of pain. PAIN 2003;106:215–19. [DOI] [PubMed] [Google Scholar]
- [40].Rahman M, Okamoto K, Thompson R, Bereiter DA. Trigeminal pathways for hypertonic saline- and light-evoked corneal reflexes. Neuroscience 2014;277:716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ren K, Hylden JLK, Williams GM, Ruda MA, Dubner R. The effects of a non-competitive NMDA receptor antagonist, MK-801, on behavioral hyperalgesia and dorsal horn neuronal activity in rats with unilateral inflammation. PAIN 1992;50:331–44. [DOI] [PubMed] [Google Scholar]
- [42].Robbins A, Kurose M, Winterson BJ, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci 2012;53:7034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci 2009;30:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf 2009;7:28–40. [DOI] [PubMed] [Google Scholar]
- [45].Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int Rev Immunol 2013;32:19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stewart P, Chen Z, Farley W, Olmos L, Pflugfelder SC. Effect of experimental dry eye on tear sodium concentration in the mouse. Eye Contact Lens 2005;31:175–8. [DOI] [PubMed] [Google Scholar]
- [47].Sullivan B. Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. Ocul Surf 2014;12:2–9. [DOI] [PubMed] [Google Scholar]
- [48].Sullivan BD, Crews LA, Messmer EM, Foulks GN, Nichols KK, Baenninger P, Geerling G, Figueiredo F, Lemp MA. Correlations between commonly used objective signs and symptoms for the diagnosis of dry eye disease: clinical implications. Acta Ophthalmol 2014;92:161–6. [DOI] [PubMed] [Google Scholar]
- [49].Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, Pepose JS, Kosheleff V, Porreco A, Lemp MA. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci 2010;51:6125–30. [DOI] [PubMed] [Google Scholar]
- [50].Suzuki M, Massingale ML, Ye F, Godbold J, Elfassy T, Vallabhajosyula M, Asbell PA. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci 2010;51:4557–61. [DOI] [PubMed] [Google Scholar]
- [51].Takemura M, Sugimoto T, Shigenaga Y. Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol 1991;111:324–31. [DOI] [PubMed] [Google Scholar]
- [52].Tashiro A, Okamoto K, Chang Z, Bereiter DA. Behavioral and neurophysiological correlates of nociception in an animal model of photokeratitis. Neuroscience 2010;169:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep 2013;1:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vehof J, Kozareva D, Hysi PG, Harris J, Nessa A, Williams FK, Bennett DL, McMahon SB, Fahy SJ, Direk K, Spector TD, Hammond CJ. Relationship between dry eye symptoms and pain sensitivity. JAMA Ophthalmol 2013;131:1304–08. [DOI] [PubMed] [Google Scholar]
- [55].Wakai A, Kohno T, Yamakura T, Okamoto M, Ataka T, Baba H. Action of isoflurane on the substantia gelatinosa neurons of the adult rat spinal cord. Anesthesiology 2005;102:379–86. [DOI] [PubMed] [Google Scholar]
- [56].Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. PAIN 2011;152:2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron 2007;55:353–64. [DOI] [PubMed] [Google Scholar]


