Highlights
-
•
All nucleocapsid (NC) basic residues in HIV-1 mutant (NC15A) were replaced with alanine.
-
•
This resulted in significantly defective Gag membrane binding, assembly, and processing.
-
•
Removal of the HIV-1 matrix (MA) globular domain led to marked improvement of NC15A assembly and processing.
-
•
Enhancement of Gag multimerization and membrane binding capacities likely improved NC15A assembly and processing.
Abbreviations: CA, capsid; MA, matrix; NC, nucleocapsid; PR, viral protease; VLP, virus-like particle
Keywords: HIV-1, Virus assembly, Gag cleavage, Nucleocapsid, Matrix, Membrane association
Abstract
Human immunodeficiency virus type 1 nucleocapsid (NC) basic residues presumably contribute to virus assembly via RNA, which serves as a scaffold for Gag–Gag interaction during particle assembly. To determine whether NC basic residues play a role in Gag cleavage (thereby impacting virus assembly), Gag processing efficiency and virus particle production were analyzed for an HIV-1 mutant NC15A, with alanine serving as a substitute for all NC basic residues. Results indicate that NC15A significantly impaired virus maturation in addition to significantly affecting Gag membrane binding and assembly. Interestingly, removal of the matrix (MA) central globular domain ameliorated the NC15A assembly and processing defects, likely through enhancement of Gag multimerization and membrane binding capacities.
1. Introduction
In most retroviruses, Gag precursor polypeptide expression is sufficient for mediating virus particle assembly [1]. During or soon after virus budding, HIV-1 Gag precursor Pr55 is cleaved by viral protease (PR) into matrix (MA; p17), capsid (CA; p24), nucleocapsid (NC; p7) and p6 domains [1,2]. This PR-mediated virus maturation process is essential for acquiring viral infectivity [3–6]. In addition to PR, enzymes required for virus replication are encoded by pol. Due to its partial overlap with the gag coding sequence, HIV-1 Pol polypeptide is initially translated as a Gag-Pol fusion protein via a ribosomal frameshifting event occurring at a frequency of approximately 5% [7], resulting in a Gag-Pol/Gag expression ratio of approximately 1:20. Maintenance of a low PR-associated Gag-Pol expression level is considered critical, since the artificial overexpression of Gag-Pol or PR triggers reduced virion yield due to the enhanced cleavage of Gag precursors prior to virus assembly [8–14].
In addition to playing a key role in mediating viral genomic RNA packaging, NC contains an interaction domain that promotes Gag–Gag interaction [15–18]. Specific NC mutations either reduce overall virus particle production, or trigger the production of low-density virus particles [1]. NC-associated RNA may serve as a scaffold facilitating NC–NC interaction and Gag assembly [19–26]. Deleting the NC domain or decreasing the number of positively charged NC residues via alanine replacement has been shown to markedly reduce virion yields [17,18,27–29]; reduced virion yields associated with NC mutants may also be attributed, at least in part, to a release defect [30,31]. One research group has suggested that decreased virion production tied to NC mutants is the result of released particle instability following cell budding [28]. However, results from a separate study indicate that HIV-1 PR activity inhibition enhances virion production by NC-deletion mutants, suggesting that substantial amounts of assembly-defective Gag molecules are cleaved by PR prior to virus particle formation [27]. Accordingly, Gag that is slowly assembled may be more susceptible to cleavage by PR, thus further reducing virus release. Since NC possesses dimerization potential [21], it may contribute to PR activation by promoting Gag-Pol dimerization. Although results from an in vitro study suggest that the NC domain enhances PR-mediated Gag cleavage [32], it is unclear whether the NC contribution to Gag cleavage, if any, also impacts virus assembly.
We have two motivations for the present study: (a) to determine whether positively charged NC basic residues involved in RNA binding are required for PR-mediated Gag cleavage, and (b) to clarify whether the impacts of NC mutations on Gag cleavage (if any) affect Gag assembly and virion yields. We found that blocking NC-RNA association via the alanine replacement of all NC basic residues (NC15A) reduced Gag cleavage efficiency and significantly impaired Gag assembly. PR activity inhibition resulted in partial restoration of NC15A virion yields, suggesting that the virion deficit was in part affected by PR activity. We also observed that removal of the central MA globular domain (ΔMA) markedly reduced NC15A-induced Gag assembly and processing defects.
2. Materials and methods
2.1. Plasmid construction
The NC15A, as described previously [33], had the 15 NC basic NC residues replaced with alanines. The MA mutation was constructed by deleting a fragment from nt 831 to nt 1147 and replacing it with a SalI linker [34]. To construct substitution mutants T26S and A28S, DNA fragments containing the point mutations were first generated by the mutation-containing primers (T26S:5′-CTATTAGATTCCGGAGCAGAT-3′; A28S:5′-TAGATACAGGATCCGATGATTAC-3′) and a reverse primer, 2577-51 (5′-ACTGGTACAGTCTCAATAGGGCTAATG-3′), using an Env-deficient HIV-1 vector, HIVgpt [35] as template. Each of the resulting PCR products was then used as a megaprimer for a second round of PCR by using the forward primer G80 (5′-ATGAGAGAACCAAGGGGAAGTGTGA-3′). The PCR products were then digested with Spe1 and BclI and ligated into HIVgpt. As described previously, the ΔMA was constructed by deleting a fragment from nt 831 to nt 1147 and replacing it with a SalI linker [34]. The myristylation-minus (Myr−) mutant, in which the second glycine residue has been replaced by alanine, blocks Gag membrane binding and virus production [36]. Each of the gag mutations was also introduced into a PR-inactivated HIV-1 expression vector, HIVgptD25 [14], yielding a set of PR-defective versions. In D25, Arg is substituted for the PR catalytic residue Asp [14]. All mutation constructs were analyzed using restriction enzymes or DNA sequencing, and each mutation was subcloned into the HIV-1 expression vector HIVgpt [35].
2.2. Cell culture and transfection
293T and cells were maintained in DMEM supplemented with 10% fetal calf serum. Twenty-four hours before transfection, confluent 293T cells were trypsinized, split 1:10, and seeded onto 10-cm dishes. For each construct, 293T cells were transfected with 20 μg plasmid DNA using the calcium phosphate precipitation method, with addition of 50 μM chloroquine to enhance transfection efficiency.
2.3. Western immunoblot analysis
Unless otherwise indicated, cells and culture supernatants were harvest for proteins analysis at 48–72 h post-transfection. Cell and supernatant samples were prepared and subjected to Western immunoblot analysis as described previously [42]. For detection of HIV Gag proteins, the primary antibody was an anti-p24gag monoclonal antibody (mouse hybridoma clone 183-H12-5C) from ascites used at a dilution of 1:5000. The secondary antibody was a sheep anti-mouse horseradish peroxidase (HRP)-conjugated antibody diluted at 1:15,000. An enhanced chemiluminescence (ECL) kit (Pierce) was used to visualize the membrane-bound Gag proteins.
2.4. Membrane flotation assays
We followed the protocol as described previously [42]. Briefly, cells were pelleted and resuspended in TE buffer (10 mM Tris–HCl, pH 7.4, 1 mM EDTA) containing 10% sucrose and Complete Protease Inhibitor Cocktail (Roche). Cell suspensions were subjected to sonication followed by low-speed centrifugation to remove nuclei and cell debris. Postnuclear supernatant (200 μl) was mixed with 1.3 ml 87.5% sucrose in TE buffer containing Complete Protease Inhibitor Cocktail and placed on the bottom of a centrifuge tube. Solutions of 7.5 ml 65% sucrose and 3 ml 10% sucrose in TE buffer were layered on top of the 1.5 ml mixture. The gradients were centrifuged at 100,000g for 16–18 h at 4 °C and then ten fractions were collected from the top of the centrifuge tube. Proteins in each fraction were precipitated with ice-cold 10% trichloroacetic acid (TCA) and analyzed by Western immunoblot.
2.5. Velocity sedimentation analysis of cytoplasmic Gag proteins
As described previously [42] and above, postnuclear supernatant (500 μl) was prepared and mixed with an equal amount of TEN buffer (50 mM Tris–HCl, pH 7.4, 2 mM EDTA, 150 mM NaCl) containing Complete Protease Inhibitor Cocktail. The mixture was then centrifuged through a pre-made 25–45% discontinuous sucrose gradient at 130,000g for 1 h. Five 0.8-ml fractions were collected from the top of the centrifuge tubes. Proteins present in aliquots of each fraction were precipitated with ice-cold 10% TCA and subjected to Western blot analysis.
2.6. Virus-associated RNA quantification
Virus-containing supernatants were collected and centrifuged through a 20% sucrose cushion. Virus-associated RNA was then purified using a QIAamp Viral RNA Mini Kit (QIAGEN). Viral RNA, eluted in RNase-free buffer, was treated with RQ1 RNAase-free DNase (Promega) at 37 °C for 30 min. As described previously [42], total viral RNA was then quantified using a RiboGreen RNA Assay Kit (Invitrogen) according to the manufacturer’s protocols. Ribosomal RNA provided in the assay kit was used to establish a RNA standard curve in parallel. Ratios of RNA concentrations to Gag immunoblot band density units were determined for each mutant and normalized to that of wt in parallel experiments.
2.7. Statistical analysis
Differences between control (wt) and experimental (mutant) groups were assessed using Student’s t-tests. Data are expressed as mean ± standard deviation. Significance was defined as ∗p < 0.05, ∗∗p < 0.01.
3. Results
3.1. NC positive charge neutralization significantly affects HIV-1 Gag assembly and processing
To determine whether NC basic residues are required for Gag assembly and processing, we inserted a NC15A mutation (with all NC basic amino acid residues replaced with alanine [17,35]) into a HIV-1 proviral expression vector (Fig. 1A). Initial results indicate barely detectable Gag products in culture supernatants following the transient expression of NC15A in 293T cells, suggesting that NC positive charge elimination significantly reduced VLP yields. Given the likelihood that the PR-mediated cleavage of assembly-defective Gag molecules may contribute to virion decreases [17,35], PR activity suppression may lead to increased VLP production. We therefore tested Gag assembly and processing (a) in the presence of a PR inhibitor, and (b) via the expression of a PR activity-diminished backbone resulting from a substitution mutation (T26S or A28S) at the PR domain. The addition of T26S and A28S resulted in 4-fold and 50-fold reductions in PR activity, respectively [36]. As shown in Fig. 1, barely detectable VLP-associated NC15A became readily detectable when expressed in T26S or A28S backbones (panel B, lanes 8–10). Similarly, barely detectable VLP-associated NC15A became clearly detectable, and wt VLP yields increased following treatment with saquinavir, a PR inhibitor (Fig. 1C, lower panel, lanes 1–6). These results support the proposal that reduced VLP yield associated with NC mutants is partly due to assembly-defective Gag cleavage; further confirmation comes from NC mutant construct expression in a PR-inactivated (D25) vector (Fig. 1D, lane 3). Combined, our data suggest that PR activity suppression was responsible for increased VLP yields associated with assembly-defective NC15A.
Fig. 1.
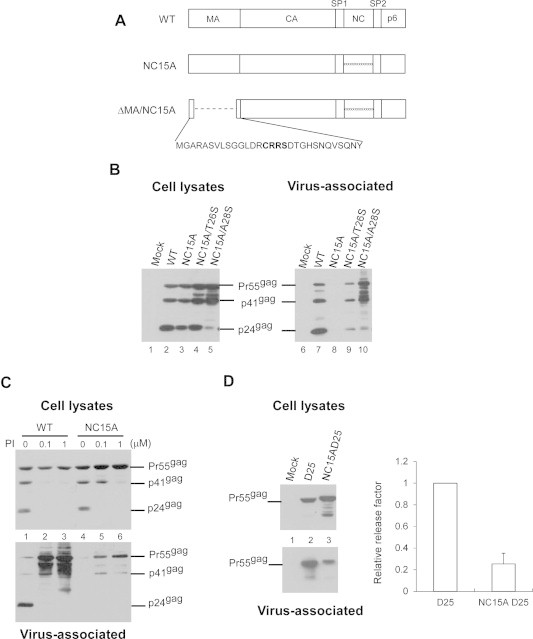
Assembly and processing of HIV-1 mutants containing alanine replacement of all NC basic residues. (A) Schematic representations of wild-type (WT) and recombinant HIV-1 mutants. Indicated are the HIV-1 Gag protein domains MA (matrix), CA (capsid), NC (nucleocapsid), p6, and two spacer peptides SP1 and SP2. “xxxxxxxxxx” denotes all NC basic residues replaced with alanines. ΔMA contains a replacement of 106 codons by 4 codons (in bold face), with myristylation and MA/CA cleavage signal retained. (B–D) Suppression of PR activity alleviates the assembly defect incurred by positive nucleocapsid charge elimination. (B) 293T cells were transfected with 20 μg of designated plasmid. T26S and A28S are PR point mutations triggering 4-fold and 50-fold reductions in PR activity, respectively [38]. At 48 h post-transfection, culture supernatants and cells were collected and prepared for protein analysis as described in Section 2. Viral pellet samples corresponding to 50% of total samples (lanes 6–10) and cell lysate samples corresponding to 5% of total samples (lanes 1–5) were fractionated using 10% SDS–PAGE and electroblotted onto nitrocellulose filters. HIV-1 Gag proteins were probed with a mouse monoclonal antibody directed at p24CA. Positions of HIV Gag proteins Pr55, p41, and p24 are indicated. (C) 293T cells were transfected with wt or mutant plasmids. At 4 h post-transfection, cells were replated on three dish plates and either left untreated (lanes 1 and 4) or treated with the HIV-1 protease inhibitor (PI) saquinavir at concentrations of 0.1 μM (lanes 2 and 5) or 1.0 μM (lanes 3 and 6). At 48–72 h post-transfection, cells and culture supernatant were collected, prepared, and subjected to Western immunoblot analysis. (D) 293T cells were transfected with the PR-inactivated version (D25) of NC15A. At 48–72 h post-transfection, culture supernatants and cells were collected and subjected to Western immunoblotting.
3.2. MA removal alleviates NC15A assembly and processing defects
MA basic residues bind with RNA [37–40], which may affect Gag–Gag or Gag–Pol/Gag–Pol interaction. We therefore tested the impacts of MA deletion on Gag assembly and processing, and repeatedly observed that NC15A exhibited a noticeable increase in VLP yields following MA removal (Fig. 2A, lane 9 versus lane 10, and B). Notably, low but detectable NC15A VLPs were largely immature—that is, virus-associated Gag remained unprocessed or incompletely processed (lane 9). In contrast, mature virus-associated p24gag associated with DMA/NC15A was readily detectable (Fig. 2A, lane 10). To minimize the impacts of PR activity on VLP assembly, all constructs were expressed in the PR-inactivated (D25) backbone. Our results also suggest that NC15A VLP yields were significantly enhanced by MA removal (Fig. 2C and D).
Fig. 2.
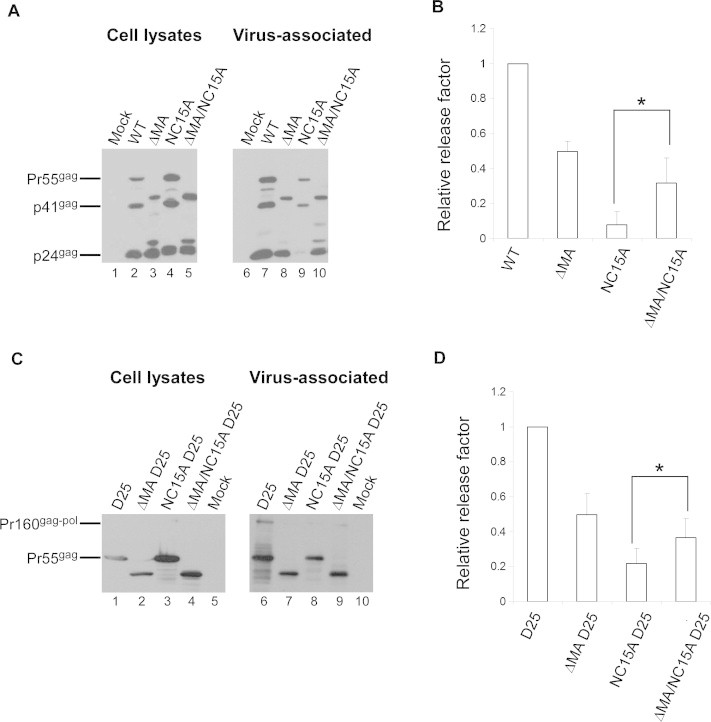
Removal of MA ameliorates NC15A assembly and processing defects. (A) 293T cells were transfected with indicated constructs. At 48 h post-transfection, cells and culture supernatants were collected, prepared, and subjected to Western immunoblotting probed with an anti-p24CA antibody. (B) Gag proteins from medium or cell samples were quantified by scanning mutant and wt p24gag-associated band densities from immunoblots. Ratios of total Gag protein levels in the media to those in cells were determined for each construct and compared with wt release levels by dividing the release ratio for each mutant by the wt ratio in parallel experiments. Error bars indicate standard deviation. ∗p < 0.05. (C) 293T cells were transfected with indicated constructs. At 48–72 h post-transfection, culture supernatants and cells were collected and subjected to Western immunoblotting. (D) Total Gag proteins (wt or mutant Gag precursor) from medium or cell samples were detected with an anti-p24CA monoclonal antibody and quantified by scanning wt and mutant Gag precursor band densities from immunoblots. Ratios of Gag in media to Gag in cells were determined for each construct, and compared with wt release levels as described above. Error bands indicate standard deviation. ∗p < 0.05.
Although NC15A and ΔMA/NC15A both exhibited similar levels of unprocessed Gag precursor at 48 h post-transfection (Fig. 2A, lanes 4 and 5), results from a time-course analysis of Gag processing suggest that removal of the MA globular domain significantly enhanced NC15A Gag processing efficiency (Fig. 3). It is likely that the Gag mutation that disrupts VLP assembly also affects Gag–Pol molecular interaction, which in turn impairs PR activation. To further determine if MA removal impacts VLP release and maturation, accumulated VLPs in supernatants were collected at different time intervals following treatment with cycloheximide. Our results indicate significant increases in wt virus-associated Gag accumulation at 4 and 8 h host-treatment, but not at 12 h, suggesting that most VLP assembly and release in the Gag molecules was completed by 8 h post-treatment (Fig. 4). Likewise, we did not observe significant changes in virus-associated Gag cleavage profiles, suggesting that PR quickly mediates virus maturation following virus budding. Mutations at HIV-1 NC basic residues can lead to PR-dependent virion instability, with virions tending to fall apart with little recovery [28,35]. This may partly explain why ΔMA/NC15A exhibited slight accumulation decreases rather than increases at 8 and 12 h. Some of mature wt VLPs might also be unstable after release, which leads to a decrease accumulated VLPs at 12 h. A faster release rate in ΔMA compared to the wt was not observed in repeat independent experiments. However, we consistently observed that ΔMA/NC15A exhibited faster virus release and processing rates compared to NC15A.
Fig. 3.
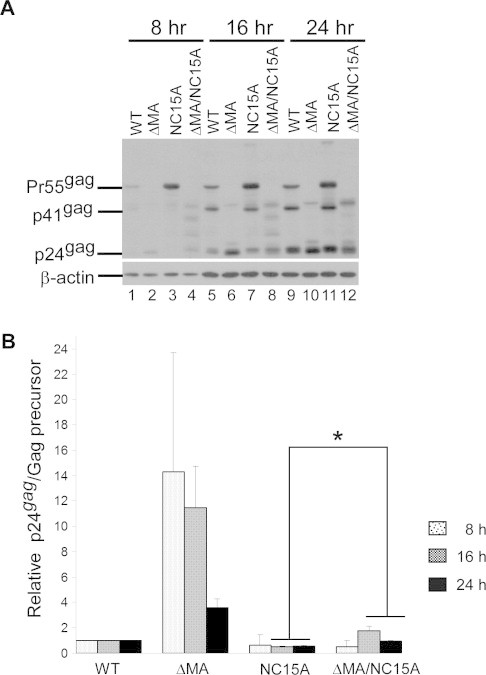
NC15A-meidated Gag processing efficiency is significantly enhanced following removal of MA. 293T cells were transfected with designated constructs. At 4 h post-transfection, equal amounts of cells were plated on three dishes. Cell were collected at 8, 16 or 24 h post-transfection and subjected to Western immunoblotting. Cellular Pr55gag and p24gag levels were quantified by scanning immunoblot band densities. Ratios of p24gag to Pr55gag or to mutant Gag precursor were determined for each mutant and normalized to those of the wt in parallel experiments. Values were derived from three independent experiments. Bars indicate standard deviations. ∗p < 0.05.
Fig. 4.
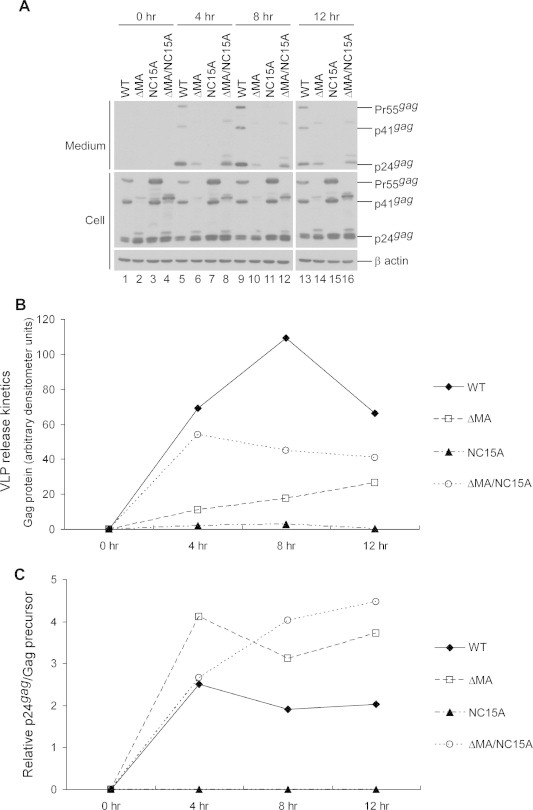
VLP release and processing kinetics 293T cells grown in 10 cm culture dishes in triplicate were transfected with the designated plasmid, pooled, and divided equally onto four dish plates. At 16 h post-transfection, supernatants were collected and fed medium containing 30 μg/ml of cycloheximide. Cells and supernatants were collected at 0, 4, 8 and 12 h following cycloheximide treatment. Supernatants were pelleted through 20% sucrose cushions and subjected to Western immunoblotting (panel A). Gag proteins were quantified by scanning p24gag-associated band densities from immunoblots. Densitometric units representing total Gag proteins in medium and the ratio of p24gag to Pr55gag band densities were plotted against time (panels B and C, respectively).
3.3. MA deletion enhances NC15A membrane binding and multimerization capacities
We performed velocity sedimentation centrifugation analyses of cellular Gag molecules, using a myristylation-deficient (myr-) Gag mutant (defective in membrane binding and incapable of multimerizing into high-molecular weight complexes) as a control [34]. Results indicate that wt Gag and the assembly-competent mutant DMA tended to be found in high-sucrose density fractions, while most myr- Gag was recovered in fractions 1 and 2 (Fig. 5); this is in agreement with previously reported results [41,42]. We consistently observed substantial shifts of NC15A to higher sucrose density fractions following MA deletion (Fig. 5, panel 3 versus 4 from top), suggesting that MA removal mitigates the NC15A multimerization defect, which is compatible with improved VLP yields (Fig. 2). It should be noted that some slightly overexposed blots were purposely presented to make it easier for readers to see the detected signals. Not all of the Western blots shown in the figures were used for quantification.
Fig. 5.
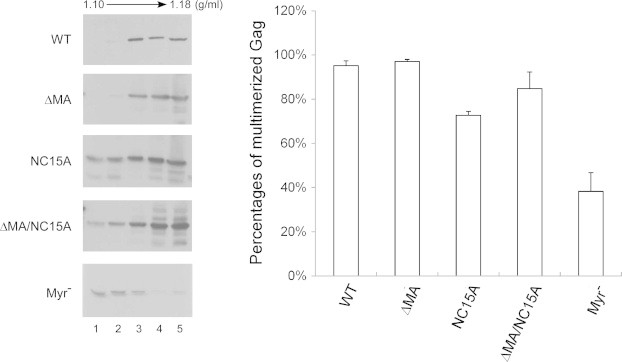
Velocity sedimentation analysis of cytoplasmic Gag precursor complexes. 293T cells were transfected with 20 μg of the PR-inactivated versions of the indicated plasmids. Two days post-transfection, cells were homogenized and their extracted cytoplasmic lysates centrifuged through 25%, 35% and 45% sucrose step gradients at 130,000g for 1 h. Fractions were collected from gradient tops, and fraction aliquots were subjected to 10% SDS–PAGE and probed with a monoclonal antibody directed at p24CA. Total Gag proteins were quantified by scanning the immunoblot band densities of fractions 1–5. Multimerized Gag protein percentages were determined by dividing multimerized Gag protein density units (fractions 3–5) by total Gag density units (fractions 1–5) and normalizing the results to that of the wt.
Since membrane binding is required for efficient Gag multimerization, we searched for correlations between Gag membrane-binding capacity and multimerization efficiency using membrane flotation assays for each mutant and wt as described in the Section 2. As expected, approximately 70% of the intracellular wt or ΔMA Gag was membrane-bound, compared to 10% of the myr- Gag (Fig. 6); this is consistent with previous research results [41,42]. Markedly decreased membrane-binding capacities were found for the multimerization-defective NC15A compared to the wt, but NC15A membrane binding capacity increased significantly following MA removal (Fig. 6B), which is compatible with improvements in both Gag assembly and VLP yield (Figs. 2 and 5).
Fig. 6.
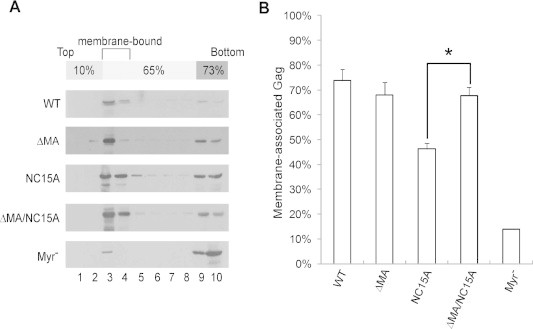
Membrane flotation centrifugation of HIV-1 Gag proteins. (A) 293T cells were transfected with PR-inactivated (D25) versions of the indicated constructs. Two days post-transfection, cells were harvested and homogenized. Crude membrane extracted from cell lysates was subjected to equilibrium flotation centrifugation as described in Section 2. In all, 10 fractions were collected from top downwards; fraction aliquots were analyzed by Western immunoblotting using an anti-p24CA monoclonal antibody. During ultracentrifugation, membrane-bound Gag proteins floated to the 10–65% sucrose interface. (B) Quantification of membrane-bound Gag proteins. Total Gag proteins were quantified by scanning the immunoblot band densities of fractions 1–10. Percentages of membrane-bound Gag proteins were determined by dividing the membrane-bound Gag protein density unit (fractions 2–4) by total Gag protein density unit and multiplying by 100. Error bands indicate standard deviation. ∗p < 0.05.
3.4. RNA binding is not sufficient for efficient virus assembly
Since RNA is required for efficient Gag assembly, virus-associated total RNAs for the NC15A and ΔMA/NC15A mutants (all expressed in the PR-inactivated [D25] backbone) were measured and normalized to those of wt (D25) in parallel experiments. Results indicate that RNA quantities in both mutants were significantly lower than that in the wt (Fig. 7). However, they still contained measurable amounts of RNA, suggesting that the CA domain and/or remaining MA and NC basic residues in the ΔMA region may contribute to RNA association. No positive relationships were noted between RNA packaging and VLP assembly capacity in the assembly-defective mutants. For example, both ΔMA/NC15A and NC15 contained similar small amounts of RNA, but the former produced VLPs more efficiently than the latter. Our results suggest that RNA binding is insufficient for efficient virus assembly.
Fig. 7.
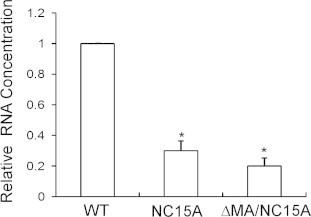
Relative levels of virus-associated RNA. 293T cells were transfected with PR-inactivated (D25) versions of the indicated constructs. Virus-containing supernatants were pelleted and resuspended in PBS buffer. About 40% of the resulting suspensions were subjected to Western immunoblot analysis, and the remaining suspensions were subjected to RNA extraction and quantification as described in Section 2. Ratios of RNA amounts to Gag protein levels obtained by quantifying immunoblot band densities were determined for each mutant and normalized to those of wt (D25) in parallel experiments. Bars indicate standard deviations. ∗p < 0.05.
4. Discussion
Our data indicate that (a) eliminating HIV-1 NC positive charges by replacing all NC basic residues with alanine (NC15A) produced a significant defect in VLP assembly associated with reduced Gag cleavage efficiency and membrane binding capacity, and (b) removing the MA central globular domain significantly reduced NC15A-incurred assembly and processing defects. That NC15A still contains measurable amounts of RNA provides further support for the proposal that RNA association is insufficient for efficient Gag assembly.
We noted that virus-associated NC15A significantly increased following PR activity inhibition, thus supporting the idea that a reduced virus-associated NC mutant is partly due to the cleavage of delayed-assembly Gag mutants prior to assembly and cell budding [29]. This may help explain our observation that virus-associated NC15A was incompletely processed (Fig. 2A lane 9). Intracellular NC15A Gag cleavage efficiency was also markedly reduced (Fig. 3). Since PR activation is triggered by Gag-Pol dimerization, the observed reduction in Gag cleavage efficiency suggests that the NC15A mutation, which impairs Gag–Gag interaction, may also affect Gag–Pol/Gag–Pol interaction. Improved NC15A assembly and processing efficiency following MA removal is likely tied to enhanced Gag assembly and Gag–Pol/Gag–Pol interaction.
In vitro, polypeptide NC-SP2-p6 cleavage by PR is significantly enhanced by RNA association [30], therefore NC association with RNA may facilitate PR-mediated Gag cleavage. However, we observed that barely detectable p24gag in NC15A transfectant supernatant became readily detectable following the removal of MA (Fig. 2A, lanes 9 versus 10), indicating that Gag can still be efficiently processed even if it lacks RNA binding capability, and suggesting that RNA association is not essential for PR-mediated Gag cleavage.
Although NC15A produced low levels of VLPs, substantial amounts of NC15A Gag were capable of membrane binding and multimerization (Figs. 5 and 6). It is likely that the low NC15A VLP yields were largely due to a release defect. In support of this hypothesis, Dussupt et al. found that HIV-1 NC basic residues play a role in combination with p6gag in virus budding by recruiting host fission machinery (ESCRT) to facilitate HIV-1 release [44,45]. Accordingly, substitution mutations at NC basic residues may contribute to reduced NC15A VLP yields as a result of a release defect. Further studies are required to test this possibility.
The inactivation of PR activity is known to restore VLP yields associated with a NC-deleted (ΔNC) mutant to wt levels [27,29]. However, we found that NC15A VLP levels were still lower than that of the wt when Gag cleavage was blocked (Fig. 2D). A possible explanation for these conflicting results may be that the NC15A mutation induces interactions that impair or delay Gag assembly processing, with potentially deleterious interaction regions being removed from the ΔNC mutant. It is also possible that NC domain binding to plasma membranes may interfere with the NC15A Gag conformational change process (from bent to straight), thus inhibiting Gag assembly [43]. NC removal may support VLP assembly by allowing Gag to be in a straight shape at the beginning of Gag assembly. These scenarios may partly explain why ΔNC is capable of producing VLPs at a wt level while NC15A is not.
Membrane association is required for HIV-1 Gag assembly and virus particle production [46–49]. According to the myristyl switch model of Gag membrane binding and multimerization [50–54], Gag multimerization induces a conformational change that exposes N-terminal myristic acid moiety, which is largely sequestered when Gag takes monomeric or lower-order oligomeric forms. The exposed myristic acid promotes Gag membrane binding capacity, which in turn enhances Gag multimerization. Further, removal of the MA globular domain containing basic residues may enhance Gag membrane-binding capacity, since MA loses that capacity once RNA binds to those residues [38,40,55]. These scenarios may partly explain why MA removal significantly mitigates NC15A membrane binding and assembly efficiency. However, the extent of improved membrane binding and multimerization capacity does not completely account for the increased NC15A virion yields following MA removal. In conclusion, our findings suggest that in addition to Gag multimerization and membrane binding deficits, a budding defect makes a significant contribution to poor NC15A virus particle production.
Acknowledgments
The authors wish to thank SW Wang for reagents and technical assistance. LJK and CTW conceived and designed the project, FHY and KJH acquired the data, LJK and FHY analyzed and interpreted the data, LJK and CTW wrote the paper. This work was supported by Grants V100C-002 and V101C-059 from Taipei Veterans General Hospital, by Grants NSC 97-2320-B-010-002-MY3 and 100-2320-B-010-015-MY3 from the Ministry of Science and Technology, Taiwan, and by a grant from the Ministry of Education, Aiming for the Top University Plan.
References
- 1.Swanstrom R., Wills J. Synthesis, assembly and processing of viral proteins. In: Coffin J., Hughes S., Varmus H., editors. Retroviruses. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 1997. pp. 263–334. [PubMed] [Google Scholar]
- 2.Freed E.O. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 3.Gottlinger H.G., Sodroski J.G., Haseltine W.A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan A.H., Zack J.A., Knigge M., Paul D.A., Kempf D.J., Norbeck D.W., Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J. Virol. 1993;67:4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pettit S.C., Moody M.D., Wehbie R.S., Kaplan A.H., Nantermet P.V., Klein C.A., Swanstrom R. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 1994;68:8017–8027. doi: 10.1128/jvi.68.12.8017-8027.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohl N.E., Emini E.A., Schleif W.A., Davis L.J., Heimbach J.C., Dixon R.A., Scolnick E.M., Sigal I.S. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacks T., Power M.D., Masiarz F.R., Luciw P.A., Barr P.J., Varmus H.E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 8.Arrigo S.J., Huffman K. Potent inhibition of human immunodeficiency virus type 1 (HIV-1) replication by inducible expression of HIV-1 PR multimers. J. Virol. 1995;69:5988–5994. doi: 10.1128/jvi.69.10.5988-5994.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill M.K., Hooker C.W., Harrich D., Crowe S.M., Mak J. Gag-Pol supplied in trans is efficiently packaged and supports viral function in human immunodeficiency virus type 1. J. Virol. 2001;75:6835–6840. doi: 10.1128/JVI.75.15.6835-6840.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krausslich H. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3213–3217. doi: 10.1073/pnas.88.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park J., Morrow C.D. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J. Virol. 1991;65:5111–5117. doi: 10.1128/jvi.65.9.5111-5117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose J.R., Babe L.M., Craik C.S. Defining the level of human immunodeficiency virus type 1 (HIV-1) protease activity required for HIV-1 particle maturation and infectivity. J. Virol. 1995;69:2751–2758. doi: 10.1128/jvi.69.5.2751-2758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shehu-Xhilaga M., Crowe S.M., Mak J. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J. Virol. 2001;75:1834–1841. doi: 10.1128/JVI.75.4.1834-1841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C.-T., Chou Y.-C., Chiang C.-C. Assembly and processing of human immunodeficiency virus Gag mutants containing a partial replacement of the matrix domain by the viral protease domain. J. Virol. 2000;74:3418–3422. doi: 10.1128/jvi.74.7.3418-3422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelick R.J., Chabot D.J., Rein A., Henderson L.E., Arthur L.O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J. Virol. 1993;67:4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berkowitz R.D., Ohagen A., Hoglund S., Goff S.P. Retroviral nucleocapsid domains mediate the specific recognition of genomic viral RNAs by chimeric Gag polyproteins during RNA packaging in vivo. J. Virol. 1995;69:6445–6456. doi: 10.1128/jvi.69.10.6445-6456.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimarelli A., Sandin S., Hoglund S., Luban J. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J. Virol. 2000;74:3046–3057. doi: 10.1128/jvi.74.7.3046-3057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poon D.T., Wu J., Aldovini A. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J. Virol. 1996;70:6607–6616. doi: 10.1128/jvi.70.10.6607-6616.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldovini A., Young R.A. Mutations of RNA and protein sequences involved in human immunodeficiency virus type 1 packaging result in production of noninfectious virus. J. Virol. 1990;64:1920–1926. doi: 10.1128/jvi.64.5.1920-1926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkowitz R.D., Luban J., Goff S.P. Specific binding of human immunodeficiency virus type 1 gag polyprotein and nucleocapsid protein to viral RNAs detected by RNA mobility shift assays. J. Virol. 1993;67:7190–7200. doi: 10.1128/jvi.67.12.7190-7200.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burniston M.T., Cimarelli A., Colgan J., Curtis S.P., Luban J. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J. Virol. 1999;73:8527–8540. doi: 10.1128/jvi.73.10.8527-8540.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell S., Rein A. In vitro assembly properties of human immunodeficiency virus type 1 Gag protein lacking the p6 domain. J. Virol. 1999;73:2270–2279. doi: 10.1128/jvi.73.3.2270-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell S., Vogt V.M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J. Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Rocquigny H., Gabus C., Vincent A., Fournie-Zaluski M.C., Roques B., Darlix J.L. Viral RNA annealing activities of human immunodeficiency virus type 1 nucleocapsid protein require only peptide domains outside the zinc fingers. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6472–6476. doi: 10.1073/pnas.89.14.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogue I.B., Hoppe A., Ono A. Quantitative fluorescence resonance energy transfer microscopy analysis of the human immunodeficiency virus type 1 Gag–Gag interaction: relative contributions of the CA and NC domains and membrane binding. J. Virol. 2009;83:7322–7336. doi: 10.1128/JVI.02545-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H., Dou J., Ding L., Spearman P. Myristoylation is required for human immunodeficiency virus type 1 Gag-Gag multimerization in mammalian cells. J. Virol. 2007;81:12899–12910. doi: 10.1128/JVI.01280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott D.E., Coren L.V., Chertova E.N., Gagliardi T.D., Nagashima K., Sowder R.C., 2nd, Poon D.T., Gorelick R.J. Elimination of protease activity restores efficient virion production to a human immunodeficiency virus type 1 nucleocapsid deletion mutant. J. Virol. 2003;77:5547–5556. doi: 10.1128/JVI.77.10.5547-5556.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S.-W., Noonan K., Aldovini A. Nucleocapsid–RNA interactions are essential to structural stability but not to assembly of retroviruses. J. Virol. 2004;78:716–723. doi: 10.1128/JVI.78.2.716-723.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ott D.E., Coren L.V., Shatzer T. The nucleocapsid region of human immunodeficiency virus type 1 Gag assists in the coordination of assembly and Gag processing: role for RNA-Gag binding in the early stages of assembly. J. Virol. 2009;83:7718–7727. doi: 10.1128/JVI.00099-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dussupt V., Javid M.P., Abou-Jaoudé G., Jadwin J.A., de La Cruz J., Nagashima K., Bouamr F. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPX<sub>n</sub>L late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 2009;5:e1000339. doi: 10.1371/journal.ppat.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dussupt V., Sette P., Bello N.F., Javid M.P., Nagashima K., Bouamr F. Basic residues in the nucleocapsid domain of Gag are critical for late events of HIV-1 budding. J. Virol. 2011;85:2304–2315. doi: 10.1128/JVI.01562-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng N., Pettit S., Tritch R., Ozturk D., Rayner M., Swanstrom R., Erickson-Viitanen S. Determinants of the human immunodeficiency virus type 1 p15NC–RNA interaction that affect enhanced cleavage by the viral protease. J. Virol. 1997;71:5723–5732. doi: 10.1128/jvi.71.8.5723-5732.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Y.-Y., Wang S.-M., Huang K.-J., Chiang C.-C., Wang C.-T. Placement of leucine zipper motifs at the carboxyl terminus of HIV-1 protease significantly reduces virion production. PLoS One. 2012;7:e32845. doi: 10.1371/journal.pone.0032845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C.T., Zhang Y., McDermott J., Barklis E. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J. Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Page K.A., Landau N.R., Littman D.R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 1990;64:5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C.T., Barklis E. Assembly, processing, and infectivity of human immunodeficiency virus type 1 Gag mutants. J. Virol. 1993;67:4264–4273. doi: 10.1128/jvi.67.7.4264-4273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S.-W., Aldovini A. RNA incorporation is critical for retroviral particle integrity after cell membrane assembly of Gag complexes. J. Virol. 2002;76:11853–11865. doi: 10.1128/JVI.76.23.11853-11865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babé L.M., Rosé J., Craik C.S. Trans-dominant inhibitory human immunodeficiency virus type 1 protease monomers prevent protease activation and virion maturation. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10069–10073. doi: 10.1073/pnas.92.22.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott D.E., Coren L.V., Gagliardi T.D. Redundant roles for nucleocapsid and matrix RNA-binding sequences in human immunodeficiency virus type 1 assembly. J. Virol. 2005;79:13839–13847. doi: 10.1128/JVI.79.22.13839-13847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfadhli A., Still A., Barklis E. Analysis of human immunodeficiency virus type 1 matrix binding to membranes and nucleic acids. J. Virol. 2009;83:12196–12203. doi: 10.1128/JVI.01197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jewell N.A., Mansky L.M. In the beginning: genome recognition, RNA encapsidation and the initiation of complex retrovirus assembly. J. Gen. Virol. 2000;81:1889–1899. doi: 10.1099/0022-1317-81-8-1889. [DOI] [PubMed] [Google Scholar]
- 42.Chukkapalli V., Oh S.J., Ono A. Opposing mechanisms involving RNA and lipids regulate HIV-1 Gag membrane binding through the highly basic region of the matrix domain. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1600–1605. doi: 10.1073/pnas.0908661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang Y.-F., Wang S.-M., Huang K.-J., Wang C.-T. Mutations in capsid major homology region affect assembly and membrane affinity of HIV-1 Gag. J. Mol. Biol. 2007;370:585–597. doi: 10.1016/j.jmb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Chang C.-Y., Chang Y.-F., Wang S.-M., Tseng Y.-T., Huang K.-J., Wang C.-T. HIV-1 matrix protein repositioning in nucleocapsid region fails to confer virus-like particle assembly. Virology. 2008;378:97–104. doi: 10.1016/j.virol.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kempf N., Postupalenko V., Bora S., Didier P., Arntz Y., de Rocquigny H., Mély Y. The HIV-1 nucleocapsid protein recruits negatively charged lipids to ensure its optimal binding to lipid membranes. J. Virol. 2015;89:1756–1767. doi: 10.1128/JVI.02931-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou W., Parent L.J., Wills J.W., Resh M.D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan X., Yu X., Lee T.H., Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J. Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ono A., Freed E.O. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spearman P., Wang J.J., Vander Heyden N., Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paillart J.C., Gottlinger H.G. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of gag membrane targeting. J. Virol. 1999;73:2604–2612. doi: 10.1128/jvi.73.4.2604-2612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spearman P., Horton R., Ratner L., Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W., Resh M.D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hermida-Matsumoto L., Resh M.D. Human immunodeficiency virus type 1 protease triggers a myristoyl switch that modulates membrane binding of Pr55 gag and p17MA. J. Virol. 1999;73:1902–1908. doi: 10.1128/jvi.73.3.1902-1908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang C., Loeliger E., Luncsford P., Kinde I., Beckett D., Summers M.F. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. U.S.A. 2004;101:517–522. doi: 10.1073/pnas.0305665101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perez-Caballero D., Hatziioannou T., Martin-Serrano J., Bieniasz P.D. Human immunodeficiency virus type 1 matrix inhibits and confers cooperativity on gag precursor-membrane interactions. J. Virol. 2004;78:9560–9563. doi: 10.1128/JVI.78.17.9560-9563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


