Abstract
Background
Malnutrition and underfeeding are major challenges in caring for critically ill patients. Our goal was to characterize interruptions in enteral nutrition (EN) delivery and their impact on caloric debt in the surgical intensive care unit (ICU).
Materials and Methods
We performed a prospective, observational study of adults admitted to surgical ICUs at a Boston teaching hospital (March–December 2012). We categorized EN interruptions as “unavoidable” vs “avoidable” and compared caloric deficit between patients with ≥1 EN interruption (group 1) vs those without interruptions (group 2). Multivariable logistic regression was used to investigate the association of EN interruption with the risk of underfeeding. Poisson regression was used to investigate the association of EN interruption with length of stay (LOS) and mortality.
Results
Ninety-four patients comprised the analytic cohort. Twenty-six percent of interruptions were deemed “avoidable.” Group 1 (n = 64) had a significantly higher mean daily and cumulative caloric deficit vs group 2 (n = 30). Patients in group 1 were at a 3-fold increased risk of being underfed (adjusted odds ratio, 2.89; 95% confidence interval [CI], 1.03–8.11), had a 30% higher risk of prolonged ICU LOS (adjusted incident risk ratio [IRR], 1.27; 95% CI, 1.14–1.42), and had a 50% higher risk of prolonged hospital LOS (adjusted IRR, 1.53; 95% CI, 1.41–1.67) vs group 2.
Conclusions
In our cohort of critically ill surgical patients, EN interruption was frequent, largely “unavoidable,” and associated with undesirable outcomes. Future efforts to optimize nutrition in the surgical ICU may benefit from considering strategies that maximize nutrient delivery before and after clinically appropriate EN interruptions.
Keywords: malnutrition, caloric debt, ICU nutrition, critical illness
The prevalence of baseline malnutrition has been reported to be as high as 30%–50% in hospitalized patients.1-3 Moreover, hospital admission itself is a risk factor for further deterioration in nutrition status.4 Critical illness, infections, surgical stress, and inadequate delivery of nutrition only exacerbate this problem.1 Indeed, underfeeding remains a major challenge in the intensive care unit (ICU), where 30%–50% of patients do not meet their daily protein and energy requirements.5,6 The detrimental effects of malnutrition are most pronounced in the patients who are most critically ill.7
Both baseline malnutrition and worsening nutrition status during hospitalization (regardless of admission nutrition status) are associated with a higher likelihood of complications and significantly higher costs to the healthcare system.2,8,9 Failure to thrive/malnutrition is the third most common reason for 30-day hospital readmission in surgical patients.10 Furthermore, early and sufficient delivery of proteins as well as calories in ICU patients has been shown to influence clinically relevant outcomes such as ventilator-free days (VFDs), ICU and hospital lengths of stay (LOS), wound healing, incidence of nosocomial infections, and mortality.11-14 Yet, worldwide, critically ill patients receive only about 50% of prescribed enteral nutrition (EN) in the first 2 weeks after ICU admission,12,15-17 with considerable geographic variation, marked differences in practice between types of ICUs (medical vs surgical), and considerable dissimilarity among healthcare providers.18
Previous studies have described inadequate nutrient delivery in medical ICU patients19 and have classified over 65% of the reasons for stopping EN infusions as “avoidable” events. Although consultation with a registered dietitian and establishing an evidence-based EN protocol increase the percentage of prescribed calories actually delivered in clinical practice,20-22 an assessment of root causes is fundamental to understanding why ICU patients are underfed.18 And while surgical patients are clearly underfed to a greater degree than their medical counterparts,17,18 the root cause(s) of why such discrepancies exist remains largely unexplored. As such, the primary goal of our study was to perform a prospective qualitative study to characterize interruptions in EN delivery in the surgical ICU. Our secondary goal was to quantitatively assess the impact of EN interruptions on caloric deficit in critically ill surgical patients. We hypothesized that within the context of an aggressive EN protocol, interruptions in EN are largely unavoidable and that patients with interrupted nutrition would accumulate large caloric deficits.
Materials and Methods
Patients and Settings
We performed a prospective, observational cohort study involving patients from 2 surgical ICUs in a large teaching hospital in Boston, Massachusetts. Participants were recruited between March 2012 and December 2012. During the study period, the surgical ICU admitted patients from the trauma and emergency surgery service, as well as a wide variety of other surgical teams, including transplant, vascular, urology, orthopedics, colorectal, and surgical oncology. In addition, postoperative patients with medical (not directly related to surgery) indications such as sepsis or rapid atrial fibrillation were admitted. All patients, 18 years and older, who received EN for more than 72 hours were eligible for inclusion (all patients started on EN were initially considered for study inclusion, but only those who continued past 72 hours were retained in the final analytic cohort). We excluded patients who (1) had an ICU stay <72 hours, (2) had a previous ICU stay within the same hospitalization, (3) received EN prior to ICU admission, and (4) had an admission diagnosis of intestinal obstruction (mechanical or paralytic ileus). Due to the observational study design, our local institutional review board determined that a waiver of consent was acceptable.
Clinical Management
EN was administered via a nasogastric, nasoenteric, or gastrostomy tube. As per our local standard of care, gastric feedings were first initiated and postpyloric feeding was attempted only if delayed gastric emptying became evident. Our standard protocol also couples a mandatory ICU registered dietitian consultation at initiation of EN; given staffing issues, these consultations are typically completed with 24 hours and a formal reassessment is performed every 2–3 days while patients are still in the ICU. When no absolute contraindications are present, EN is initiated within 48 hours of admission to the surgical ICU. EN is typically started at a rate of 10 mL/h and advanced by 10 mL/h every 2 hours until the target infusion rate is achieved. The EN infusion goal rate is based on the formal consult by an ICU registered dietitian and is based on the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient.8
Tolerance to enteral feeding was evaluated by recording the gastric residual volume (GRV). Every 4 hours, GRV was measured and clinical decisions were based on the following protocol:
GRV <250 mL: (a) reinfuse residuals and (b) continue EN per protocol.
GRV 250–500 mL: (a) reinfuse residuals, (b) continue EN per protocol, and (c) initiate a promotility agent (metoclopramide and/or erythromycin).
GRV >500 mL: (a) reinfuse residuals, (b) hold EN, (c) initiate promotility agents (metoclopramide and erythromycin), (d) recheck residuals every 4 hours, and (e) resume EN at previous rate if subsequent GRV <500 mL.
The standard operating procedures in our ICUs dictate that in patients undergoing a surgical or radiological intervention after surgical ICU admission, periprocedural EN is allowed if the following criteria are satisfied: (1) a controlled airway (endotracheal or cuffed tracheostomy tube) is in place, (2) a supine operative position will be maintained, and (3) interventions do not involve the airway or gastrointestinal (GI) tract.
Definitions
Transient EN interruptions for ICU-related activities (eg, bed to chair transfer using a patient lift), which lasted 10 minutes or less, were not recorded. For all other EN interruption episodes, the reason for interruption was recorded as (1) (re)intubation/ extubation, (2) bedside tracheostomy/percutaneous endoscopic gastrostomy (PEG) tube placement, (3) GI surgery, (4) orthopedic surgery, (5) other surgical procedures, (6) interventional radiology (IR) procedure, (7) body imaging study, and/or (8) high GRV. Interruptions for reasons of (re)intubation/extubation, tracheostomy/PEG tube placement, GI surgery, and GRV >500 mL were considered unavoidable. For imaging studies where the radiologist specifically requested fasting for optimal visualization (eg, abdominal ultrasounds), interruptions were also considered unavoidable. For IR procedures, orthopedic surgery, and other surgical procedures, the interruption was considered unavoidable if the patient did not have a controlled airway or was not in the supine position for the procedure.
If EN was interrupted for an operation or IR procedure that met all 3 periprocedural nutrition criteria listed above (eg, orthopedic extremity fixation, soft tissue dressing change, etc), then that episode was considered avoidable. Likewise, if EN was interrupted for an imaging study in which the radiologist did not specifically request fasting, then that episode was considered avoidable. Finally, if EN was interrupted for a GRV <500 mL, that interruption was considered avoidable.
Clinical Data Collection
Additional data collected for analysis included (1) ICU admission diagnosis, (2) age, (3) sex, (3) body mass index (BMI), (4) admission serum albumin level, (5) variables to calculate the Acute Physiology and Chronic Health Evaluation (APACHE II) score and the Deyo-Charlson Comorbidity Index (DCCI), (6) incidence of acute ICU complications (ear infection, myocardial infarction, cerebrovascular accident, and renal replacement therapy), (7) ICU LOS, (8) hospital LOS, (9) 30-day VFD, (10) in-hospital mortality, and (11) 30-day mortality. Data on protein and energy intake from enteral and parenteral feedings were recorded daily throughout the surgical ICU admission for a maximum of 14 days, until initiation of oral intake, discharge from the ICU, or death (whichever occurred first). If parenteral nutrition (PN) was given simultaneously with EN, the exact number of parenteral calories received was calculated and included in the daily nutrition assessments. Calories contained in propofol infusions and in protein supplements were included when calculating the daily EN prescription. Resting energy expenditure, nitrogen balance, prealbumin, and C-reactive protein levels were obtained only in a selected group of patients requiring long-term critical care services and were not routinely measured in the first 2 weeks after ICU admission.
Outcomes
Our 2 primary outcomes were the percentage of EN interruption episodes that were considered unavoidable and the cumulative caloric deficit. Secondary outcomes of interest included 30-day mortality, surgical ICU (SICU) LOS, hospital LOS, VFDs, and total complications per patient.
Statistical Analysis
Normally distributed descriptive data were reported as means ± standard deviations (SD), while nonparametric data were reported as medians and interquartile ranges (IQRs) or as frequencies (%), when appropriate. Outcomes between those with at least 1 interrupted EN episode and those without any EN interruption episodes were compared using 2-sample t tests, Wilcoxon rank sum tests, or Fisher exact tests, respectively. Based on published studies and our own preliminary data, we assumed that 60% of patients with no EN interruptions achieved their prescribed protein and caloric needs. We also assumed that in patients with 1 or more EN interruptions, only 20% achieved their prescribed protein and caloric needs. To detect this difference with a power of 0.9 and assuming an α of 0.05 would require a minimum of 30 patients in each group. In addition, we calculated risk ratios for underfeeding, ICU LOS, hospital LOS, 30-day VFD, in-hospital mortality, and 30-day mortality in patients with 1 or more EN interruptions vs those with no interruptions. Underfeeding (defined as <66% of total prescribed calories during an ICU stay),23,24 mortality, and EN interruption were considered binary variables. ICU LOS, hospital LOS, and 30-day VFD were considered continuous, nonparametric variables. To test the association of EN interruptions with underfeeding, we developed a logistic regression model while controlling for biologically plausible covariates, including (1) age, (2) sex, (3) BMI, (4) APACHE II score, (5) type of surgical patient (trauma, orthopedics, vascular, etc), and (6) ICU complications. Similar models were constructed to test the association of EN interruptions with in-hospital as well as 30-day mortality. To test the association of EN interruptions with ICU LOS, we developed a zero-truncated Poisson regression model while controlling for biologically plausible covariates, including (1) age, (2) sex, (3) BMI, (4) APACHE II score, (5) type of surgical patient, and (6) ICU complications. Similar models were constructed to test the association of EN interruptions with hospital LOS as well as 30-day VFD. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). Two-sided P < .05 and adjusted odds ratios (ORs) and incident risk ratios (IRRs) with 95% confidence intervals (CIs) not including 1 were considered statistically significant.
Results
A total of 94 SICU patients who met eligibility criteria were included in the cohort. The mean age was 63 ± 17 years, and most (71%) patients were male. Baseline characteristics of the study cohort are shown in Table 1. When comparing patients who had 1 or more EN interruptions (group 1, n = 64) with those who had no interruptions (group 2, n = 30), there was no significant difference in age, admission serum albumin level, APACHE II and DCCI scores, total number of days receiving EN, total complications, ICU LOS, VFD, the use of PN, initiation of EN within 48 hours of ICU admission, and mortality. Patients in group 1 had a higher mean daily caloric deficit (608 ± 473 kcal vs 346 ± 276 kcal, P = .001), a greater mean cumulative caloric deficit (5834 ± 4641 kcal vs 3066 ± 3223 kcal, P = .001), but an equivalent median hospital LOS (33 [20–44] days vs 25 [18–38] days, P = .065). Sixty-four patients had a total of 106 episodes of EN interruption. The reasons for EN interruption are shown in Table 2, with the 3 most common reasons being (1) (re)intubation/extubation, (2) tracheostomy/PEG tube placement, and (3) the need for an imaging study. When the EN interruption episodes were analyzed in greater detail and the definitions of “avoidable” vs “unavoidable” were applied, approximately 26% of all episodes were considered avoidable events.
Table 1.
Demographics, Nutrition Delivery, and Outcomes.
| Characteristic | Group 1 (1 or More EN Interruptions) | Group 2 (No EN Interruptions) | P Value |
|---|---|---|---|
| Age, y | 61.3 ± 17.5 | 65.4 ± 16.0 | .27 |
| Male sex, % | 73 | 70 | .81 |
| Serum albumin | 3.0 ± 0.5 | 2.9 ± 0.4 | .30 |
| APACHE II | 14.6 ± 6.5 | 12.8 ± 5.8 | .20 |
| DCCI | 2.3 ± 2.1 | 2.1 ± 2.0 | .66 |
| BMI | 26.9 ± 6.3 | 25.8 ± 6.1 | .43 |
| EN started within 48 h of ICU admission, % | 67 | 67 | .89 |
| Days receiving EN | 10.2 ± 4.2 | 8.7 ± 4.2 | .14 |
| PN, % | 6.3 | 16.7 | .14 |
| Calories prescribed, kcal/d | 1755 ± 422 | 1607 ± 285 | .049 |
| Proteins prescribed, g/d | 88 ± 21 | 85 ± 19 | .46 |
| Calories received, kcal/d | 1148 ± 418 | 1261 ± 374 | .19 |
| Proteins received, g/d | 54 ± 23 | 56 ± 21 | .68 |
| Daily caloric deficit, kcal/d | 608 ± 473 | 346 ± 276 | .001 |
| Daily protein deficit, g/d | 33 ± 23 | 28 ± 19 | .26 |
| Cumulative caloric deficit, kcal | 5834 ± 4641 | 3066 ± 3223 | .001 |
| Cumulative protein deficit, g | 326 ± 248 | 238 ± 187 | .061 |
| % Calories received | 63 | 76 | .002 |
| % Proteins received | 63 | 68 | .25 |
| VFD, median (IQR) | 18.5 (12–23) | 22.5 (13–26) | .08 |
| SICU LOS, median (IQR), d | 15 (10–28) | 13.5 (8–23) | .24 |
| Hospital LOS, median (IQR), d | 33 (20–44) | 25 (18–38) | .065 |
| Total complications per patient, median (IQR) | 2 (1–3) | 2 (1–3) | .86 |
| 30-Day mortality, % | 7.8 | 6.7 | .99 |
Values are expressed as mean ± SD unless otherwise indicated. APACHE II, Acute Physiology and Chronic Health Evaluation II; BMI, body mass index; DCCI, Deyo-Charlson Comorbidity Index; EN, enteral nutrition; ICU, intensive care unit; IQR, interquartile range; LOS, length of stay; PN, parenteral nutrition; SICU, surgical intensive care unit; VFD, ventilator-free days.
Table 2.
Number of Potentially Avoidable Interruption Episodes by Indication.
| Reason for EN Interruption | n | Potentially Avoidable | % |
|---|---|---|---|
| (Re)intubation/extubation | 29 | 0 | 0 |
| Tracheostomy/PEG | 23 | 0 | 0 |
| Imaging study | 16 | 14 | 87.5 |
| Ortho procedures | 12 | 6 | 50.0 |
| High GRV | 10 | 0 | 0 |
| Other | 6 | 4 | 66.7 |
| IR procedure | 6 | 4 | 66.7 |
| GI surgery | 4 | 0 | 0 |
| Total | 106 | 28 | 26.4 |
EN, enteral nutrition; GI, gastrointestinal; GRV, gastric residual volume; IR, interventional radiology; PEG, percutaneous endoscopic gastrostomy.
Patients who experienced at least 1 interruption in their EN infusions were approximately 3 times more likely to be underfed (ie, <66% of total prescribed calories) during their ICU admission compared with their counterparts who did not experience any interruptions (adjusted OR, 2.89; 95% CI, 1.03–8.11) and accumulated a greater cumulative caloric deficit (Figure 1). Patients who experienced at least 1 interruption in their EN infusions were also more likely to have a prolonged ICU LOS (adjusted IRR, 1.27; 95% CI, 1.14–1.42) as well as a prolonged hospital LOS (adjusted IRR, 1.53; 95% CI, 1.41– 1.67) compared with their counterparts who did not experience any interruptions. Nonsignificant trends were observed regarding an association of EN interruptions with 30-day VFD (adjusted IRR, 0.95; 95% CI, 0.85–1.06), in-hospital mortality (adjusted OR, 1.16; 95% CI, 0.23–5.88), and 30-day mortality (adjusted OR, 2.78; 95% CI, 0.38–10). Substitution of DCCI for APACHE II scores or addition of the DCCI to the models did not materially change any of the results. Similarly, the addition of admission serum albumin levels to the models did not materially change any of the results.
Figure 1.
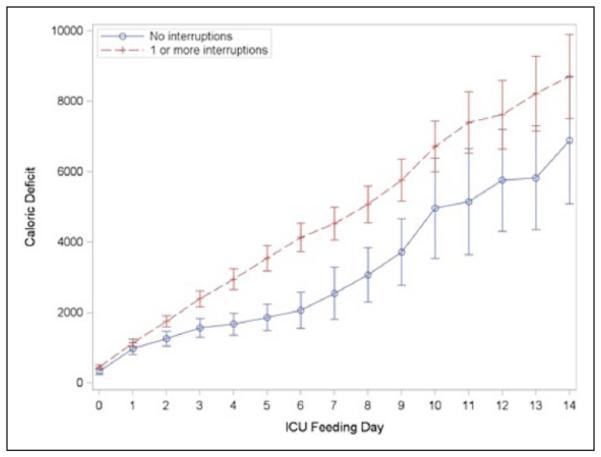
Cumulative caloric deficit (kcal).
Discussion
In this prospective observational study, we investigated the causes and consequences of EN interruptions in SICU patients. We demonstrated that the most common reasons for interruptions in EN were (1) (re)intubation/extubation, (2) major bedside interventions (tracheostomy/PEG tube placement), and (3) for imaging studies. Only 26% of these interruptions were considered avoidable. Patients who had interruptions in their EN infusions experienced a significantly higher caloric deficit during their ICU stay and had longer ICU as well as hospital LOS compared with patients who did not have any feeding interruptions. While others have highlighted the importance of feeding protocols in the ICU,14,20,22,25,26 our work provides novel insights into specific areas of improvement for existing protocols that may help optimize nutrition status in critically ill surgical patients. However, due to the observational design of this study, causal inferences about the relationship between EN interruptions, caloric debt, and clinical outcomes are limited.
Previous studies in mixed ICU cohorts (medical and surgical patients) have identified underprescription and high GRV as major factors contributing to interruptions in EN.17,27,28 McClave et al19 reported that despite existing policies for caloric needs to be estimated between 25 and 35 kcal/d, only 65% of patients were prescribed an appropriate goal EN rate. Furthermore, of the volume ordered, only 78% was actually delivered, and as such, patients ultimately received only 51% of estimated caloric needs. Unlike our findings, McClave et al reported that interruptions of EN occurred in 83% of patients, most commonly for “high” GRVs, which accounted for 45% of cessation time. It is important to note that while McClave et al defined high GRV as >200 mL, in almost half of the interruption episodes for “unacceptable” GRV, the volumes were recorded as <200 mL. Feeding tube displacement was the second most common reason for interrupted EN (affecting 41% of patients) in their study. While interruptions for diagnostic procedures and routine nursing care were common, affecting 27% and 30%, respectively, the duration of interruption was generally short, and therefore lost infusion time was <1% in both cases. Overall, the authors concluded that EN interruptions were “avoidable” in 66% of cases. In a larger study of mixed medical/surgical patients, Heyland et al29 also report that the most common reason for EN interruption was “high” GRVs (estimated to have occurred in 51% of patients).
In contrast to previous studies in medical patients, our cohort focused on critically ill surgical patients, and our GRV threshold for holding EN infusions was >500 mL. Accordingly, “high” GRVs accounted for only 9% of all EN interruptions. In all instances of interrupted EN for GRV indications, the measured volume was >500 mL and were thus considered unavoidable. While prolonged interruptions for routine nursing care did not occur, we did find that interruption for imaging procedures occurred frequently, with the majority deemed avoidable. Overall, 68% of our patients had at least 1 episode of EN interruption. Patients who had their EN interrupted (group 1) accumulated almost double the caloric deficit, stayed in the ICU for an additional 1.5 days, and remained hospitalized 8 days longer than patients without a single interruption (group 2). Although we did not find a statistical difference in 30-day VFDs, in-hospital mortality, and 30-day mortality between the 2 groups, the present study was grossly underpowered to assess these outcomes. Our results do, however, provide critical information to help plan for adequately powered clinical trials to assess the impact of caloric intake on important ICU outcome measures in critically ill surgical patients.
Of particular note, despite no EN interruptions, group 2 patients still accumulated a significant caloric deficit, which was likely due to delayed initiation (33% of patients started EN >48 hours after ICU admission) of EN and our use of a conservative “ramp-up” feeding protocol to gradually arrive at goal rates. This “ramp-up” practice was also occasionally employed upon resumption of interrupted tube feeds in the group 1 patients (despite having previously tolerated higher rates), further contributing to the cumulative caloric deficit. An additional reason for caloric deficit in group 2 patients was the debatable “purposeful” underprescription of EN (eg, maintaining a patient only at a “trophic” rate of 10 or 20 mL/h for several days before increasing to goal). Although there were no interruptions, nutrition delivery was suboptimal; in most cases, this underprescription was at the explicit request of the surgical teams. Others have reported similar findings; in a large cluster-randomized study,28 early initiation of EN failed to demonstrate improved clinical outcomes likely due to an inability to provide sufficient calories despite early initiation. In another cluster-randomized clinical trial, patients in intervention hospitals who were fed according to evidence-based algorithms received significantly more EN, had a shorter hospital LOS, and showed a trend toward reduced mortality compared with patients in hospitals that did not use evidence-based feeding algorithms.26 Current evidence therefore suggests that while early nutrition is necessary, sufficient nutrition is required to optimize benefits.
We found that most EN interruptions in our surgical patients were unavoidable, and as such, attention in this cohort should perhaps be focused on methods to enhance nutrition delivery in the setting of expected interruptions for appropriate indications. For example, Heyland et al30 recently described their “PEP uP Protocol,” which features a multifaceted, aggressive approach to ICU nutrition to include (1) initiating EN at a higher rate, (2) prophylactic use of prokinetic agents at the initiation of EN, (3) use of semi-elemental formulas to improve tolerance, (4) initial protein supplementation, and (5) encouraging the use of “catch-up” EN boluses to achieve daily goals when nutrition is interrupted. When tested in a cluster-randomized trial, the PEP uP Protocol resulted in a significant increase in calorie and protein delivery.31 Whether these findings are generalizable to other ICUs and whether these results can be replicated in different patient cohorts will need to be explored through large randomized controlled trials.
While our findings are compelling, a number of study limitations must be discussed. It is important to note that the current study was performed at a single, tertiary-care, level 1 trauma center, and therefore, our results may not be generalizable to all hospitals. Moreover, since our study cohort was heavily skewed toward trauma and general surgery patients, other surgical subspecialties (eg, cardiac, neurosurgery, urology, gynecology) are underrepresented. Although our sample size was adequately powered to address the primary study objectives, we were underpowered to detect potential differences in some major clinically relevant outcomes (eg, ICU LOS, VFD, 30-day mortality). It is also worth noting that due to our study inclusion/exclusion criteria, the mean APACHE II scores in both groups of patients were not very high and therefore the study findings may not be applicable to patients with a high severity of illness. Although we attempted to limit confounding due to patient comorbidities by including APACHE II scores, DCCI scores, and admission serum albumin levels in our analyses, there are likely aspects of “frailty” or “malnutrition” that we are unable to control for. We were also unable to control for individual ICU provider or surgeon behavior regarding timing, rate, and decision to hold/continue EN infusions. Although the duration of cessation events may have provided an interesting dimension to the data analysis, we did not collect this information at the outset of the study and therefore cannot control for the length of EN interruptions. In addition, our calculation of caloric debt was based on estimated caloric needs according to A.S.P.E.N. guidelines and was not routinely based on calorimetry or nitrogen balance studies to accurately quantify deficits. And finally, due to the observational design of the study, our results should not be interpreted as causation but rather as an exploration of well-controlled associations that will lead to adequately powered, carefully designed, randomized controlled clinical trials.
Conclusions
In this prospective observational study, we report that despite implementation of an aggressive evidence-based feeding protocol, EN infusions in critically ill surgical patients occur frequently. The main reasons for interruption are related to (1) (re) intubation/extubation, (2) bedside procedures involving the airway and proximal GI tract, and (3) imaging studies. Although patients who experience EN interruptions are more likely to be underfed, most of these interruptions are unavoidable. Therefore, in this cohort of patients, efforts should perhaps focus on maximizing nutrient delivery before and after EN interruptions, rather than on trying to eradicate interruptions. Future randomized controlled clinical trials are needed to determine whether increased attention to nutrition delivery around episodes of “unavoidable” EN interruptions will translate to improved clinical outcomes.
Footnotes
Financial disclosures: S.A.Q. received support from NIH 5T32GM007592-33.
References
- 1.Barker LA, Gout BS, Crowe TC. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. Int J Environ Res Public Health. 2011;8(2):514–527. doi: 10.3390/ijerph8020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim SL, Ong KC, Chan YH, Loke WC, Ferguson M, Daniels L. Malnutrition and its impact on cost of hospitalization, length of stay, readmission and 3-year mortality. Clin Nutr. 2012;31(3):345–350. doi: 10.1016/j.clnu.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Hiesmayr M. Nutrition risk assessment in the ICU. Curr Opin Clin Nutr Metab Care. 2012;15(2):174–180. doi: 10.1097/MCO.0b013e328350767e. [DOI] [PubMed] [Google Scholar]
- 5.Cahill NE, Dhaliwal R, Day AG, Jiang X, Heyland DK. Nutrition therapy in the critical care setting: what is “best achievable” practice? An international multicenter observational study. Crit Care Med. 2010;38(2):395–401. doi: 10.1097/CCM.0b013e3181c0263d. [DOI] [PubMed] [Google Scholar]
- 6.Binnekade JM, Tepaske R, Bruynzeel P, Mathus-Vliegen EM, de Hann RJ. Daily enteral feeding practice on the ICU: attainment of goals and interfering factors. Crit Care. 2005;9(3):R218–R225. doi: 10.1186/cc3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006;129(4):960–967. doi: 10.1378/chest.129.4.960. [DOI] [PubMed] [Google Scholar]
- 8.Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100(11):1316–1324. doi: 10.1016/S0002-8223(00)00373-4. [DOI] [PubMed] [Google Scholar]
- 9.Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22(3):235–239. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 10.Kassin MT, Owen RM, Perez SD, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322–330. doi: 10.1016/j.jamcollsurg.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumayer LA, Smout RJ, Horn HG, Horn SD. Early and sufficient feeding reduces length of stay and charges in surgical patients. J Surg Res. 2001;95(1):73–77. doi: 10.1006/jsre.2000.6047. [DOI] [PubMed] [Google Scholar]
- 12.Alberda C, Gramlich L, Jones N, et al. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35(10):1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 13.Allingstrup MJ, Esmailzadeh N, Wilkens Knudsen A, et al. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin Nutr. 2012;31(4):462–468. doi: 10.1016/j.clnu.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest. 2004;125(4):1446–1457. doi: 10.1378/chest.125.4.1446. [DOI] [PubMed] [Google Scholar]
- 15.O’Meara D, Mireles-Cabodevila E, Frame F, et al. Evaluation of delivery of enteral nutrition in critically ill patients receiving mechanical ventilation. Am J Crit Care. 2008;17(1):53–61. [PubMed] [Google Scholar]
- 16.Heyland DK, Stephens KE, Day AG, McClave SA. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. 2011;30(2):148–155. doi: 10.1016/j.clnu.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Heyland DK, Schroter-Noppe D, Drover JW, et al. Nutrition support in the critical care setting: current practice in Canadian ICUs—opportunities for improvement? JPEN J Parenter Enteral Nutr. 2003;27(1):74–83. doi: 10.1177/014860710302700174. [DOI] [PubMed] [Google Scholar]
- 18.Drover JW, Cahill NE, Kutsogiannis J, et al. Nutrition therapy for the critically ill surgical patient: we need to do better! JPEN J Parenter Enteral Nutr. 2010;34(6):644–652. doi: 10.1177/0148607110372391. [DOI] [PubMed] [Google Scholar]
- 19.McClave SA, Sexton LK, Spain DA, et al. Enteral tube feeding in the intensive care unit: Factors impeding adequate delivery. Crit Care Med. 1999;27(7):1252–1256. doi: 10.1097/00003246-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Soguel L, Revelly JP, Schaller MD, Longchamp C, Berger MM. Energy deficit and length of hospital stay can be reduced by a two-step quality improvement of nutrition therapy: the intensive care unit dietitian can make the difference. Crit Care Med. 2012;40(2):412–419. doi: 10.1097/CCM.0b013e31822f0ad7. [DOI] [PubMed] [Google Scholar]
- 21.Spain DA, McClave SA, Sexton LK, et al. Infusion protocol improves delivery of enteral tube feeding in the critical care unit. JPEN J Parenter Enteral Nutr. 1999;23(5):288–292. doi: 10.1177/0148607199023005288. [DOI] [PubMed] [Google Scholar]
- 22.Mackenzie SL, Zygun DA, Whitmore BL, Doig CJ, Hameed SM. Implementation of a nutrition support protocol increases the proportion of mechanically ventilated patients reaching enteral nutrition targets in the adult intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29(2):74–80. doi: 10.1177/014860710502900274. [DOI] [PubMed] [Google Scholar]
- 23.Weijs PJ, Wischmeyer PE. Optimizing energy and protein balance in the ICU. Curr Opin Clin Nutr Metab Care. 2013;16(2):194–201. doi: 10.1097/MCO.0b013e32835bdf7e. [DOI] [PubMed] [Google Scholar]
- 24.Heyland DK, Cahill N, Day AG. Optimal amount of calories for critically ill patients: depends on how you slice the cake! Crit Care Med. 2011;39(12):2619–2626. doi: 10.1097/CCM.0b013e318226641d. [DOI] [PubMed] [Google Scholar]
- 25.Adam S, Batson S. A study of problems associated with the delivery of enteral feed in critically ill patients in five ICUs in the UK. Intensive Care Med. 1997;23:261–266. doi: 10.1007/s001340050326. [DOI] [PubMed] [Google Scholar]
- 26.Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ. Multicentre, cluster-randomized clinical trial of algorithms for critical-care enteral and parenteral therapy (ACCEPT) CMAJ. 2004;170(2):197–204. [PMC free article] [PubMed] [Google Scholar]
- 27.De Jonghe B, Appere-De-Vechi C, Fournier M, et al. A prospective survey of nutritional support practices in intensive care unit patients: what is prescribed? What is delivered? Crit Care Med. 2001;29(1):8–12. doi: 10.1097/00003246-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Stotts NA, Froelicher ES, Engler MM, Porter C. Why patients in critical care do not receive adequate enteral nutrition? A review of the literature. J Crit Care. 2012;27(6):702–713. doi: 10.1016/j.jcrc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Heyland D, Cook DJ, Winder B, Brylowski L, Van deMark H, Guyatt G. Enteral nutrition in the critically ill patient: a prospective survey. Crit Care Med. 1995;23(6):1055–1060. doi: 10.1097/00003246-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Heyland DK, Cahill NE, Dhaliwal R, et al. Enhanced protein-energy provision via the enteral route in critically ill patients: a single center feasibility trial of the PEP uP protocol. Crit Care. 2010;14(2):R78. doi: 10.1186/cc8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyland DK, Murch L, Cahill N, et al. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: results of a cluster randomized trial. Crit Care Med. 2013;41(12):2743–2753. doi: 10.1097/CCM.0b013e31829efef5. [DOI] [PubMed] [Google Scholar]


