Abstract
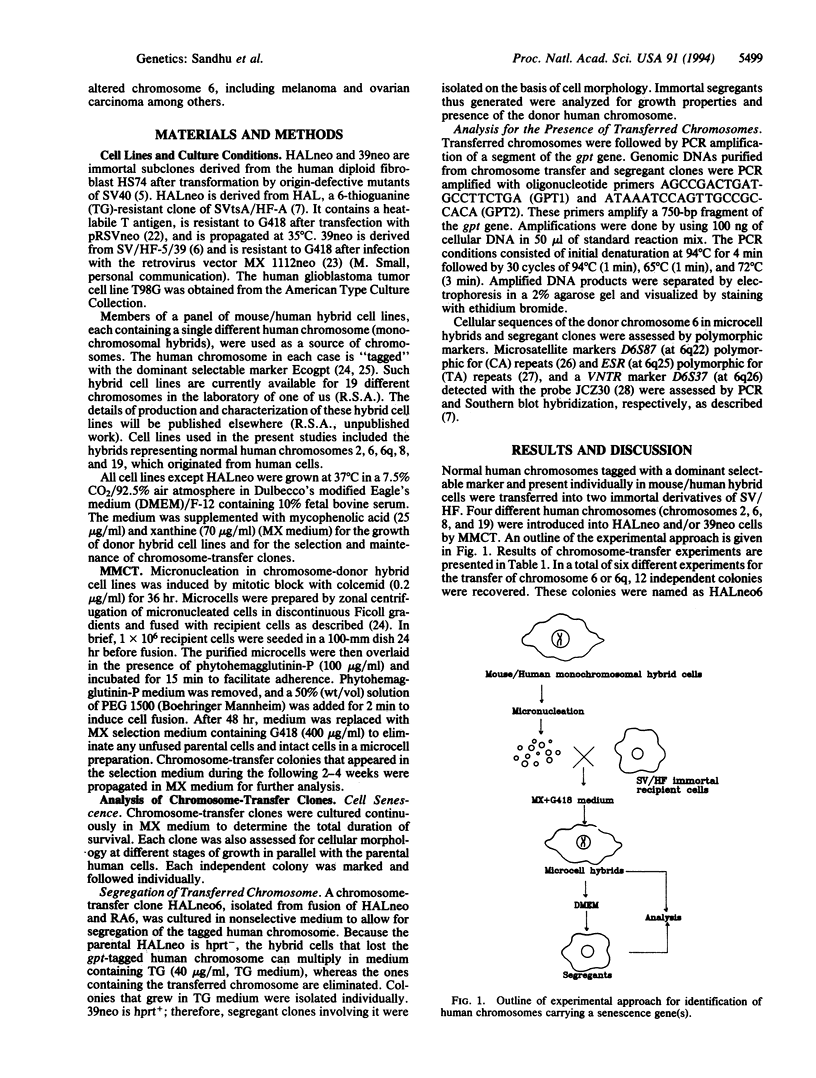
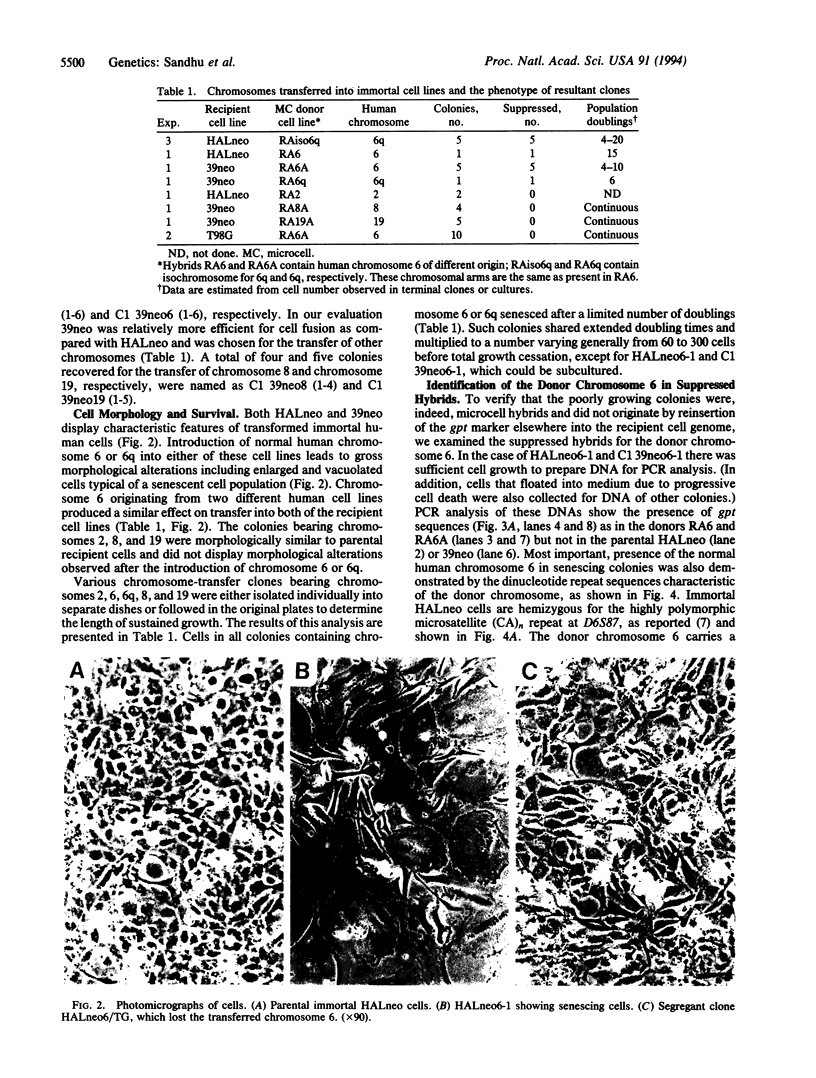
In these studies we show that introduction of a normal human chromosome 6 or 6q can suppress the immortal phenotype of simian virus 40-transformed human fibroblasts (SV/HF). Normal human fibroblasts have a limited life span in culture. Immortal clones of SV/HF displayed nonrandom rearrangements in chromosome 6. Single human chromosomes present in mouse/human monochromosomal hybrids were introduced into SV/HF via microcell fusion and maintained by selection for a dominant selectable marker gpt, previously integrated into the human chromosome. Clones of SV/HF cells bearing chromosome 6 displayed limited potential for cell division and morphological characteristics of senescent cells. The loss of chromosome 6 from the suppressed clones correlated with the reappearance of immortal clones. Introduced chromosome 6 in the senescing cells was distinguished from those of parental cells by the analysis for DNA sequences specific for the donor chromosome. Our results further show that suppression of immortal phenotype in SV/HF is specific to chromosome 6. Introduction of individual human chromosomes 2, 8, or 19 did not impart cellular senescence in SV/HF. In addition, introduction of chromosome 6 into human glioblastoma cells did not lead to senescence. Based upon these results we propose that at least one of the genes (SEN6) for cellular senescence in human fibroblasts is present on the long arm of chromosome 6.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Athwal R. S., Smarsh M., Searle B. M., Deo S. S. Integration of a dominant selectable marker into human chromosomes and transfer of marked chromosomes to mouse cells by microcell fusion. Somat Cell Mol Genet. 1985 Mar;11(2):177–187. doi: 10.1007/BF01534706. [DOI] [PubMed] [Google Scholar]
- Church S. L., Grant J. W., Ridnour L. A., Oberley L. W., Swanson P. E., Meltzer P. S., Trent J. M. Increased manganese superoxide dismutase expression suppresses the malignant phenotype of human melanoma cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3113–3117. doi: 10.1073/pnas.90.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Goyette M. C., Cho K., Fasching C. L., Levy D. B., Kinzler K. W., Paraskeva C., Vogelstein B., Stanbridge E. J. Progression of colorectal cancer is associated with multiple tumor suppressor gene defects but inhibition of tumorigenicity is accomplished by correction of any single defect via chromosome transfer. Mol Cell Biol. 1992 Mar;12(3):1387–1395. doi: 10.1128/mcb.12.3.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X. Y., Meltzer P. S., Cao J., Trent J. M. Rapid generation of region-specific genomic clones by chromosome microdissection: isolation of DNA from a region frequently deleted in malignant melanoma. Genomics. 1992 Nov;14(3):680–684. doi: 10.1016/s0888-7543(05)80168-5. [DOI] [PubMed] [Google Scholar]
- Gyuris J., Golemis E., Chertkov H., Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993 Nov 19;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hoffschir F., Ricoul M., Lemieux N., Estrade S., Cassingena R., Dutrillaux B. Jumping translocations originate clonal rearrangements in SV40-transformed human fibroblasts. Int J Cancer. 1992 Aug 19;52(1):130–136. doi: 10.1002/ijc.2910520123. [DOI] [PubMed] [Google Scholar]
- Hubbard-Smith K., Patsalis P., Pardinas J. R., Jha K. K., Henderson A. S., Ozer H. L. Altered chromosome 6 in immortal human fibroblasts. Mol Cell Biol. 1992 May;12(5):2273–2281. doi: 10.1128/mcb.12.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huschtscha L. I., Holliday R. Limited and unlimited growth of SV40-transformed cells from human diploid MRC-5 fibroblasts. J Cell Sci. 1983 Sep;63:77–99. doi: 10.1242/jcs.63.1.77. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990 Jun 1;1032(1):119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Loh W. E., Jr, Scrable H. J., Livanos E., Arboleda M. J., Cavenee W. K., Oshimura M., Weissman B. E. Human chromosome 11 contains two different growth suppressor genes for embryonal rhabdomyosarcoma. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1755–1759. doi: 10.1073/pnas.89.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Cowen J., Martin C., Leppert M., O'Connell P., Lathrop G. M., Lalouel J. M., White R. Isolation and mapping of a polymorphic DNA sequence (pJCZ30) on chromosome 6 [D6S37]. Nucleic Acids Res. 1988 May 25;16(10):4743–4743. doi: 10.1093/nar/16.10.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld D. S., Ripley S., Henderson A., Ozer H. L. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987 Aug;7(8):2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y., Weber J. L., Killary A. M., Ledbetter D. H., Smith J. R., Pereira-Smith O. M. Genetic analysis of indefinite division in human cells: evidence for a cell senescence-related gene(s) on human chromosome 4. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5635–5639. doi: 10.1073/pnas.88.13.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A., Ning Y., Venable S. F., Pereira-Smith O. M., Smith J. R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994 Mar;211(1):90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Smith J. R. Genetic analysis of indefinite division in human cells: identification of four complementation groups. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6042–6046. doi: 10.1073/pnas.85.16.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radna R. L., Caton Y., Jha K. K., Kaplan P., Li G., Traganos F., Ozer H. L. Growth of immortal simian virus 40 tsA-transformed human fibroblasts is temperature dependent. Mol Cell Biol. 1989 Jul;9(7):3093–3096. doi: 10.1128/mcb.9.7.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray F. A., Kraemer P. M. Frequent deletions in nine newly immortal human cell lines. Cancer Genet Cytogenet. 1992 Mar;59(1):39–44. doi: 10.1016/0165-4608(92)90155-2. [DOI] [PubMed] [Google Scholar]
- Sack G. H., Jr Human cell transformation by simian virus 40--a review. In Vitro. 1981 Jan;17(1):1–19. doi: 10.1007/BF02618025. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Sugawara O., Oshimura M., Koi M., Annab L. A., Barrett J. C. Induction of cellular senescence in immortalized cells by human chromosome 1. Science. 1990 Feb 9;247(4943):707–710. doi: 10.1126/science.2300822. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Wang X. W., Lin X., Klein C. B., Bhamra R. K., Lee Y. W., Costa M. A conserved region in human and Chinese hamster X chromosomes can induce cellular senescence of nickel-transformed Chinese hamster cell lines. Carcinogenesis. 1992 Apr;13(4):555–561. doi: 10.1093/carcin/13.4.555. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Kwitek A. E., May P. E., Killary A. M. Dinucleotide repeat polymorphism at the D4S174 locus. Nucleic Acids Res. 1990 Aug 11;18(15):4636–4636. [PMC free article] [PubMed] [Google Scholar]
- Weissman B. E., Saxon P. J., Pasquale S. R., Jones G. R., Geiser A. G., Stanbridge E. J. Introduction of a normal human chromosome 11 into a Wilms' tumor cell line controls its tumorigenic expression. Science. 1987 Apr 10;236(4798):175–180. doi: 10.1126/science.3031816. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Senno L., Aguiari G. L., Piva R. Dinucleotide repeat polymorphism in the human estrogen receptor (ESR) gene. Hum Mol Genet. 1992 Aug;1(5):354–354. doi: 10.1093/hmg/1.5.354. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]