Abstract
AIM: To investigate the reduction in hepatitis B virus (HBV) covalently closed-circular DNA (cccDNA) with entecavir (ETV) or lamivudine (LAM).
METHODS: This analysis included patients who had participated in the randomized Phase III study ETV-022 comparing ETV vs LAM in nucleos(t)ide-naive, HBeAg-positive patients. Patients received ETV (0.5 mg daily) or LAM (100 mg daily) for a minimum of 52 wk. Patients were eligible to participate in this sub-study if they had paired biopsies at baseline and week 48 with evaluable measurements for hepatic HBV cccDNA and total hepatic HBV DNA. The main objective was to compare changes in hepatic HBV cccDNA and total hepatic HBV DNA at week 48 of ETV or LAM treatment, which was a secondary endpoint of study ETV-022. Additional post hoc analyses included linear regression analyses to assess associations of baseline levels and on-treatment changes of cccDNA with other baseline factors [sex, age, serum HBV DNA, alanine aminotransferase (ALT), Knodell necroinflammatory score, Ishak fibrosis score, total hepatic HBV DNA, and HBV genotype], or on-treatment factors (changes from baseline at week 48 in serum HBV DNA, ALT, Knodell necroinflammatory score, Ishak fibrosis score, total hepatic HBV DNA, and HBeAg loss at week 48).
RESULTS: Overall, 305 patients (ETV = 159; LAM = 146) of ETV-022 had paired baseline and week 48 liver biopsies with evaluable measurements for hepatic HBV cccDNA and total hepatic HBV DNA, and were included in this analysis. Baseline demographics and disease characteristics were comparable between the two arms. After 48 wk, ETV resulted in significantly greater reductions in hepatic HBV cccDNA [-0.9 log10 copies/human genome equivalent (HGEq) vs -0.7 log10 copies/HGEq; P = 0.0033] and total hepatic DNA levels (-2.1 log10 copies/HGEq vs -1.6 log10 copies/HGEq; P < 0.0001) than LAM. Virologic, biochemical, and histologic response rates at week 48 were also greater with ETV than with LAM. Baseline HBV cccDNA levels were positively associated with baseline levels of serum HBV DNA and total hepatic HBV DNA, and negatively associated with HBV genotype F. On-treatment changes in HBV cccDNA levels were negatively associated with baseline levels of serum HBV DNA and baseline ALT, and were positively associated with on-treatment changes in the levels of serum HBV DNA, total hepatic HBV DNA levels, and ALT, change in Knodell necroinflammatory score, and HBeAg loss.
CONCLUSION: Forty-eight weeks of ETV resulted in greater reductions in cccDNA and total hepatic HBV DNA than LAM, but long-term therapy may be needed for cccDNA elimination.
Keywords: Hepatitis B virus, Nucleos(t)ide analog therapy, Intrahepatic hepatitis B virus DNA, Antiviral suppression, Virologic cure
Core tip: In chronic hepatitis B, persistence of hepatitis B virus (HBV) infection is due to an intrahepatic pool of stable, HBV covalently closed-circular DNA (cccDNA), whose elimination is a limiting factor in anti-HBV treatment. This study shows that 48 wk of treatment with entecavir resulted in a greater reduction of hepatic HBV cccDNA and total hepatic HBV DNA than lamivudine. However, cccDNA was still detectable in most biopsies.
INTRODUCTION
Worldwide, chronic hepatitis B (CHB) is the leading cause of cirrhosis and hepatocellular carcinoma (HCC), with around 50% of all HCC cases attributable to the hepatitis B virus (HBV)[1]. Persistence of HBV infection in CHB is due to a reservoir of covalently closed-circular HBV DNA (cccDNA) within the nuclei of infected hepatocytes, which serves as template for viral transcription[2-5]. Low levels of cccDNA can persist even after clinical resolution of the infection, thereby allowing HBV reactivation after hepatitis B surface antigen (HBsAg) clearance[6,7]. Although a direct causal relationship has not been shown, hepatic HBV cccDNA and chromosomal HBV integration-together with other factors such as immune-mediated liver inflammation-may also be associated with HCC development[8-10]. Reduction or elimination of cccDNA with anti-HBV therapy may provide important clinical benefits such as lower risks of HBV reactivation after seroclearance or HCC-associated liver resection or transplantation[11,12], as well as a potential decrease in the risk of HCC development[13-16].
Antiviral therapy with nucleos(t)ide analogs inhibits HBV DNA polymerase, thereby blocking synthesis of serum HBV DNA, but does not directly target cccDNA[17]. However, there is some evidence from small studies suggesting that cccDNA can be reduced with nucleos(t)ide analog treatment[7,15,18], but larger studies are needed to confirm these findings.
Entecavir (ETV) is a potent antiviral nucleoside analog that demonstrated superior efficacy to lamivudine (LAM) in virologic, biochemical, and histologic responses[19,20]. During long-term use, ETV maintained high rates of virologic suppression and a low rate of resistance with a favorable safety profile[21-24]. Long-term treatment with ETV has also been shown to reduce the risk of HBV-related HCC[25-29].
Here, we describe the results of an analysis of hepatic HBV cccDNA and total hepatic HBV DNA reductions in a large subgroup of nucleos(t)ide-naive hepatitis B e antigen-positive [HBeAg(+)] patients with paired liver biopsies before and after 48 wk of treatment with ETV or LAM. Patients included in this analysis represent a subset of a randomized sample of patients included in the ETV Phase III study ETV-022, which assessed the efficacy of ETV vs LAM for virologic, biochemical, serologic, and histologic outcomes.
MATERIALS AND METHODS
Study design, patients, and antiviral treatment
This report describes the results of a secondary endpoint analysis and an exploratory post hoc analysis of the Phase III trial ETV-022. ETV-022 was a multi-center, double-blind, randomized, comparative trial of ETV (0.5 mg daily) vs LAM (100 mg daily) in nucleos(t)ide-naive, HBeAg(+) patients, the primary results of which have been previously described[20]. In ETV-022, patients received a minimum of 52 wk of treatment. At week 52, patients who achieved a protocol-defined response [HBV DNA < 0.7 mEq/mL by branched-chain DNA assay (about 0.7 × 106 copies/mL) and HBeAg loss] or a virologic non-response at week 48 discontinued study therapy; virologic responders (HBV DNA < 0.7 mEq/mL but without HBeAg loss) could continue blinded treatment for up to 44 additional weeks[20].
Patients who participated in study ETV-022 and had paired baseline and week 48 liver biopsies with evaluable measurements for hepatic HBV cccDNA and total hepatic HBV DNA were eligible for inclusion in this analysis. Therefore, patients included in this analysis represent a subset of the original randomized sample. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Analysis
The main objective of this analysis was to assess the mean changes from baseline in hepatic HBV cccDNA and total hepatic HBV DNA at week 48 of treatment, which were protocol-defined secondary endpoints in ETV-022. Furthermore, the following post hoc analyses were conducted for this sub-group of patients: proportion of patients at week 48 with HBV DNA < 50 IU/mL (approximately 300 copies/mL), alanine aminotransferase (ALT) normalization [ALT < 1.25 × upper limit of normal (ULN)], histologic improvement [≥ 2-point decrease in Knodell necroinflammatory score with no worsening (≥ 1-point increase from baseline) of Knodell fibrosis score], and with Ishak fibrosis score improvement (≥ 1-point decrease from baseline), as well as the mean changes from baseline at week 48 in serum HBV DNA, Knodell necroinflammatory score, and Ishak fibrosis score. In order to better understand how cccDNA relates to other measures, additional post hoc analyses involving linear regression analyses were performed to assess associations of baseline levels and on-treatment changes of cccDNA with other baseline or on-treatment factors.
Serum HBV DNA was measured using the COBAS® AMPLICOR polymerase chain reaction (PCR) assay [version 2.0, Roche; lower limit of quantification: 300 copies/mL (57 IU/mL)]. Total hepatic HBV DNA was extracted from frozen liver biopsy samples (MasterPure™ DNA Purification Kit, Epicentre Biotechnologies). Hepatic HBV cccDNA and total hepatic HBV DNA were quantified by real-time PCR (LightCycler®, Roche, Mannheim, Germany) as described elsewhere[30]. Copy numbers of hepatic HBV cccDNA and total hepatic HBV DNA per human genome equivalent (HGEq) were determined by normalizing samples to the cellular beta-globin gene (limit of detection: 0.002 copies/HGEq). Hepatic HBV cccDNA values < 0.002 copies/HGEq were replaced by a value of 0.0019 copies/HGEq, and hepatic HBV cccDNA values > 1000 copies/HGEq were replaced by a value of 1001 copies/HGEq. Beta-globin DNA was quantified using the LightCycler® Roche DNA control kit (Roche Diagnostic, Penzberg, Germany).
Statistical analysis
Differences (ETV vs LAM) in mean log10 changes in hepatic HBV cccDNA and total hepatic HBV DNA were estimated using linear regression adjusted for baseline levels. Binary endpoints are presented as non-completer = missing (NC = M) analyses. Associations of cccDNA with baseline factors (sex, age, serum HBV DNA, ALT, Knodell necroinflammatory score, Ishak fibrosis score, total hepatic HBV DNA, and HBV genotype), or on-treatment factors (changes from baseline at week 48 in serum HBV DNA, ALT, Knodell necroinflammatory score, Ishak fibrosis score, total hepatic HBV DNA, and HBeAg loss at week 48) were examined with linear regression analyses. HBV genotyping was conducted by Delft Diagnostic Laboratory, Delft, The Netherlands.
RESULTS
Study population
Overall, 709 patients were randomized and treated in study ETV-022; of these, 159 patients in the ETV arm and 146 patients in the LAM arm had paired baseline and week 48 liver biopsies with evaluable measurements for hepatic HBV cccDNA and total hepatic HBV DNA. Baseline demographics and disease characteristics were comparable between the two arms (Table 1).
Table 1.
Patient demographics and disease characteristics n (%)
| Baseline | ETV (n = 159) | LAM (n = 146) |
| Age, mean ± SE, yr (range) | 35 ± 1.1 (17-76) | 34 ± 1.1 (16-71) |
| Male | 116 (73) | 104 (71) |
| Race | ||
| Asian | 92 (58) | 87 (60) |
| White | 64 (40) | 53 (36) |
| HBV genotype | ||
| A | 45 (28) | 41 (28) |
| B | 28 (18) | 28 (19) |
| C | 44 (28) | 36 (25) |
| D | 18 (11) | 23 (16) |
| F | 12 (8) | 5 (3) |
| Mixed | 4 (3) | 3 (2) |
| Indeterminate | 7 (4) | 8 (5) |
| Missing | 1 (< 1) | 2 (1) |
| Serum HBV DNA, mean ± SE, log10 IU/mL | 9.52 ± 0.16 | 9.66 ± 0.16 |
| Hepatic HBV cccDNA, mean ± SE, log10 copies/HGEq | 1.90 ± 0.11 | 2.0 ± 0.11 |
| Hepatic HBV DNA, mean ± SE, log10 copies/HGEq | 0.3 ± 0.05 | 0.4 ± 0.04 |
| ALT (U/L) | ||
| mean ± SE | 134.9 (9.84) | 132.8 (10.07) |
| Median (range) | 96.0 (23.0-915.0) | 90.0 (27.0-715.0) |
| International normalized ratio | 1.1 (0.01) | 1.1 (0.01) |
| Albumin (g/dL) | 4.3 (0.04) | 4.2 (0.04) |
| Total bilirubin (mg/dL) | 0.8 (0.03) | 0.8 (0.03) |
| Prothrombin time (s) | 13.2 (0.16) | 13.3 (0.16) |
| Knodell necroinflammatory score, mean ± SE | 7.8 ± 0.23 | 7.5 ± 0.24 |
| Ishak fibrosis score, mean ± SE | 2.4 ± 0.11 | 2.1 ± 0.11 |
CccDNA: Covalently closed-circular DNA; ETV: Entecavir; LAM: Lamivudine; HGEq: Human genome equivalent.
Efficacy
ETV treatment for 48 wk resulted in significantly greater reductions in hepatic HBV cccDNA and total hepatic HBV DNA levels than LAM (Figure 1). The mean change from baseline in hepatic HBV cccDNA at week 48 was -0.9 log10 copies/HGEq for ETV and -0.7 log10 copies/HGEq for LAM [difference estimate (adjusted for baseline hepatic cccDNA level) -0.2 log10 copies/HGEq; 95%CI: -0.3, -0.1; P = 0.0033]. For total hepatic HBV DNA, the mean change from baseline at week 48 was -2.1 log10 copies/HGEq for ETV, and -1.6 log10 copies/HGEq for LAM [difference estimate (adjusted for baseline total hepatic HBV DNA level) -0.5 log10 copies/HGEq; 95%CI: -0.6, -0.3; P < 0.0001].
Figure 1.
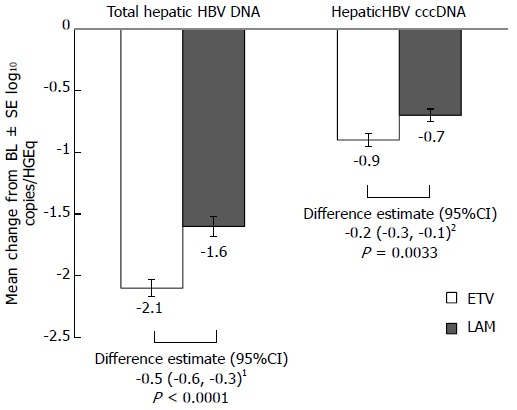
Change from baseline at week 48 in total hepatic hepatitis B virus DNA and hepatitis B virus covalently closed-circular DNA. 1Adjusted for baseline total hepatic HBV DNA level; 2Adjusted for baseline hepatic cccDNA level. BL: Baseline; CI: Confidence interval; HGEq: Human genome equivalent; SE: Standard error; CccDNA: Covalently closed-circular DNA; ETV: Entecavir; LAM: Lamivudin.
The proportions of patients with total hepatic HBV DNA < 0.002 copies/HGEq (limit of quantification) at week 48 were 6% (10/159) with ETV and 5% (8/146) with LAM; one patient in each arm had HBV cccDNA < 0.002 copies/HGEq at week 48.
Virologic, biochemical, and histologic response rates at week 48 were also greater with ETV than with LAM (Table 2 and Figure 2). The results for these endpoints in this cohort were comparable to those observed in the overall ETV-022 randomized samples[20].
Table 2.
Virologic and histologic efficacy at week 48 among treated patients with evaluable hepatic hepatitis B virus DNA pairs
| Week 48 efficacy (NC = M) | ETV (n = 159) | LAM (n = 146) |
| Serum HBV DNA, mean ± SE, change from baseline log10 IU/mL | -6.85 ± 0.16 | -5.30 ± 0.22 |
| Knodell necroinflammatory score, mean ± SE change from baseline | -3.4 ± 0.26 | -3.0 ± 0.29 |
| Ishak fibrosis score, mean ± SE change from baseline | -0.5 ± 0.08 | -0.3 ± 0.08 |
NC = M: Non-completer = missing; ETV: Entecavir; LAM: Lamivudine.
Figure 2.
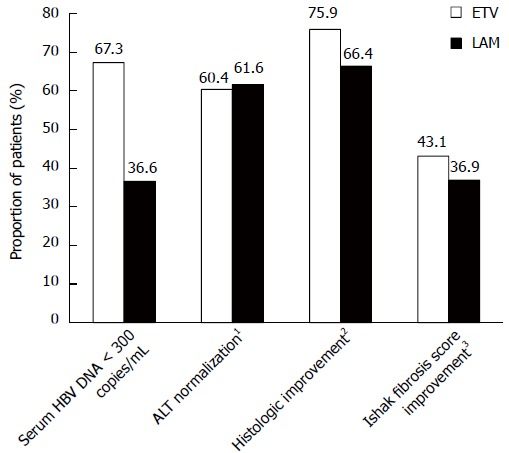
Virologic, biochemical, and histologic efficacy at week 48 among treated patients with evaluable hepatic hepatitis B virus DNA pairs. 1ALT < 1.25 × upper limit of normal; 2≥ 2-point decrease in Knodell necroinflammatory score with no worsening (≥ 1-point increase from baseline) of Knodell fibrosis score; 3≥ 1-point decrease in Ishak fibrosis score from baseline.
Association of HBV cccDNA with baseline and on-treatment factors
In univariate linear regression analyses, baseline HBV cccDNA was significantly associated with baseline serum HBV DNA, baseline total hepatic HBV DNA, and HBV genotype F (Table 3). For baseline serum HBV DNA and total hepatic HBV DNA, an increase by 1 log10 IU/mL was associated with an increase in baseline cccDNA of 0.11 log10 copies/HGEq. In patients with HBV genotype F, baseline cccDNA levels were lower by 0.41 log10 copies/HGEq compared with non-F genotypes. Baseline HBV cccDNA was not associated with any of the other baseline factors (sex, age, ALT, Knodell necroinflammatory score, Ishak fibrosis score, or HBV genotypes A, B, C, D, or “other”).
Table 3.
Significant associations of baseline factors with baseline hepatic hepatitis B virus covalently closed-circular DNA in univariate linear regression analysis
| Estimate1 | 95%CI | P value | |
| Baseline serum HBV DNA, per 1 log10 IU/mL | 0.11 | 0.08, 0.13 | < 0.0001 |
| Baseline total hepatic HBV DNA, per 1 log10 IU/mL | 0.11 | 0.07, 0.16 | < 0.0001 |
| HBV genotype F: Not F | -0.41 | -0.67, -0.15 | 0.0023 |
log10 copies/HGEq. HBV: Hepatitis B virus.
HBV cccDNA reduction at week 48 was also significantly associated with baseline levels of serum HBV DNA and ALT, and with on-treatment changes in serum HBV DNA, total hepatic HBV DNA, Knodell necroinflammatory score, ALT, and HBeAg loss (Table 4), whereas there was no association with on-treatment improvement in Ishak fibrosis score. The significance among the on-treatment changes confirms the collinearity expected among response measures.
Table 4.
Significant associations of baseline and on-treatment factors with hepatic hepatitis B virus cccDNA change from baseline at week 48 in univariate linear regression analysis (adjusted for treatment)
| Estimate1 | 95%CI | P value | ||
| Baseline | Serum HBV DNA, per 1 log10 IU/mL | -0.05 | -0.08, -0.01 | 0.0106 |
| ALT ≥ 2.6 × ULN : < 2.6 × ULN | -0.42 | -0.56, -0.28 | < 0.0001 | |
| On-treatment | Serum HBV DNA, per 1 log10 IU/mL change from baseline at week 48 | 0.11 | 0.08, 0.14 | < 0.0001 |
| Total hepatic HBV DNA, per 1 log10 IU/mL change from baseline at week 48 | 0.35 | 0.28, 0.42 | < 0.0001 | |
| Knodell necroinflammatory score, per 1 unit change from baseline at week 48 | 0.04 | 0.01, 0.06 | 0.0062 | |
| ALT, per 1 U/L change from baseline at week 48 | 1.51 × 10-3 | 0.98 × 10-3, 2.05 × 10-3 | < 0.0001 | |
| No HBeAg loss at week 48 : HBeAg loss at week 48 | -0.20 | -0.38, -0.02 | 0.0338 | |
log10 copies/HGEq. ULN: Upper limit of normal; HBV: Hepatitis B virus; HBeAg: Hepatitis B e antigen; ALT: Alanine aminotransferase.
For baseline serum HBV DNA, every increase by 1 log10 IU/mL was associated with an on-treatment cccDNA reduction of 0.05 log10 copies/HGEq, whereas baseline ALT ≥ 2.6 × ULN was associated with a cccDNA reduction of 0.42 log10 copies/HGEq.
For on-treatment changes in serum HBV DNA and total hepatic DNA, a decline by 1 log10 IU/mL was associated with a cccDNA reduction of 0.11 and 0.35 log10 copies/HGEq, respectively. Similar associations were seen for on-treatment changes in Knodell score (1 unit reduction associated with a cccDNA decline by 0.04 log10 copies/HGEq) and ALT (1 IU/mL reduction associated with a cccDNA decline by 1.51 × 10-3 log10 copies/HGEq). Finally, HBeAg loss by week 48 was associated with a cccDNA reduction of 0.20 log10 copies/HGEq.
DISCUSSION
In the present study, 48 wk of ETV treatment resulted in reductions of approximately 2 log10 copies/HGEq for hepatic HBV DNA, and approximately 1 log10 copies/HGEq for cccDNA. Both cccDNA and total hepatic HBV DNA reductions were significantly greater with ETV than with LAM. This finding can be explained by the greater potency of ETV vs LAM in suppressing viral replication. Nucleos(t)ide analogs inhibit HBV DNA polymerase, thereby reducing the levels of circulating virions carrying viral DNA that infect or re-infect hepatocytes; as a result, fewer copies of relaxed circular genomic HBV DNA are available that can be converted into cccDNA[13,14]. In addition, the greater treatment effect observed with ETV may also be a reflection of its higher barrier to resistance compared with LAM, which may contribute indirectly to cccDNA reduction by limiting the risk of virologic breakthrough in serum HBV DNA levels. In study ETV-022, 6 (2%) patients in the ETV group and 63 (18%) patients in the LAM group experienced virologic breakthrough during the first year of treatment; of these, none of the ETV-treated patients had evidence of emergent ETV resistance, whereas 45 (71%) of the LAM-treated patients had mutations in the YMDD motif[20]. Therefore, an antiviral agent that achieves greater suppression of HBV replication - through greater potency and a lower resistance rate - such as ETV, is expected to be more efficient in reducing cccDNA levels compared with an agent of lower potency and higher resistance rate such as LAM.
Despite substantial improvements in CHB management, HBV-associated HCC remains an important problem, as evidenced by increasing trends of HCC incidences recently reported in Western countries such as France, Australia, and the United States[31]. In clinical practice, surrogate markers for HCC risk are used that correlate with clinical outcomes and can be measured over short periods of time[14,32-34]. Among these, HBV cccDNA is one of the most important, as it is the stable template for all HBV transcripts, and is responsible for persistent infection of hepatocytes[14].
The effect of ETV vs other nucleos(t)ide analogs on HBV cccDNA levels has been assessed previously in a few small, single-center studies, which generally reported no differences in cccDNA reduction between ETV and other NUCs after 1 year. However, the numbers of patients in these studies may have been too small to see a significant difference[15,18,35]. For example, a sub-study of 39 patients participating in the Phase III studies ETV-022 and ETV-027[19,20] at a single center in Hong Kong reported no difference between ETV and LAM after 48 wk[15]; however, the treatment effects for virologic and serologic endpoints were also not consistent between this cohort and the overall Phase III study populations[15,19,20].
In studies assessing adefovir alone or combined with peg-interferon in HBeAg(+) patients, reductions of -1.63 to -2.69 log10 copies/cell for total hepatic HBV DNA and -0.80 to -1.03 log10 copies/cell for cccDNA were seen after 48 wk of therapy, with higher values achieved with the combined treatment[7,36].
In the present study, the reduction in cccDNA levels was considerably lower than the reduction in serum HBV DNA, an observation that has also been reported before[7,15,18,36,37], and which is probably related to the fact that the main, direct target of nucleos(t)ide analogs is the inhibition of HBV DNA polymerase, whereas the effect on cccDNA is indirect. However, levels of HBV cccDNA levels were positively associated with those of serum HBV DNA, consistent with previous reports showing a correlation between these two viral DNA species[7,30]. Baseline HBV cccDNA was also associated with HBV genotype F (in a negative fashion), but not with any of the other genotypes tested (although these results may need to be interpreted cautiously due to the relatively high number of statistical tests performed). Lower baseline cccDNA in HBV genotype F might be related to lower levels of serum HBV DNA; however there are currently no data on viral loads across HBV genotypes, including HBV genotype F. HBV genotype F is most prevalent in South America and the Arctic Circle; interestingly, it has been linked to a more aggressive course of liver disease and one of the highest (8 × higher) HCC risks compared with other genotypes[38].
The reduction in hepatic HBV cccDNA was greater among patients with higher baseline ALT and on-treatment improvements in Knodell necroinflammatory score and ALT. High baseline ALT is indicative of a high turnover of hepatocytes[39], and on-treatment ALT normalization and liver histology improvements are indicative of liver regeneration; these associations therefore suggest that cccDNA reduction also involves cytolytic destruction of infected hepatocytes, rather than just suppression of new cccDNA formation.
The reduction in HBV cccDNA was also greater in patients who achieved HBeAg loss at week 48. Similar observations were made in studies assessing the impact of adefovir alone or combined with peg-interferon on cccDNA levels, which showed that patients who underwent HBeAg or HBsAg seroconversion at week 48 had lower baseline levels or on-treatment changes of cccDNA levels than patients who did not achieve these endpoints[7,36,37]. Further studies are needed to assess whether cccDNA reduction with nucleos(t)ide analog therapy is associated with achievement of a durable serologic response.
A limitation of the exploratory analysis is that the patients included in this analysis were a subset of the originally randomized treatment arms of ETV-022, based on the logistic feasibility of performing cccDNA analysis on their biopsy material, and were therefore no longer balanced by stratification factors; however, baseline characteristics appeared to be balanced between ETV- and LAM-treated patients. A second limitation is the short duration of follow-up. Clearance of cccDNA shows considerably slower kinetics than serum HBV DNA clearance; therefore, a study with longer follow-up may show a greater reduction in cccDNA levels. Another limitation is that quantitative HBsAg (qHBsAg) levels, which reflect transcriptional activity of cccDNA[40], were not measured in this cohort. qHBsAg levels have been shown to correlate with intrahepatic cccDNA levels, and some studies described an association between cccDNA and qHBsAg reductions during nucleos(t)ide analog therapy[7,18]. Although this association has not been assessed in the present study, a separate sub-study of ETV-022 in patients with qHBsAg measurements through week 48 demonstrated that qHBsAg levels declined during 48 wk of ETV, in particular among patients who achieved seroclearance of HBsAg or HBeAg[41].
In conclusion, the results of this analysis, which was carried out as part of a large, multi-center, randomized, controlled trial, show that for patients with HBeAg(+) CHB, ETV resulted in a greater reduction in hepatic HBV cccDNA and total hepatic HBV DNA than LAM after 48 wk of treatment. Although cccDNA levels were reduced, they were still detectable after 48 wk of treatment in the majority of patients; this suggests that despite potent suppression of serum HBV DNA, short-term nucleos(t)ide analog therapy is not able to eliminate intrahepatic cccDNA. Further studies are needed to provide evidence on the long-term impact of nucleos(t)ide analog therapy on HBV cccDNA levels. In addition, the development of new CHB treatments, including new agents that directly degrade cccDNA or inhibit its formation[42,43], or combination therapies targeting multiple steps in the viral life cycle[14], might provide alternative treatment strategies with the potential of achieving a virologic cure of the infection, in which viral load, HBsAg, and cccDNA are durably suppressed[14].
ACKNOWLEDGMENTS
Writing assistance was provided by Isabelle Kaufmann of Articulate Science.
COMMENTS
Background
Covalently closed-circular DNA (cccDNA) is the stable genetic form of hepatitis B virus (HBV) that serves as the template for viral transcription. It can persist within infected hepatocytes even after clinical resolution of the infection, thereby allowing HBV reactivation, and it may also be associated with the development of HBV-related hepatocellular carcinoma (HCC). Therefore, reduction or elimination of cccDNA may provide important clinical benefits. However, antiviral therapy with nucleos(t)ide analogs blocks the synthesis of serum HBV DNA but does not directly target cccDNA, making HBV cccDNA clearance a particular challenge in antiviral therapy.
Research frontiers
There is some evidence from small studies suggesting that HBV cccDNA can be reduced with nucleos(t)ide analog treatment, but larger studies are needed.
Innovations and breakthroughs
This analysis compared the impact of entecavir (ETV), a currently recommended first-line antiviral agent, vs lamivudine (LAM) on levels of HBV cccDNA and total intrahepatic HBV DNA. The analysis was carried out as part of a large, multi-center, randomized, controlled trial. This report also identified predictors of cccDNA baseline levels and on-treatment reduction.
Applications
The study shows that in HBeAg(+) patients, ETV resulted in a greater reduction in HBV cccDNA and total hepatic HBV DNA than LAM after 48 wk of treatment. Although cccDNA levels were reduced, they were still detectable after 48 wk of treatment in the vast majority of patients. Thus, despite potent suppression of serum HBV DNA, short-term nucleos(t)ide analog therapy is not able to eliminate intrahepatic cccDNA. Further studies are needed to provide evidence on the long-term impact of nucleos(t)ide analog therapy on HBV cccDNA levels. In addition, the development of new chronic hepatitis B treatments that directly target cccDNA might provide alternative treatment strategies with the potential of achieving a virologic cure of the infection, in which viral load, hepatitis B surface antigen, and cccDNA are durably suppressed.
Terminology
cccDNA is short for covalently closed-circular hepatitis B virus DNA. It is generated by filling in the gap in the relaxed, partially double-stranded viral DNA by the cellular replicative machinery, followed by ligation of both strand extremities and supercoiling of the viral DNA.
Peer-review
The present study opens a window to the possibility that cccDNA and hence the virus, can be eradicated. It is currently not known if the decrease in cccDNA has an influence on HCC development. As the authors stated in their conclusions, additional studies assessing long-term treatment and post-treatment outcomes are necessary.
Footnotes
Supported by Bristol-Myers Squibb.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 9, 2014
First decision: October 14, 2014
Article in press: January 16, 2015
P- Reviewer: Frider B, Lesmana CRA, Sunbul M S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Nguyen VT, Law MG, Dore GJ. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J Viral Hepat. 2009;16:453–463. doi: 10.1111/j.1365-2893.2009.01117.x. [DOI] [PubMed] [Google Scholar]
- 2.Caruntu FA, Molagic V. CccDNA persistence during natural evolution of chronic VHB infection. Rom J Gastroenterol. 2005;14:373–377. [PubMed] [Google Scholar]
- 3.Zoulim F. New insight on hepatitis B virus persistence from the study of intrahepatic viral cccDNA. J Hepatol. 2005;42:302–308. doi: 10.1016/j.jhep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Laras A, Koskinas J, Dimou E, Kostamena A, Hadziyannis SJ. Intrahepatic levels and replicative activity of covalently closed circular hepatitis B virus DNA in chronically infected patients. Hepatology. 2006;44:694–702. doi: 10.1002/hep.21299. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311–322. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen MF, Wong DK, Sablon E, Tse E, Ng IO, Yuan HJ, Siu CW, Sander TJ, Bourne EJ, Hall JG, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology. 2004;39:1694–1701. doi: 10.1002/hep.20240. [DOI] [PubMed] [Google Scholar]
- 7.Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Tan YJ. Hepatitis B virus infection and the risk of hepatocellular carcinoma. World J Gastroenterol. 2011;17:4853–4857. doi: 10.3748/wjg.v17.i44.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuzaki T, Tatsuki I, Otani M, Akiyama M, Ozawa E, Miuma S, Miyaaki H, Taura N, Hayashi T, Okudaira S, et al. Significance of hepatitis B virus core-related antigen and covalently closed circular DNA levels as markers of hepatitis B virus re-infection after liver transplantation. J Gastroenterol Hepatol. 2013;28:1217–1222. doi: 10.1111/jgh.12182. [DOI] [PubMed] [Google Scholar]
- 12.Hosaka T, Suzuki F, Kobayashi M, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Akuta N, Suzuki Y, Saitoh S, et al. HBcrAg is a predictor of post-treatment recurrence of hepatocellular carcinoma during antiviral therapy. Liver Int. 2010;30:1461–1470. doi: 10.1111/j.1478-3231.2010.02344.x. [DOI] [PubMed] [Google Scholar]
- 13.Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res. 2004;64:1–15. doi: 10.1016/j.antiviral.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Block TM, Gish R, Guo H, Mehta A, Cuconati A, London WT, Guo JT. Chronic hepatitis B: what should be the goal for new therapies? Antiviral Res. 2013;98:27–34. doi: 10.1016/j.antiviral.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong DK, Yuen MF, Ngai VW, Fung J, Lai CL. One-year entecavir or lamivudine therapy results in reduction of hepatitis B virus intrahepatic covalently closed circular DNA levels. Antivir Ther. 2006;11:909–916. [PubMed] [Google Scholar]
- 16.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 17.Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong DK, Seto WK, Fung J, Ip P, Huang FY, Lai CL, Yuen MF. Reduction of hepatitis B surface antigen and covalently closed circular DNA by nucleos(t)ide analogues of different potency. Clin Gastroenterol Hepatol. 2013;11:1004–1010.e1. doi: 10.1016/j.cgh.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 20.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 21.Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422–430. doi: 10.1002/hep.23327. [DOI] [PubMed] [Google Scholar]
- 22.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 23.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 24.Manns MP, Akarca US, Chang TT, Sievert W, Yoon SK, Tsai N, Min A, Pangerl A, Beebe S, Yu M, Wongcharatrawee S. Long-term safety and tolerability of entecavir in patients with chronic hepatitis B in the rollover study ETV-901. Expert Opin Drug Saf. 2012;11:361–368. doi: 10.1517/14740338.2012.653340. [DOI] [PubMed] [Google Scholar]
- 25.Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–1547. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 26.Zoutendijk R, Reijnders JG, Zoulim F, Brown A, Mutimer DJ, Deterding K, Hofmann WP, Petersen J, Fasano M, Buti M, et al. Virological response to entecavir is associated with a better clinical outcome in chronic hepatitis B patients with cirrhosis. Gut. 2013;62:760–765. doi: 10.1136/gutjnl-2012-302024. [DOI] [PubMed] [Google Scholar]
- 27.Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98–107. doi: 10.1002/hep.26180. [DOI] [PubMed] [Google Scholar]
- 28.Yang SC, Lee CM, Hu TH, Wang JH, Lu SN, Hung CH, Changchien CS, Chen CH. Virological response to entecavir reduces the risk of liver disease progression in nucleos(t)ide analogue-experienced HBV-infected patients with prior resistant mutants. J Antimicrob Chemother. 2013;68:2154–2163. doi: 10.1093/jac/dkt147. [DOI] [PubMed] [Google Scholar]
- 29.Kumada T, Toyoda H, Tada T, Kiriyama S, Tanikawa M, Hisanaga Y, Kanamori A, Niinomi T, Yasuda S, Andou Y, et al. Effect of nucleos(t)ide analogue therapy on hepatocarcinogenesis in chronic hepatitis B patients: a propensity score analysis. J Hepatol. 2013;58:427–433. doi: 10.1016/j.jhep.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Sung JJ, Wong ML, Bowden S, Liew CT, Hui AY, Wong VW, Leung NW, Locarnini S, Chan HL. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology. 2005;128:1890–1897. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.McClune AC, Tong MJ. Chronic hepatitis B and hepatocellular carcinoma. Clin Liver Dis. 2010;14:461–476. doi: 10.1016/j.cld.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 32.European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 34.Liaw YF, Kao JH, Piratvisuth T, Chan HLY, Chien RN, Liu CJ, Gane E, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: A 2012 update. Hepatol Int. 2012;6:531–561 [DOI 10.1007/s12072-012-9365-4]. doi: 10.1007/s12072-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 35.Cheng PN, Liu WC, Tsai HW, Wu IC, Chang TT, Young KC. Association of intrahepatic cccDNA reduction with the improvement of liver histology in chronic hepatitis B patients receiving oral antiviral agents. J Med Virol. 2011;83:602–607. doi: 10.1002/jmv.22014. [DOI] [PubMed] [Google Scholar]
- 36.Takkenberg B, Terpstra V, Zaaijer H, Weegink C, Dijkgraaf M, Jansen P, Beld M, Reesink H. Intrahepatic response markers in chronic hepatitis B patients treated with peginterferon alpha-2a and adefovir. J Gastroenterol Hepatol. 2011;26:1527–1535. doi: 10.1111/j.1440-1746.2011.06766.x. [DOI] [PubMed] [Google Scholar]
- 37.Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler F, Zankel M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 38.Marciano S, Galdame OA, Gadano AC. HBV genotype F: natural history and treatment. Antivir Ther. 2013;18:485–488. doi: 10.3851/IMP2604. [DOI] [PubMed] [Google Scholar]
- 39.Senior JR. Alanine aminotransferase: a clinical and regulatory tool for detecting liver injury-past, present, and future. Clin Pharmacol Ther. 2012;92:332–339. doi: 10.1038/clpt.2012.108. [DOI] [PubMed] [Google Scholar]
- 40.Chan HL, Thompson A, Martinot-Peignoux M, Piratvisuth T, Cornberg M, Brunetto MR, Tillmann HL, Kao JH, Jia JD, Wedemeyer H, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55:1121–1131. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Gish RG, Chang TT, Lai CL, de Man RA, Gadano A, Llamoso C, Tang H. Quantitative hepatitis B surface antigen analysis in hepatitis B e antigen-positive nucleoside-naive patients treated with entecavir. Antivir Ther. 2013;18:691–698. doi: 10.3851/IMP2559. [DOI] [PubMed] [Google Scholar]
- 42.Cai D, Mills C, Yu W, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu X, Guo JT, Block TM, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucifora J, Xia Y, Reisinger F, Zhang K, Stadler D, Cheng X, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343:1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]


