Abstract
Objective
To investigate whether preadmission 25-hydroxyvitamin D (25(OH)D) levels are associated with the risk of hospital-acquired Clostridium difficile infection (HACDI).
Materials and Methods
Our retrospective cohort study focused on 568 adult patients from 2 Boston teaching hospitals between August 1993 and November 2006. All patients had 25(OH)D levels measured before hospitalization and were at risk for HACDI (defined as the presence of C difficile toxin A or B in stool samples obtained >48 hours after hospitalization). We performed multivariable regression analyses to test the association of prehospital 25(OH)D levels with HACDI while adjusting for clinically relevant covariates.
Results
In these 568 patients, mean (SD) 25(OH)D level was 19 (12) ng/mL, and 11% of patients met criteria for incident HACDI. Following adjustment for age, sex, race (nonwhite vs white), patient type (medical vs surgical), and Deyo-Charlson index, patients with 25(OH)D levels <10 ng/mL had higher odds of HACDI (odds ratio [OR], 2.90; 95% confidence interval [CI], 1.01–8.34) compared with patients with 25(OH)D levels ≥30 ng/mL. When patients with HACDI were analyzed relative to a larger patient cohort without HACDI (n = 5047), those with 25(OH)D levels <10 ng/mL (OR, 4.96; 95% CI, 1.84–13.38) and 10–19.9 ng/mL (OR, 3.36; 95% CI, 1.28–8.85) had higher adjusted odds of HACDI compared with patients with 25(OH)D levels ≥30 ng/mL.
Conclusions
In our cohort of adult patients, vitamin D status before hospital admission was inversely associated with the risk of developing HACDI. These data support the need for randomized, controlled trials to test the role of vitamin D supplementation to prevent HACDI.
Keywords: vitamin D, 25-hydroxyvitamin D, Clostridium difficile, hospital-acquired infection, nosocomial infection
Introduction
In the United States, the incidence of hospital-acquired Clostridium difficile infections (HACDIs) has almost tripled over the past decade.1 Approximately 350,000 new cases of nosocomial C difficile infections are associated with roughly 15,000 potentially avoidable fatalities each year.1,2 Excess annual healthcare expenditures attributable to HACDIs range from $1.1 to $3.2 billion, and the average hospital length of stay is prolonged by 3–6 days among patients who develop C difficile infections during acute care hospitalizations.3-5 Despite the existence of national guidelines, the adoption of recognized preventative strategies has not resulted in the eradication of HACDIs.6,7
C difficile is typically an opportunistic pathogen, resulting in disease if the normal gastrointestinal (GI) flora is perturbed and when host immune responses are suboptimal.8-10 Risk factors for acquiring C difficile–associated infection include age, antibiotic exposure, and GI procedures.11 Recent evidence suggests that vitamin D is a key regulator of the immune system,12 and as such, it may play an important role in patient susceptibility to hospital-acquired infections,13 including HACDIs.14 Given that the prevalence of suboptimal levels of 25-hydroxyvitamin D (25(OH)D) has increased in the general population,15-19 we performed a 2-center retrospective cohort study of hospitalized adult patients among whom 25(OH)D was measured within 1 year of hospitalization. Our objective was to test the hypothesis that vitamin D status before hospital admission is associated with the risk of developing HACDIs.
Materials and Methods
Source Population
We abstracted laboratory and administrative data from the electronic medical records of individuals admitted to 2 teaching hospitals in Boston, Massachusetts: Brigham and Women’s Hospital (BWH), with 793 beds, and Massachusetts General Hospital (MGH), with 902 beds. These 2 hospitals provide primary and tertiary care to a diverse population within eastern Massachusetts and the surrounding region. BWH and MGH are both level 1 trauma centers, and both have 45,000–47,000 hospital admissions per year. BWH and MGH are members of Partners HealthCare, which is the largest healthcare provider in Massachusetts.
Data Sources
We obtained data on all patients admitted to BWH or MGH between August 1993 and November 2006 through the Research Patient Data Registry (RPDR). RPDR is a computerized registry, which serves as a central data warehouse for all inpatient and outpatient records at Partners HealthCare facilities (including BWH and MGH). The registry has been used for multiple clinical research studies.20-24 Institutional review board approval for the study was granted by the Partners Human Research Committee.
Study Population
We identified 5341 individual patient admissions (age ≥18 years) over the study period that were assigned a Diagnostic Related Group (DRG) and that had documented serum 25(OH) D measurements between 7 and 365 days before hospitalization. We then excluded 5 foreign patients without Social Security numbers since vital status in this study was determined by the Social Security Administration Death Master File, 44 patients who received high-dose vitamin D supplementation (ergocalciferol, 50,000 IU) between the 25(OH)D level draw and hospital admission, 153 patients who had evidence of prior C difficile infection, 92 patients who had C difficile toxin A or B detected within 48 hours of hospital admission, and 4479 patients who did not have stool sample testing for C difficile toxin >48 hours after hospital admission. We did not exclude patients with diarrhea at hospital presentation. The final study cohort was therefore composed of 568 patients.
Exposure of Interest and Comorbidities
The exposure of interest was preadmission serum 25(OH)D level obtained 7–365 days prior to the date of hospitalization. 25(OH)D levels were categorized a priori as <10 ng/mL, 10–19.9 ng/mL, 20–29.9 ng/mL, and ≥30 ng/mL (to convert from ng/mL to nmol/L, multiply by 2.496). All cut points were adapted from existing national clinical guidelines.25
We used the International Classification of Diseases, Ninth Revision coding algorithms, which are well studied and validated,26,27 to derive the Deyo-Charlson index to assess the burden of chronic illness in our study cohort.28 “Patient type” was defined as medical or surgical and incorporated the DRG method.29 Inpatient antibiotic use was determined by pharmacy records with exclusion of antibiotics given following HACDI testing. Prior use of vitamin D supplementation in the year prior to hospitalization was determined by outpatient pharmacy records for cholecalciferol, calcitriol, and ergocalciferol (but excluding ergocalciferol ≥50,000 units given following 25(OH)D draw). Critical care services were determined by the assignment of Current Procedural Terminology (CPT) code 99291 (critical care, first 30–74 minutes) during hospital admission. The use of CPT code 99291 in this manner has been previously validated in the RPDR database.24
Assessment of Mortality
Information on vital status for the study cohort was obtained from the Social Security Administration Death Master File, which has a reported sensitivity for mortality up to 92% and a specificity of 99.9%.30-33 Utilization of the Death Master File allows for long-term follow-up of patients following hospital discharge.
Serum 25(OH)D Assay
Serum 25(OH)D in all cohort subjects was determined by radioimmunoassay (RIA). Between 1993 and 2006, at both hospitals, RIA was performed using the 25-Hydroxyvitamin D 125I RIA kit (DiaSorin Corporation, Stillwater, MN).34
End Points
The primary end point was incident HACDI. Microbiology reports on stool samples for the study cohort were obtained from the computerized registry at the hospitals under study. All cohort patients had stool sample testing for C difficile toxin A and B by an enzyme-linked immunosorbent assay (ELISA). A positive toxin result was defined as the presence of toxin A or B in at least 1 stool sample. To be considered a HACDI, the first positive toxin result must have been on a stool sample obtained >48 hours after hospital admission in patients with no known history of C difficile infection. Thus, HACDI was defined as the presence of C difficile toxin A or B in a stool sample obtained >48 hours after hospital admission in patients without a record of previous C difficile toxin A or B positivity. The secondary outcome was an assessment of 30-day and 90-day mortality.
Power Calculations and Statistical Analysis
On the basis of our prior study of bloodstream infection susceptibility among hospitalized patients,35 we assumed HACDI incidence would decrease from 15% in patients with a prehospital 25(OH)D <20 ng/mL to 7% in those with a prehospital 25(OH)D ≥20 ng/mL. With an α error level of 5% and a power of 80%, the minimum sample size required for our primary end point (HACDI) was 530 total patients.
Categorical variables were described by frequency distribution and compared across 25(OH)D groups using contingency tables and χ2 testing. Continuous variables were examined graphically (eg, histogram, box plot) and in terms of summary statistics (ie, mean and standard deviation or median and interquartile range) and then compared across exposure groups using 1-way analysis of variance.
Unadjusted associations between 25(OH)D levels and HACDI were estimated by bivariable logistic regression models. Adjusted odds ratios (ORs) were estimated by multivariable logistic regression models with a priori inclusion of covariates thought to be linked with both 25(OH)D level and HACDI. In this manner, we sought to create a parsimonious model that did not unnecessarily adjust for variables that do not affect bias or the causal relation between exposure and outcome.36 For the primary model (HACDI), specification of each continuous covariate (as a linear vs categorical term) was adjudicated by the empiric association with the primary outcome using the Akaike information criterion; overall model fit was assessed using the Hosmer-Lemeshow test. Models for secondary analyses were similar to the primary model. Unadjusted event rates were calculated with the use of the Kaplan-Meier methods and compared with the use of the log-rank test. Locally weighted scatter plot smoothing (LOWESS) was used to graphically represent37,38 the relationship between prehospital 25(OH)D level and risk of HACDI. A secondary analysis was performed to investigate the 25(OH)D-HACDI association in the parent cohort of 5047 inpatients with serum 25(OH) D measured between 7 and 365 days before hospital admission (568 from the study cohort and the 4479 patients who did not have a C difficile toxin assay determined >48 hours after hospital admission). All P values are 2-tailed, with values <.05 considered statistically significant. All analyses were performed using Stata 12.0MP statistical software (StataCorp LP, College Station, TX).
Results
The mean (SD) age at hospital admission was 63 (18) years (Table 1). Most patients were female, were white, and had a medically related DRG. The mean (SD) 25(OH)D level was 19 (12) ng/mL. Approximately half (53%) of the 25(OH)D measurements occurred in the 3 months before hospital admission. Over the hospital stay, 11% of the cohort met criteria for HACDI (n = 64). There was no statistical difference between HACDI incidence and season of hospital admission (χ2(3, N = 568) = 3.45, P = .33) or year of hospital admission (χ2(3, N = 568) = 7.62, P = .054). Over the years of the study, there does not appear to be a significant difference in 25(OH)D serum levels (χ2(9, N = 568) = 16.18, P = .063), but there appears to be a pattern where fewer cases of 25(OH)D <10 ng/mL are found over time.
Table 1.
Baseline Demographic Characteristics of the Study Population.
| Characteristic | Total | HACDI | Non-HACDI |
|---|---|---|---|
| Total number of cases | 568 | 64 | 504 |
| Age, mean (SD), years | 63 (18) | 71 (15) | 62 (17) |
| Sex, No. (%) | |||
| Female | 353 (62) | 44 (69) | 309 (61) |
| Male | 215 (38) | 20 (31) | 195 (39) |
| Race, No. (%) | |||
| Nonwhite | 88 (15) | 9 (14) | 79 (16) |
| White | 480 (85) | 55 (86) | 425 (84) |
| Patient type, No. (%) | |||
| Medical | 401 (71) | 50 (78) | 351 (70) |
| Surgical | 167 (29) | 14 (22) | 153 (30) |
| Deyo-Charlson index, No. (%) | |||
| 0–3 | 147 (26) | 20 (31) | 127 (25) |
| 4–6 | 169 (30) | 19 (30) | 150 (30) |
| ≥7 | 252 (44) | 25 (39) | 227 (45) |
| Antibiotic use, No. (%) | 403 (71) | 45 (70) | 358 (71) |
| 25(OH)D, mean (SD), ng/mL |
19 (12) | 17 (10) | 19 (12) |
| 25(OH)D, mean (SD) by season, ng/mL | |||
| Fall (n = 151) | 18 (9) | 20 (9) | 18 (10) |
| Spring (n = 147) | 17 (10) | 16 (8) | 17 (10) |
| Summer (n = 132) | 21 (11) | 19 (10) | 21 (11) |
| Winter (n = 138) | 19 (9) | 15 (12) | 19 (15) |
| Vitamin D | 78 (14) | 6 (9) | 72 (14) |
| supplemental use, No. (%) |
|||
HACDI, hospital-acquired Clostridium difficile infection; 25(OH)D, 25-hydroxyvitamin D. Antibiotic use is exposure of antibiotics during the hospitalization prior to measurement of Clostridium difficile toxin A or B in stool samples. Vitamin D supplemental use is vitamin D supplementation in the year prior to hospitalization.
Patient characteristics were stratified according to pread- mission 25(OH)D levels (Table 2). Factors that significantly differed between stratified groups included sex and race. The most common admission diagnosis categories were ill-defined conditions (13%); circulatory system (13%); endocrine, nutrition, and metabolic (12%); digestive system (9%); genitourinary system (7%); neoplasms (6%); and respiratory system (6%). Age and 25(OH)D levels were significant predictors of HACDI (Table 3). Thirty-day and 90-day mortality rates were 10% and 17%, respectively.
Table 2.
Patient Characteristics by Prehospital Vitamin D Status.
| Prehospital 25(OH)D |
|||||
|---|---|---|---|---|---|
| Characteristic | <10 ng/mL | 10–19.9 ng/mL | 20–29.9 ng/mL | ≥30 ng/mL | P Value |
| Number of cases | 135 | 232 | 137 | 74 | |
| Age, mean (SD), y | 63 (17) | 61 (18) | 64 (17) | 65 (17) | .35a |
| Sex, No. (%) | <.0001 | ||||
| Female | 82 (61) | 118 (53) | 94 (69) | 59 (80) | |
| Male | 53 (39) | 104 (47) | 43 (31) | 15 (20) | |
| Race, No. (%) | .04 | ||||
| Nonwhite | 30 (22) | 31 (14) | 21 (15) | 6 (8) | |
| White | 105 (78) | 191 (86) | 116 (85) | 68 (92) | |
| Patient type, No. (%) | .94 | ||||
| Medical | 96 (71) | 157 (71) | 98 (72) | 50 (68) | |
| Surgical | 39 (29) | 65 (29) | 39 (28) | 24 (32) | |
| Deyo-Charlson index, No. (%) | .42 | ||||
| 0–3 | 27 (20) | 56 (25) | 39 (28) | 25 (34) | |
| 4–6 | 40 (30) | 68 (31) | 40 (29) | 21 (28) | |
| ≥7 | 68 (50) | 98 (44) | 58 (42) | 28 (49) | |
| Antibiotic use, No. (%) | 89 (66) | 166 (75) | 101 (73) | 47 (65) | .21 |
| Vitamin D supplemental use, No. (%) | 14 (10) | 35 (16) | 18 (13) | 11 (15) | .52 |
| White blood cells, × 103/μL, No. (%) | .81 | ||||
| 0–3.9 | 9 (7) | 10 (5) | 9 (6) | 4 (5) | |
| 4.0–9.9 | 50 (37) | 89 (40) | 61 (45) | 28 (38) | |
| >10 | 76 (56) | 123 (55) | 67 (49) | 42 (57) | |
| Red blood cell distribution width, mean (SD) |
16.7 (2.5) | 15.7 (2.4) | 15.2 (2.5) | 15.1 (2.1) | <.0001 |
| ICU admission, No. (%) | 24 (18) | 44 (20) | 34 (25) | 13 (18) | .45 |
ICU, intensive care unit; 25(OH)D, 25-hydroxyvitamin D. White blood cells and red cell distribution width measured at hospital admission. Antibiotic use is the exposure of antibiotics during the hospitalization prior to measurement of Clostridium difficile toxin A or B in stool samples. Vitamin D supplemental use is vitamin D supplementation in the year prior to hospitalization.
P value was determined by Kruskal-Wallis (all other P values were determined by χ2).
Table 3.
Multivariable-Adjusted Associations Between Covariates and Hospital-Acquired Clostridium difficile Infections.
| Characteristic | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age (per 1 year) | 1.04 | 1.02–1.06 | <.0001 |
| Sex | |||
| Female | 1.00 | Reference | .37 |
| Male | 0.76 | 0.42–1.38 | |
| Race | |||
| Nonwhite | 1.00 | Reference | .93 |
| White | 0.96 | 0.44–2.08 | |
| Patient type | |||
| Medical | 1.00 | Reference | .13 |
| Surgical | 0.61 | 0.32–1.15 | |
| Deyo-Charlson index | |||
| 0–3 | 1.00 | Reference | |
| 4–6 | 0.62 | 0.31–1.25 | .18 |
| ≥7 | 0.58 | 0.30–1.14 | .11 |
| Prehospital 25(OH)D, ng/mL | |||
| <10 | 2.90 | 1.01–8.34 | .048 |
| 10–19.9 | 2.14 | 0.76–5.98 | .15 |
| 20–29.9 | 1.70 | 0.58–5.03 | .34 |
| ≥30 | 1.00 | Reference | |
CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D. Adjusted odds ratios were estimated by a multivariable logistic regression model with inclusion of covariate terms thought to plausibly associate with vitamin D status and hospital-acquired Clostridium difficile infections. Estimates for each variable are adjusted for all other variables in the table.
Primary Outcome
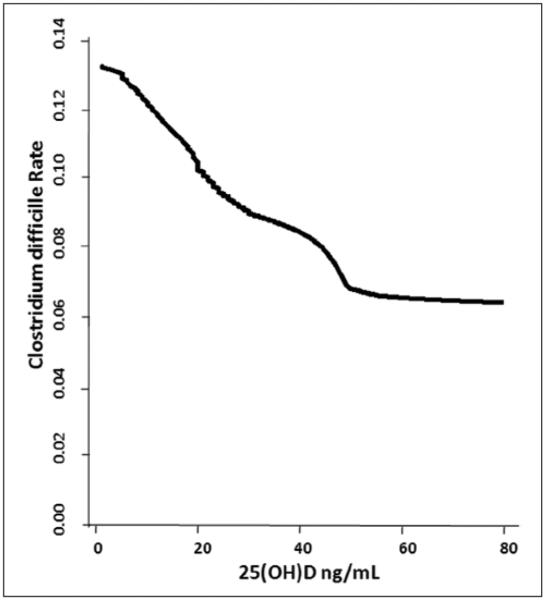
Preadmission 25(OH)D level <10 ng/mL was a strong predictor of HACDI after adjustment for age, sex, race, patient type, and Deyo-Charlson index (Table 4). The adjusted odds of HACDI in patients with 25(OH)D levels <10 ng/mL was 3-fold higher than that of patients with levels ≥30 ng/mL. Additional adjustment for white blood cell count did not materially alter the point estimates for HACDI (fully adjusted OR, 2.88; 95% confidence interval [CI], 1.01–8.30). LOWESS plot (Figure 1) demonstrated a near-inverse linear association between 25(OH)D level and risk of HACDI up to 25(OH)D levels near 30 ng/mL. Beyond serum 25(OH)D levels of 50 ng/mL, the curve appears flat.
Table 4.
Unadjusted and Adjusted Associations Between Prehospital Vitamin D Status and HACDI in Patients With Confirmed Toxin A or B in Stool Samples (n = 568).
| Prehospital 25(OH)D |
||||
|---|---|---|---|---|
| Association | <10 ng/mL | 10–19.9 ng/mL | 20–29.9 ng/mL | ≥30 ng/mL |
| Unadjusted | ||||
| OR (95% CI) | 2.40 (0.86–6.69) | 1.75 (0.66–4.75) | 1.57 (0.54–4.55) | 1.00 (Reference) |
| P value | .094 | .21 | .84 | |
| Adjusted | ||||
| OR (95% CI) | 2.90 (1.01–8.34) | 2.14 (0.76–5.98) | 1.70 (0.58–5.03) | 1.00 (Reference) |
| P value | .048 | .15 | .34 | |
CI, confidence interval; HACDI, hospital-acquired Clostridium difficile infection; OR, odds ratio; 25(OH)D, 25-hydroxyvitamin D. Unadjusted associations between 25(OH)D groups and HACDI were estimated by bivariable logistic regression models. Adjusted ORs were estimated by multivariable logistic regression models with inclusion of covariate terms thought to plausibly associate with both 25(OH)D concentrations and HACDI. Estimates adjusted for age, sex, race (nonwhite vs white), patient type (medical vs surgical), and Deyo-Charlson index.
Figure 1.
Vitamin D status vs risk of hospital-acquired Clostridium difficile infection. Locally weighted scatter plot smoothing was used to represent the near-inverse linear association between prehospital 25(OH)D level and risk of HACDI. Plot constructed with data from inpatients with prehospital vitamin D status and toxin A or B measured in stool samples (n = 568). HACDI, hospital-acquired C difficile infection; 25(OH)D, 25-hydroxyvitamin D.
Secondary Analyses
In the parent cohort of 5047 inpatients with serum 25(OH)D measured between 7 and 365 days before hospital admission (568 from the study cohort and the 4479 patients who did not have C difficile toxin ELISA determined >48 hours after hospital admission), the proportion of patients with C difficile toxin measured was highest in those with the lowest prehospital 25(OH)D level: 15%, 13%, 10%, and 7% in patients with levels <10 ng/mL, 10–19.9ng/mL, 20–29.9 ng/mL, and ≥30 ng/ mL, respectively (χ2(3, N = 5047) = 41, P < .001).
Under the assumption that vitamin D status was not a factor in the decision to order a stool C difficile toxin assay and that those who did not have ELISA testing were likely to be true negatives and not false negatives, we performed a further analysis on the 5047 inpatients with 25(OH)D levels determined prior to hospital admission to determine the association between vitamin D status and HACDI. In this subanalysis, pre- hospital 25(OH)D levels <20 ng/mL were associated with increased odds of HACDI (Table 5). Compared with the patients with 25(OH)D levels ≥30 ng/mL, multivariable adjusted odds of HACDI in those with levels <10 ng/mL and those with levels 10–19.9 ng/mL were 4.96 (95% CI, 1.84–13.38) and 3.36 (95% CI, 1.28–8.85), respectively. Furthermore, using data from this larger cohort (n = 5047), the HACDI rate in patients with prehospital 25(OH)D <10 ng/mL, 10–19.9 ng/ mL, 20–29.9 ng/mL, and ≥30 ng/mL was 27, 18, 18, and 8 per 10,000 patient-days, respectively. Finally, we examined the association of HACDI with all-cause mortality in this 5047 inpatient sample (Figure 2). When HACDI was considered as the exposure and all-cause mortality was the outcome, patients with HACDI had a 2-fold increased risk of 90-day mortality (OR, 2.02; 95% CI, 1.04–3.92) relative to those without HACDI after adjustment for age, sex, race, patient type, and Deyo-Charlson index.
Table 5.
Unadjusted and Adjusted Associations Between Prehospital Vitamin D Status and HACDI in Patients With Confirmed Toxin A or B in Stool Samples Relative to All Hospitalized Patients (n = 5047).
| Prehospital 25(OH)D |
||||
|---|---|---|---|---|
| Association | <10 ng/mL | 10–19.9 ng/mL | 20–29.9 ng/mL | ≥30 ng/mL |
| Unadjusted | ||||
| OR (95% CI) | 4.94 (1.85–13.22) | 3.11 (1.19–8.15) | 2.20 (0/79–6.12) | 1.00 (Reference) |
| P value | .001 | <.0001 | .13 | |
| Adjusted | ||||
| OR (95% CI) | 4.96 (1.84–13.38) | 3.36 (1.28–8.85) | 2.30 (0.82–6.42) | 1.00 (Reference) |
| P value | .002 | .01 | .11 | |
CI, confidence interval; HACDI, hospital-acquired Clostridium difficile infection; OR, odds ratio; 25(OH)D, 25-hydroxyvitamin D. Unadjusted associations between 25(OH)D groups and HACDI were estimated by bivariable logistic regression models. Adjusted odds ratios were estimated by multivariable logistic regression models with inclusion of covariate terms thought to plausibly associate with both 25(OH)D concentrations and HACDI. Estimates adjusted for age, sex, race (nonwhite vs white), patient type (medical vs surgical), and Deyo-Charlson index. Patients with HACDI were analyzed relative to all patients with either negative C difficile infections or those without stool samples analyzed for C difficile.
Figure 2.
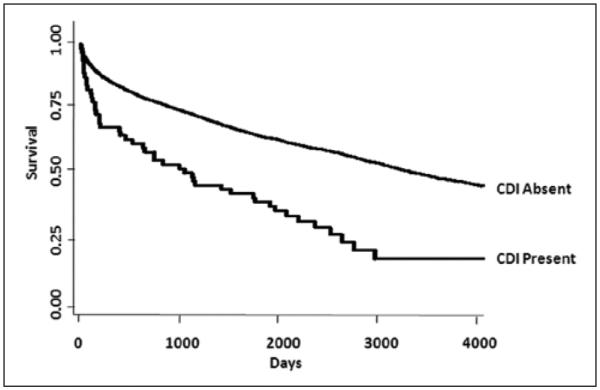
Time-to-event curves for the secondary end point (mortality). Unadjusted event rates were calculated with the use of the Kaplan-Meier methods and compared with the use of the log-rank test. The global comparison log rank P value is <.0001. Curve constructed with data from all inpatients with prehospital vitamin D status determined (n = 5047). CDI, Clostridium difficile infection.
Effect Modification
The fully adjusted model for the 25(OH)D-HACDI association was further evaluated for the presence of effect modification. We individually tested creatinine, hematocrit, white blood cell count, and season of 25(OH)D draw by adding an interaction term to the multivariable model. None of these variables emerged as an effect modifier of the association between 25(OH)D and HACDI (P interaction was >.20 for all variables tested). Further effect modification analyses showed that the association between 25(OH)D and HACDI was not modified by serum calcium >10.5 mg/dL (P interaction = .99). In addition, an analysis was performed to evaluate the data related to the 25(OH)D draw being ≥90 or <90 days before hospital admission as well as the hospital where care was received. The effect modification analysis showed that the association between vitamin D status and HACDI was neither modified by the time between 25(OH)D draw and hospital admission (P interaction = .65) nor the hospital of care (P interaction = .48).
Discussion
In this cohort study, we investigated whether prehospital vitamin D status was associated with the risk of HACDI. We demonstrated that 25(OH)D <10 ng/mL before hospital admission was indeed associated with a significant increase in the odds of developing HACDI. While others have also hypothesized that vitamin D status may play an important protective role against HACDIs,13,39 our work provides important evidence to suggest that vitamin D supplementation may provide a novel approach to lowering the risk of HACDI. However, due to the observational design of this study, causal inferences about the relationship between vitamin D status and HACDI are limited.
Clostridium difficile is a Gram-positive, anaerobic bacillus typically implicated in the progression to antibiotic-associated colitis.8 The pathogen is primarily transmitted from patient to patient through shared medical equipment or on the hands of healthcare workers.6 In the GI tract, C difficile causes disease by the production of toxins (toxins A and B), which induce characteristic inflammatory responses.9 Toxin A directly attracts neutrophils and monocytes, while toxin B is associated with a more generalized inflammatory response due its effect on colonic epithelial cell integrity.9 After alteration of the healthy colonic bacterial flora (which occurs with antibiotic usage), the immune response to C difficile toxin appears to play a major role in determining host susceptibility to C difficile infections.9,10
Recent studies have demonstrated that cells of the innate and adaptive immune system express the vitamin D receptor (VDR).12 Macrophages activated through the VDR by 1,25-dihydroxyvitamin D (the most hormonally active vitamin D metabolite) upregulate expression of cathelicidin (LL-37).40 LL-37 is an endogenous antimicrobial peptide that is active against a broad spectrum of infectious agents such as bacteria, viruses, fungi, and mycobacteria.41 Moreover, LL-37 is highly expressed at natural barrier sites (eg, skin, lungs, gut) by epithelial cells and may represent an important first line of defense for the innate immune system.42 In addition, vitamin D stimulates the expression of β-defensin in the intestinal epithelium, which has broad-spectrum antimicrobial activity.43 Since it is evident that derangements in immune function and disruption of natural barrier sites predispose patients to HACDI and that vitamin D status is essential for both optimal immune function and natural barrier integrity, our study findings raise intriguing questions that merit further investigation.
The association between prehospital vitamin D status and HACDI may also be explained by antibiotic usage for infection. We have demonstrated in prior studies that patients with hypovitaminosis D prior to hospital admission may be at higher risk for bloodstream infection35,44 and sepsis.45 Since antimicrobial therapy with quinolones, cephalosporins, macrolides, clindamycin, and intravenous β-lactam/β-lactamase inhibitors have been shown to be associated with C difficile–associated diarrhea,46 it is likely that antibiotic usage is a contributor to the prehospital vitamin D status–HACDI association.
Although cohort studies provide a high level of observational evidence and may direct future research by illustrating the existence or absence of a true effect,47 they also have several potential limitations, such as confounding, reverse causation, and/or the lack of a randomly distributed exposure. Ascertainment bias may exist in our study since our patient cohort had both prehospital vitamin D status determined and toxin A or B measured in stool samples, both related to unknown reasons that may be absent in other hospitalized patients. Furthermore, the cohort under study had a particularly high 30-day mortality rate. These differences may decrease the generalizability of our results to all hospitalized patients. Despite adjustment for multiple potential covariates, there may still be residual confounding that contributed to the observed differences in outcomes. Specifically, low 25(OH)D levels may be a marker for the general condition of patients, for which we are unable to fully adjust. As patients received care from physicians outside of our health system, we are unable to determine with confidence the number of patients who were actively taking vitamin D supplements.
Another potential limitation is related to the fact that we used 25(OH)D measurements between 7 and 365 days prior to hospitalization as a reflection of preadmission vitamin D status. Others have shown that the intraperson Pearson correlation coefficient for 25(OH)D in outpatients following adjustments for age, race, and season is 0.70 at 3 years between blood draws.48 There was no interaction of the 25(OH)D-HACDI association with regard to when 25(OH)D was obtained. Despite this observation, vitamin D status at the time of hospitalization may have been different than when prehospital values were drawn. Indeed, we know that inflammatory changes and IV fluid administration contribute significantly to the rapid drop (30%−40%) in circulating 25(OH)D levels during acute stress.49 The assumption used in the study power estimate may not be valid since there are no prior studies available on incident HACDI and vitamin D status. Furthermore, the somewhat wide confidence intervals seen in our data (Tables 4 and 5) are likely due to relatively low sample numbers in the 25(OH)D groups and reflect lower precision and more statistical instability than in a study of larger size. These issues will need to be addressed by future studies as we and other groups try to replicate and extend the current finding.
In large published studies from Quebec, Canada, the C difficile infection incidence/1000 hospital admissions was 3–12 cases/1000 hospital admissions in 1991–2002 and 25–43 cases/1000 admissions in 2003–2004.50 The incidence in our study sample (n = 568) was 113/1000 hospital admissions, and in the parent cohort of 5047 inpatients, the incidence of C difficile infection was 13/1000 hospital admissions. We are likely missing true-positive cases in the population of patients who did not have 25(OH)D measured.
The present study has several strengths. We have sufficient statistical power to detect a clinically relevant difference in HACDI. The Deyo-Charlson index accounts for chronic conditions that may alter immune function (including diabetes mellitus and chronic renal failure).51 By our design of measuring 25(OH)D prior to hospitalization, we have attempted to uncouple the influence of illness and inflammation on vitamin D status. For example, since inflammation is associated with decreased vitamin D binding protein,52 we used 25(OH)D levels that were measured prior to hospitalization and thus conceivably prior to the inflammation that may have altered 25(OH)D levels. We did not include 25(OH)D levels drawn in the 7 days prior to hospitalization to avoid any potential alterations of vitamin D status related to illness or inflammation.
Conclusion
Our results suggest that vitamin D status may be a modifiable risk for HACDI. We hypothesize that 25(OH)D levels ≥30 ng/ mL are associated with optimal expression of endogenous antimicrobial peptides. In turn, this may attenuate the effect of barrier site disruptions that are characteristic of HACDI. This is particularly important since the incidence of high virulence and antibiotic-resistant C difficile strains is increasing at an alarming rate.53,54 As a consequence, the overall severity of infections, cases of recurrence, costs, and associated mortality are expected to continue to rise.55,56 Prospective studies are needed to validate our findings, to assess the potential benefit of optimizing preadmission 25(OH)D levels, and to identify the mechanism by which vitamin D may confer protection against nosocomial infections such as C difficile infections.
Clinical Relevancy Statement.
Hospital-acquired Clostridium difficile infections are difficult to treat, require prolonged therapy, and may result in devastating consequences. The results of the current study provide novel insights on the potential immunomodulatory role of vitamin D in patients who develop nosocomial infections.
Acknowledgment
This manuscript is dedicated to the memory of our dear friend and colleague, Nathan Edward Hellman, MD, PhD.
Financial disclosure: This study was support by 5T32GM007592-33 and UL1 RR025758 (S.A.Q.), R01 AI093723 and U01 AI087881 (C.A.C.), and K08AI060881 (K.B.C.) from the National Institutes of Health.
References
- 1.Dubberke E. Clostridium difficile infection: the scope of the problem. J Hosp Med. 2012;7(suppl 3):S1–S4. doi: 10.1002/jhm.1916. [DOI] [PubMed] [Google Scholar]
- 2.Vital signs: preventing Clostridium difficile infections. MMWR Morb Mortal Wkly Rep. 2012;61(9):157–162. [PubMed] [Google Scholar]
- 3.Dubberke ER, Reske KA, Olsen MA, McDonald LC. Fraser VJ. Short- and long-term attributable costs of Clostridium difficile–associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46(4):497–504. doi: 10.1086/526530. [DOI] [PubMed] [Google Scholar]
- 4.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34(3):346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile–associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28(11):1219–1227. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 7.Butler M, Bliss D, Drekonja D, et al. Effectiveness of early diagnosis, prevention, and treatment of Clostridium difficile infection. Agency for Healthcare Research and Quality (US); Rockville, MD: 2011. [Epub ahead of print] [PubMed] [Google Scholar]
- 8.Bartlett JG. Narrative review: the new epidemic of Clostridium difficile– associated enteric disease. Ann Intern Med. 2006;145(10):758–764. doi: 10.7326/0003-4819-145-10-200611210-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sunenshine RH, McDonald LC. Clostridium difficile–associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73(2):187–197. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 10.Kelly CP. A 76-year-old man with recurrent Clostridium difficile– associated diarrhea: review of C. difficile infection. JAMA. 2009;301(9):954–962. doi: 10.1001/jama.2009.171. [DOI] [PubMed] [Google Scholar]
- 11.Sehulster L, Chinn RY. Guidelines for environmental infection control in health-care facilities: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 2003;52(RR-10):1–42. [PubMed] [Google Scholar]
- 12.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Youssef DA, Ranasinghe T, Grant WB, Peiris AN. Vitamin D’s potential to reduce the risk of hospital-acquired infections. Dermatoendocrinology. 2012;4(2):167–175. doi: 10.4161/derm.20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef D, Grant WB, Peiris AN. Vitamin D deficiency: a potential risk factor for Clostridium difficile infection. Risk Manag Healthc Policy. 2012;5:115–116. doi: 10.2147/RMHP.S36781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuriacose R, Olive KE. Prevalence of vitamin D deficiency and insufficiency in northeast Tennessee. South Med J. 2008;101(9):906–909. doi: 10.1097/SMJ.0b013e318181881b. [DOI] [PubMed] [Google Scholar]
- 16.Pellicane AJ, Wysocki NM, Schnitzer TJ. Prevalence of 25-hydroxyvitamin D deficiency in the outpatient rehabilitation population. Am J Phys Med Rehabil. 2010;89(11):899–904. doi: 10.1097/PHM.0b013e3181f71112. [DOI] [PubMed] [Google Scholar]
- 17.Fish E, Beverstein G, Olson D, Reinhardt S, Garren M, Gould J. Vitamin D status of morbidly obese bariatric surgery patients. J Surg Res. 2010;164(2):198–202. doi: 10.1016/j.jss.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300–2304. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun A, Chang D, Mahadevappa K, et al. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit Care Med. 2011;39(4):671–677. doi: 10.1097/CCM.0b013e318206ccdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hivert MF, Grant RW, Shrader P, Meigs JB. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv Res. 2009;9:170. doi: 10.1186/1472-6963-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hug BL, Lipsitz SR, Seger DL, Karson AS, Wright SC, Bates DW. Mortality and drug exposure in a 5-year cohort of patients with chronic liver disease. Swiss Med Wkly. 2009;139(51-52):737–746. doi: 10.4414/smw.2009.12686. [DOI] [PubMed] [Google Scholar]
- 23.Linder JA, Bates DW, Williams DH, Connolly MA, Middleton B. Acute infections in primary care: accuracy of electronic diagnoses and electronic antibiotic prescribing. J Am Med Inform Assoc. 2006;13(1):61–66. doi: 10.1197/jamia.M1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zager S, Mendu ML, Chang D, et al. Neighborhood poverty rate and mortality in patients receiving critical care in the academic medical center setting. Chest. 2011;139(6):1368–1379. doi: 10.1378/chest.10-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Rapoport J, Gehlbach S, Lemeshow S, Teres D. Resource utilization among intensive care patients: managed care vs traditional insurance. Arch Intern Med. 1992;152(11):2207–2212. [PubMed] [Google Scholar]
- 30.Cowper DC, Kubal JD, Maynard C, Hynes DM. A primer and comparative review of major US mortality databases. Ann Epidemiol. 2002;12(7):462468. doi: 10.1016/s1047-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 31.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schisterman EF, Whitcomb BW. Use of the Social Security Administration Death Master File for ascertainment of mortality status. Popul Health Metr. 2004;2(1):2. doi: 10.1186/1478-7954-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman TB, Brown AN. Use of commercial record linkage software and vital statistics to identify patient deaths. J Am Med Inform Assoc. 1997;4(3):233–237. doi: 10.1136/jamia.1997.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clin Chem. 1993;39(3):529–533. [PubMed] [Google Scholar]
- 35.Lange N, Litonjua A, Gibbons F, Giovannucci E, Christopher K. Prehospital vitamin D concentration, mortality and bloodstream infection in a hospitalized patient population. Am J Med. 2013;126(7):640.e19–27. doi: 10.1016/j.amjmed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 38.Cleveland W, Devlin S. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83(403):596–610. [Google Scholar]
- 39.Youssef D, Bailey B, El Abbassi A, et al. Healthcare costs of Staphylococcus aureus and Clostridium difficile infections in veterans: role of vitamin D deficiency. Epidemiol Infect. 2010;138(9):1322–1327. doi: 10.1017/S0950268809991543. [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro da Silva F, Machado MC. Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides. 2012;36(2):308–314. doi: 10.1016/j.peptides.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 42.Tollin M, Bergman P, Svenberg T, Jornvall H, Gudmundsson GH, Agerberth B. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003;24(4):523–530. doi: 10.1016/s0196-9781(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 43.Vora P, Youdim A, Thomas LS, et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173(9):5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 44.Quraishi SA, Litonjua AA, Moromizato T, et al. Association between prehospital vitamin D status and hospital-acquired bloodstream infections. Am J Clin Nutr. 2013;98(4):952–959. doi: 10.3945/ajcn.113.058909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moromizato T, Litonjua AA, Braun AB, Gibbons FK, Giovannucci E, Christopher KB. Association of low serum 25-hydroxyvitamin D levels and sepsis in the critically ill [published online August 26, 2013] Crit Care Med. doi: 10.1097/CCM.0b013e31829eb7af. [DOI] [PubMed] [Google Scholar]
- 46.Pepin J, Saheb N, Coulombe MA, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile–associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005;41(9):1254–1260. doi: 10.1086/496986. [DOI] [PubMed] [Google Scholar]
- 47.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342(25):1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15(3):255–265. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 49.Quraishi SA, Camargo CA., Jr Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15(6):625–634. doi: 10.1097/MCO.0b013e328358fc2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pepin J, Valiquette L, Alary ME, et al. Clostridium difficile–associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171(5):466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile– associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 54.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med. 2008;359(18):1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 55.Emerson CB, Eyzaguirre LM, Albrecht JS, Comer AC, Harris AD, Furuno JP. Healthcare-associated infection and hospital readmission. Infect Control Hosp Epidemiol. 2012;33(6):539–544. doi: 10.1086/665725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J Hosp Infect. 2012;81(1):1–14. doi: 10.1016/j.jhin.2012.02.004. [DOI] [PubMed] [Google Scholar]