Solid organ transplant recipients frequently suffer diarrhea. In 534 admissions over an 18-month study period 30.1% of community-onset diarrhea was found to be infectious, consisting predominantly of Clostridium difficile, norovirus, and cytomegalovirus, whereas other causes were rare.
Keywords: solid organ transplant, diarrhea, Clostridium difficile, cytomegalovirus, norovirus
Abstract
Background. Although diarrhea is a frequent complaint among solid organ transplant recipients, the contribution of infectious etiologies remains incompletely defined. We sought to define the etiologies of diarrhea and the yields of testing at our institution.
Methods. We performed a retrospective analysis over an 18-month period of hospitalized solid organ transplant recipients. We stratified diarrhea by community onset vs hospital onset of diarrhea.
Results. We identified 422 admissions (representing 314 unique patients) with community-onset diarrhea, and 112 admissions (representing 102 unique patients) with hospital-onset diarrhea. The majority of community- and hospital-onset diarrheal episodes had no identified etiology (60.9% and 75.9%, respectively; P = .03), yet were also self-limited (91% and 91%, respectively; P = .894). Thereafter, the most frequently encountered infectious etiologies were Clostridium difficile infection (13.3% and 11.8%, respectively), norovirus enteritis (8.2% and 3%), cytomegalovirus disease or colitis (6.3% and 2.7%), and bacterial enterocolitis (0.9% and 0%) (P = .03). In aggregate, these entities represented 93.7% and 90.5% of the identified infectious etiologies, respectively. Protozoan causes were rarely seen. Coinfection, or the simultaneous detection of ≥2 pathogens, occurred in 8 (1.9%) and 2 (1.8%) community- and hospital-onset diarrheal admissions, respectively (P = .99).
Conclusions. In solid organ transplant recipients who presented at our institution with diarrhea, approximately one-third had infectious etiologies identified, consisting predominantly of C. difficile, norovirus, cytomegalovirus, and bacterial enterocolitis. Other infectious etiologies were rare.
(See the Editorial Commentary by Polage on pages 738–40.)
Diarrhea in solid organ transplant (SOT) recipients has been estimated to occur in 22%–52% of patients [1–4]. At one center, gastrointestinal complaints accounted for 31% of presentations to their emergency department with an associated risk of hospitalization [5]. Furthermore, posttransplant diarrhea is associated with allograft loss and increased mortality [2, 6, 7]. The impact of diarrhea on immunosuppressive therapy may, in part, explain this association. Gastrointestinal distress is a recognized side effect of mycophenolate mofetil (MMF) and mycophenolic acid (MPA), which increase tacrolimus exposure [8]. Resulting reductions in immunosuppression increase the risk of acute rejection and ultimately graft failure [9–11].
Despite its significance, the epidemiology of diarrhea in transplant recipients is poorly defined. Previously, infectious causes of diarrhea in general accounted for 13%–19% of diarrheal episodes [2, 6]. The Diarrhea Diagnosis Aid and Clinical Treatment study prospectively investigated the etiology and management of diarrhea in renal transplant recipients and identified a specific infectious etiology in 30 of 108 (28%) patients, with Campylobacter jejuni enteritis and cytomegalovirus (CMV) colitis being the most common [1]. Later studies have found that Clostridium difficile colitis is frequent, but its incidence relative to other pathogens and noninfectious etiologies has not been compared [12–15].
Recommendations for the diagnostic evaluation and management of posttransplant diarrhea are based largely on expert opinion and generally include initial stool culture, stool C. difficile assessment, and blood CMV quantitative viral load, if necessary to be followed by consideration of empiric reduction in immunosuppression, and thereafter colonoscopy [1, 16–18]. Recommendations for additional testing are quite variable and may include fecal testing for leukocytes (or lactoferrin), ova and parasites, Cystoisospora (Isospora) and Cyclospora assessments, Giardia and Cryptosporidium antigen screen or enzyme immunoassay (EIA), and norovirus detection by polymerase chain reaction (PCR). SOT recipients are at increased risk of atypical and typical infections leading to diarrhea, but it is unclear that this immunocompromised state necessarily translates to an increased incidence of all possible etiologies of diarrhea, especially with consideration of endemic and geographic exposures. There may be more optimized testing strategies, for which evidence is required. We hypothesized that the majority of SOT recipients hospitalized with diarrheal illness were attributable to a few etiologies, predominantly C. difficile and norovirus, and that the yield for other studies is low.
MATERIALS AND METHODS
Study Design
After approval by the Northwestern University Institutional Review Board, a Structured Query Language report was created to identify hospitalized SOT recipients at Northwestern Memorial Hospital in Chicago, Illinois, with complaints of diarrhea during the period spanning from 1 March 2012 to 30 September 2013. All SOT recipients age ≥18 years were included in the study. Exclusion criteria included history of stem cell transplant, human immunodeficiency virus infection, and the absence of immunosuppressive medications in the context of a nonfunctioning allograft. Admissions were identified by International Classification of Diseases, Ninth Revision (ICD-9) codes, and diarrhea was identified by relevant ICD-9 codes, Current Procedural Terminology (CPT) codes, or laboratory analysis (Supplementary Data). Subjects who met both criteria were identified for chart review, upon which inclusion/exclusion criteria were reaffirmed. The primary endpoint was to define the etiology of diarrheal illness in the study population, with a secondary endpoint of describing the rates and yields of analyses for diarrhea.
Upon chart review, diarrheal illness was determined by history, nursing documentation, or physician assessments as per the Infectious Diseases Society of America (IDSA) practice guidelines' definition [18]; ICD-9, CPT, and/or performance of laboratory test(s) alone were insufficient. Diarrhea was stratified by community onset vs hospital onset, the latter defined as onset >72 hours after admission. Diarrheal illness that resolved during the hospitalization was considered self-limited. Inpatient and outpatient electronic medical records were reviewed up to 90 days after discharge in evaluation of recurrence and/or readmission. Chronic diarrhea was defined as per IDSA practice guidelines [18].
Past medical histories were reviewed, and patients with immediately antecedent diarrheal illnesses where a diagnosis was already made prior to admission were excluded from analysis. A past medical history of infectious diarrhea otherwise was noted and did not preclude inclusion. Patients who were admitted more than once over the study period with diarrheal complaints were included if they met study criteria. Data was collected on a per-admission basis. All aspects of clinical care, including diagnostic testing, were at the discretion of the treating physicians.
We implemented the following modified definitions with regard to CMV: CMV viremia, or asymptomatic detection of CMV in the blood without suggestion or demonstration of end-organ disease; CMV disease, or probable invasive CMV infection defined by CMV viremia, symptoms including CMV syndrome and high-clinical suspicion; and CMV enteritis or colitis, defined by surgical histopathology with evidence of invasive CMV infection [17]. Criteria for clinical diagnoses, such as antibiotic-associated diarrhea or MMF-associated diarrhea, were based on history, temporal association, resolution with cessation, and absence of infectious pathogens; clinical suspicion alone was insufficient.
Diagnostic Assays
Clostridium difficile was assessed by a toxin B gene real-time PCR assay (GeneOhm Cdiff real-time PCR, BD, Franklin Lakes, New Jersey). Two serum CMV diagnostic assays were in use during the study period. From 1 March through 11 November 2012, the laboratory employed the Amplicor CMV Monitor Assay (Roche Molecular Diagnostics, Pleasanton, California), with a lower limit of detection (LLD) of 545 IU/mL. The Cobas TaqMan CMV Test (Roche Molecular Diagnostics, Pleasanton, California), with an LLD of 137 IU/mL, replaced this assay from 12 November 2012 through September 2013. Fecal norovirus detection by PCR was performed at a reference laboratory (ViraCor IBT Laboratories, Lee's Summit, Missouri). Giardia lamblia and Cryptosporidium were assessed by EIA (Immunocard STAT Crypto/Giardia, Meridian Bioscience, Cincinnati, Ohio). Stool culture was performed using standard media including blood agar, MacConkey agar, Hektoen enteric agar, Campylobacter agar, and Selenite broth. Bloody stools or when Escherichia coli O157:H7 assessment was requested underwent additional culture with Sorbitol MacConkey agar. Requested assessments for Vibrio were performed with additional thiosulphate citrate–bile salts–sucrose agar. Standard methodology was employed [19, 20]. Assessment for ova and parasites included microscopic analysis of concentrated wet mount and stained smears. Cyclospora assessment was by ultraviolet epifluorescence microscopy, Cystoisospora by modified acid-fast stains, and Microsporidia by fluorochrome calcofluor screening with confirmatory modified trichrome staining. Fecal leukocytes were assessed by methylene blue wet mount preparation.
The Division of Organ Transplantation protocol for the evaluation of diarrhea in the SOT recipient includes blood quantitative CMV viral load, stool C. difficile assessment, stool culture, stool Giardia/Cryptosporidium EIA assessment, and stool norovirus PCR. Implementation of the protocol, however, was at the discretion of the treating physician.
Statistical Analysis
All statistical analysis was performed on Stata version 13.1 (StataCorp, College Station, Texas). Univariate analysis with continuous variables was analyzed by Student t test or the Wilcoxon signed-rank test, and categorical variables were tested in aggregate by χ2 or Fisher exact test where appropriate. An α level of .05 was considered statistically significant.
RESULTS
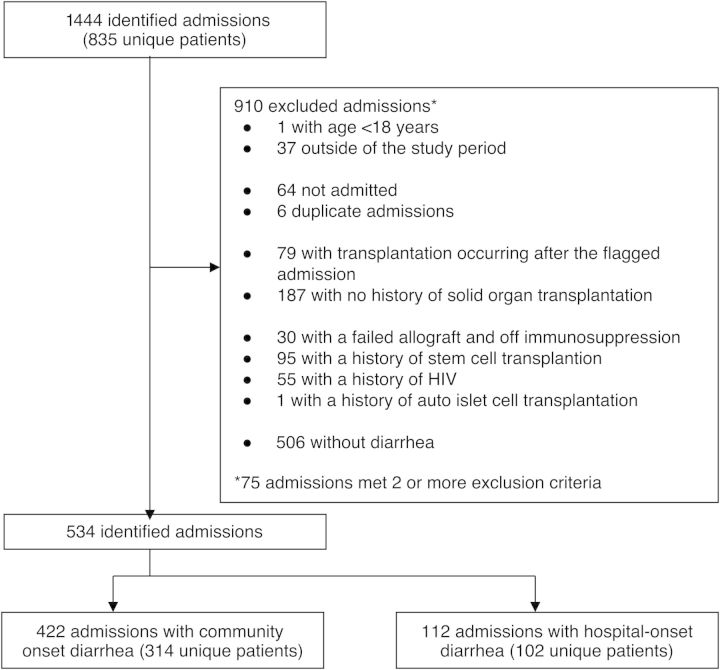
From 1444 initially identified admissions, 544 met criteria for inclusion (Figure 1). Four hundred twenty-two SOT admissions had community-onset diarrhea (representing 314 unique patients), and 112 admissions had hospital-onset diarrhea (representing 102 unique patients). Table 1 summarizes the demographic characteristics.
Figure 1.
Patient selection, inclusion, and exclusion. Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Demographic Characteristics of Patients
| Characteristic | Community-Onset Diarrhea (n = 422) |
Hospital-Onset Diarrhea (n = 112) |
P Value | ||
|---|---|---|---|---|---|
| Unique patients | 314 | 74.40% | 102 | 91.10% | <.005 |
| Age on admission, years, median (IQR) | 55.9 | 41.7–64.3 | 57.5 | 49.2–66.1 | .273 |
| Race | .004 | ||||
| White | 213 | 50.5 | 72 | 64.3 | |
| Black | 112 | 26.5 | 17 | 15.2 | |
| Asian | 21 | 5 | 6 | 5.4 | |
| Other | 57 | 13.5 | 7 | 6.3 | |
| Declined | 16 | 3.8 | 7 | 6.3 | |
| Unknown | 3 | 0.7 | 3 | 2.7 | |
| Transplant type | .003 | ||||
| Kidney alone | 169 | 40.1 | 23 | 20.5 | |
| Liver alone | 95 | 22.5 | 37 | 33 | |
| Heart alone | 47 | 11.1 | 13 | 11.6 | |
| Bowel alone | 3 | 0.7 | 0 | 0 | |
| Simultaneousa | 60 | 14.2 | 24 | 21.4 | |
| Recurrentb | 46 | 10.9 | 14 | 12.5 | |
| Other transplantc | 2 | 0.5 | 1 | 0.89 | |
| Transplant occurred during admission | 0 | 0 | 38 | 33.9 | <.005 |
| Days from first transplant to diarrhea, median (IQR) | 1028.6 | 240.4–2372.1 | 242.9 | 0.3–2091 | <.005 |
| Past medical history of diarrhea | |||||
| NOS | 101 | 23.9 | 17 | 15.2 | .054 |
| Clostridium difficile | 59 | 14 | 11 | 9.82 | .274 |
| CMV viremia, disease, or enteritis | 17 | 4.03 | 2 | 1.79 | .39 |
| Norovirus | 17 | 4.03 | 4 | 3.57 | .99 |
| Other pathogend | 5 | 1.18 | 0 | 0 | .589 |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: CMV, cytomegalovirus; IQR, interquartile range; NOS, not otherwise specified.
a Simultaneous includes pancreas and kidney (38 [9.0%] vs 7 [6.3%]); liver and kidney (22 [5.2%] vs 16 [14.3%]); heart and kidney (0 vs 1 [0.9%]).
b Recurrent includes pancreas after kidney (10 [2.4%] vs 3 [2.7%]); kidney after kidney (11 [2.6%] vs 2 [1.8%]); liver after liver (9 [2.1%] vs 3 [2.7%]); other (16 [3.8%] vs 6 [5.4%]).
c Other transplant includes pancreas only (1 [0.2%] vs 0) and lung only (1 [0.2%] vs 1 [0.9%]).
d Other pathogen includes Campylobacter (n = 3), Giardia (n = 2), Salmonella (n = 1).
When community-onset diarrhea admissions were compared to hospital-onset admissions, SOT recipients were of similar age and sex, but differed by race (P = .004) and the organ transplanted (P = .003). The majority of community-onset diarrhea admissions occurred in kidney recipients (169 [40.1%]), whereas hospital-onset diarrhea predominantly occurred in kidney (23 [20.5%]) and liver recipients (37 [33%]). Community-onset presentations were more likely to include a past history of diarrhea where the etiology was not otherwise specified (NOS) (23.9% vs 15.2%; P = .054). There were no differences insofar as previously identified infectious etiologies. No patients presenting with community-onset diarrhea were transplanted during the same hospitalization; in hospital-onset diarrheal episodes, 38 (33.9%) patients developed diarrhea during the same admission as their transplant procedure (P < .005). The 2 groups differed by time from transplant to occurrence of diarrhea: median, 1029 days (interquartile range [IQR], 240–2372 days) vs median, 243 days (IQR, 0.3–2091 days) (P = .760).
Clinical outcomes are described in Table 2. Ninety-one percent of community- and hospital-onset episodes of diarrhea were self-limited (P = .835). Length of stay was greater among those with hospital-onset diarrhea (median, 17.7 days [IQR, 7.63–28.4 days] vs median, 3.17 days [IQR, 1.91–5.50 days]) in admissions with community-onset diarrhea (P < .005). Differences in MMF and MPA usage are described in Table 2. Among patients utilizing maintenance MMF or MPA, 105 (32.6%) underwent medication dosage decreases, changes, or discontinuations.
Table 2.
Clinical Characteristics and Outcomes
| Characteristic | Community-Onset Diarrhea (n = 422) |
Hospital-Onset Diarrhea (n = 112) |
P Value | ||
|---|---|---|---|---|---|
| Admission length of stay, days, median (IQR) | 3.17 | 1.91–5.50 | 17.7 | 7.63–28.4 | <.005 |
| Course | .835 | ||||
| Self-limited | 386 | 91.50 | 102 | 91.10 | |
| Continued diarrhea | 31 | 7.35 | 8 | 7.14 | |
| Bowel resection | 5 | 1.18 | 2 | 1.79 | |
| Mortality during admission | 4 | 0.95 | 10 | 8.93 | <.005 |
| Readmission with acute diarrhea | .397 | ||||
| Within 30 days | 43 | 59.70 | 16 | 69.60 | |
| Within 30–90 days | 29 | 40.30 | 7 | 30.40 | |
| MPA and MMF usage on admission (n = 405) | <.005 | ||||
| MPA | 185 | 57.90 | 12 | 14.10 | |
| MMF | 135 | 42.20 | 73 | 85.90 | |
| MMF management | .861 | ||||
| No change | 85 | 63.00 | 46 | 63.00 | |
| Decrease | 24 | 17.78 | 13 | 17.81 | |
| Change to MPA | 7 | 5.19 | 4 | 5.48 | |
| Increase | 5 | 3.70 | 4 | 5.48 | |
| Change to azathioprine | 1 | 0.74 | 0 | 0.00 | |
| Discontinued | 13 | 9.63 | 5 | 6.85 | |
| Added | 0 | 0.00 | 1 | 1.37 | |
| MPA management | .008 | ||||
| No change | 126 | 67.38 | 6 | 46.15 | |
| Decrease | 37 | 19.79 | 2 | 15.38 | |
| Change to MMF | 0 | 0.00 | 0 | 0.00 | |
| Increase | 1 | 0.53 | 2 | 15.38 | |
| Change to azathioprine | 0 | 0.00 | 0 | 0.00 | |
| Discontinued | 23 | 12.30 | 3 | 23.08 | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: IQR, interquartile range; MMF, mycophenolate mofetil; MPA, mycophenolic acid.
There were similar proportions of diarrhea persisting until discharge. Readmission with acute diarrhea within 30 days or 90 days did not differ between community- and hospital-onset diarrhea. In-hospital mortality was greater with hospital-onset diarrhea (8.9% vs 0.9%; P < .005). Diarrhea-associated attributable mortality was negligible.
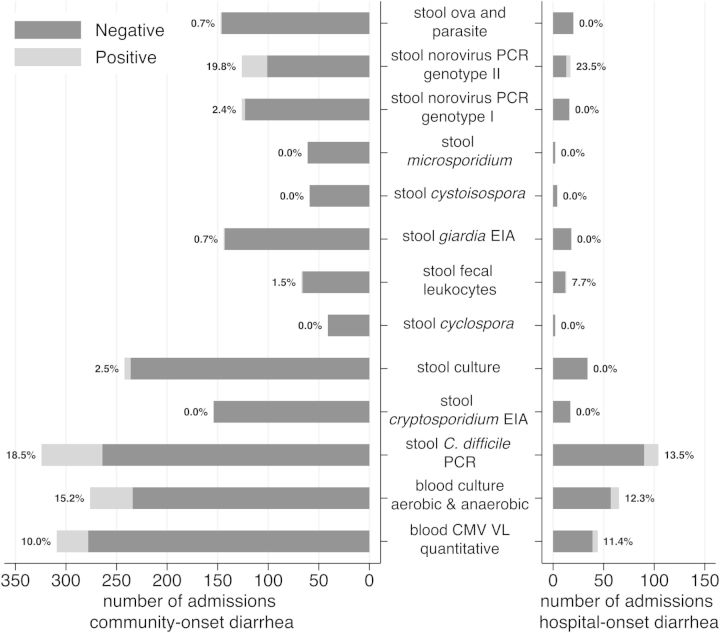
The median number of tests performed for those with community-onset diarrhea was significantly more than those with hospital-onset diarrhea (4 [IQR, 2–6] vs 3 [IQR, 1–4]; P < .005; Figure 2). There was no significant difference in median number of tests preformed between those with an eventual diagnosis and those with a diagnosis of NOS in both groups.
Figure 2.
Diagnostic studies for community-onset diarrhea. The most frequently performed tests included blood cytomegalovirus (CMV) quantitative viral load (VL) (73% tested of which 10.0% was positive), blood culture (65% tested with 15.2% positive), stool Clostridium difficile polymerase chain reaction (PCR) (77% tested with 18.5% positive), stool culture (58% tested with 2.5% positive and 1 rejected by the laboratory as inconclusive), fecal leukocytes (16% tested with 1.5% positive), stool ova and parasite (35% tested with 1 positive for Blastocystis hominis), stool Giardia and Cryptosporidium enzyme immunoassay (EIA) (27% underwent combination testing with 1 positive for Giardia lamblia), and stool norovirus PCR (30% tested with 22.2% positive for genotype I or II). Additional tests included blood adenovirus antibody (4 tested, 0 positive), blood adenovirus PCR (3 tested, 0 positive), blood CMV qualitative VL (10 tested, 0 positive), blood mycobacterial culture (5 tested, 0 positive), blood fungal culture (3 tested, 0 positive), stool adenovirus qualitative PCR (2 tested, 0 positive), stool mycobacterial smear (2 tested, 0 positive), stool CMV DNA (7 tested, 0 positive), stool norovirus EIA (4 tested, 1 positive), stool norovirus PCR genotype not otherwise specified (5 tested, 2 positive), stool rotavirus antigen (5 tested, 0 positive), stool rotavirus PCR (1 tested, 0 positive), and stool total lipids (0 tested, 0 positive). Diagnostic studies for hospital-onset diarrhea. The most frequently ordered tests included blood CMV quantitative VL (39% tested with 11% positive), blood culture (58% tested with 12.3% positive), stool C. difficile PCR (93% tested with 13.5% positive), stool culture (31% tested with 0 positive and 1 rejected for being ordered >72 hours after hospitalization), fecal leukocytes (12% tested with 7.7% positive), stool norovirus PCR (15% tested with 23.5% positive, all for genotype II), and stool ova and parasite (18% tested with 0 positive). Additional tests included blood adenovirus PCR (1 tested, 0 positive), blood CMV qualitative VL (1 tested, 0 positive), blood mycobacterial culture (3 tested, 0 positive), and blood fungal culture (4 tested, 0 positive).
The most frequently encountered etiologies of diarrhea among community- and hospital-onset admissions were NOS (62.2% vs 77.3%), C. difficile (13.3% vs 11.8%), norovirus (8.2% vs 2.7%), and CMV disease/colitis (6.3% vs 2.7%) (P = .03; Table 3). Coinfection, or the simultaneous identification of ≥2 pathogens, was identified in 8 (1.9%) community-onset and 2 (1.8%) hospital-onset admissions (P = .99). Among 24 patients with community-onset diarrhea admitted with self-reported chronic diarrhea, the following etiologies were identified: 6 cases of norovirus, 1 C. difficile, 1 colonic adenocarcinoma, and 1 posttransplant lymphoproliferative disorder; the remainder had no etiology identified.
Table 3.
Etiology of Diarrhea Among Hospitalized Solid Organ Transplant Recipients
| Etiology | Community-Onset Diarrhea (n = 422) |
Hospital-Onset Diarrhea (n = 112) |
P Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Single diagnosis (n = 523) | .03 | ||||
| NOS | 257 | 60.9 | 85 | 75.9 | |
| Clostridium difficile infection | 55 | 13.0 | 13 | 11.6 | |
| Norovirus | 34 | 8.1 | 3 | 2.7 | |
| CMV disease/colitis | 26 | 6.2 | 3 | 2.7 | |
| Othera | 42 | 10.0 | 6 | 5.4 | |
Multiple diagnoses (n = 10) consisted of C. difficile and CMV infection (4 [40%] vs 1 [50%]); CMV infection and norovirus (2 [20%] vs 1 [50%]); C. difficile and bacterial enterocolitis (1 [10%] vs 0); and C. difficile, norovirus, and Giardia (1 [10%] vs 0); P = .99, Fisher exact test.
Abbreviations: CMV, cytomegalovirus; NOS, not otherwise specified.
a Diagnoses that comprised <5% of the study population were analyzed in aggregate. Other includes adenocarcinoma (1 [0.2%] vs 0); antibiotic-associated (4 [0.9%] vs 0); appendicitis (1 [0.2%] vs 1 [0.9%]); bacterial enterocolitis (4 [0.9%] vs 0); chronic mesenteric ischemia (1 [0.2%] vs 0); Crohn disease first flare (1 [0.2%] vs 0); diverticulitis (2 [0.5%] vs 0); incarcerated hernia (2 [0.5%] vs 0); influenza A/H1N1 (1 [0.2%] vs 0); influenza A/H3N2 (1 [0.2%] vs 0); ischemic colitis (3 [0.7%] vs 0); lactulose titration (1 [0.2%] vs 0); lower gastrointestinal bleed (1 [0.2%] vs 1 [0.9%]); mycophenolate mofetil associated (4 [0.9%] vs 0); mycophenolic acid associated (1 [0.2%] vs 0); opioid withdrawal (1 [0.2%] vs 0); pneumoperitoneum (1 [0.2%] vs 0); postileostomy high-output diarrhea (4 [0.9%] vs 1 [0.9%]); posttransplant lymphoproliferative disorder (3 [0.7%] vs 0); small bowel obstruction (2 [0.2%] vs 1 [0.9%]); upper gastrointestinal bleed (2 [0.5%] vs 2 [1.8%]); ulcerative colitis (1 [0.2%] vs 0).
In community-onset diarrhea admissions, there was a median of 1 admission per patient (IQR, 1–2) during the study period: 240 patients with a single admission (etiology NOS for 152 [63%]), 48 patients with 2 admissions (55 [57%] NOS), 20 patients with 3 admissions (34 [57%] NOS), 4 patients with 4 admissions (12 [75%] NOS), and 2 patients with 5 admissions (4 [40%] NOS). With hospital-onset diarrhea, there was a median of 1 admission per patient (IQR, 1–1) during the study period: 93 patients had a single admission (71% [76%] NOS), 8 patients had 2 admissions (13% [81%] NOS), and 1 patient had 3 admissions (1 was NOS).
DISCUSSION
In this single-center study utilizing contemporary diagnostic modalities, 127 of 422 (30.1%) community-onset diarrheal admissions and 21 of 112 (18.8%) hospital-onset diarrheal admissions had an associated infectious etiology. The most commonly diagnosed infections were C. difficile, norovirus, and CMV. Ours is one of the first studies to describe the epidemiology of diarrhea utilizing molecular diagnostics for all 3 of the most commonly identified pathogens; earlier studies ranged from 13% to 28% [1, 2, 6].
The majority of both community-onset and hospital-onset admissions had no causative etiology for the diarrhea identified. Our criteria for clinical diagnoses such as antibiotic-, MMF-, or MPA-associated diarrheal illness likely precluded instances where these may have contributed. The variety of concurrent treatments, illnesses (eg, peritonitis, pyelonephritis, pneumonia), and adjustments to immunosuppressives complicates isolation of such contributions. Measurement of antibiotic exposure and drug levels may help to elucidate this question. Ultimately, 91% of diarrheal admissions had self-limited courses. Unfortunately, our observations do not relate causality, and given heterogeneity in diagnoses and empiric treatments, the significance may be limited. Validation of a testing and treatment algorithm is necessary with assessment of effects on diagnostic accuracy, immunosuppressive modification, allograft outcomes, and relapse. Ideally, current strategies (at the discretion of the treating physician or center) would be compared to a modeled paradigm.
Clostridium difficile alone was identified in 13.3% of community-onset presentations and 11.8% of hospital-onset episodes. Whereas there are wide ranges of reported incidences, our rates approximate descriptions of an increasing trend. Unfortunately, we cannot exclude a component of our greater rate as due to the use of a PCR testing modality (ie, detection of C. difficile carriage as opposed to C. difficile infection). The sensitivity, specificity, and advantages of PCR testing, compared with toxin EIA testing, have been widely described [21–23]. Both through routine laboratory protocol and chart review, we sought to only include patient encounters with diarrheal illness. As such, we do not consider these results to be representative of asymptomatic carriage. Nonetheless, we cannot discount alternative or concurrent causes of diarrhea such as adverse drug effects where confirmation is limited. Given the retrospective nature of our study, we did not have the opportunity to perform confirmatory C. difficile stool cultures or cytotoxicity neutralization assays.
Norovirus is the leading agent of gastroenteritis in the United States [24]. In immunocompromised persons, it has the potential to evade the immune response and persist longer, sometimes chronically [25]. The extent of illness among SOT recipients is incompletely characterized. To date, the majority of reports encompass case reports and series [26, 27]. Therein, our study encompasses the first measure of the extent of this burden with respect to other etiologies of diarrhea among hospitalized SOT recipients. It is important to note, however, that our study period ran concurrent to the 2012–2013 epidemic norovirus season comprising the GII.4 2012/Sydney variant, and we cannot exclude an overestimation of this burden.
CMV is a major etiology of early and late disease among SOT recipients. Overall, CMV disease and colitis accounted for 6.3% of community-onset and 2.7% of hospital-onset diarrhea. Additionally, there were instances of codetection, including CMV with C. difficile occurring in 5 (1.2%) admissions, and CMV with norovirus in 2 (0.5%). The significance of identification of a copathogen is unclear: Does this signify excessive immunosuppression, overly sensitive testing modalities, a synergistic or attenuated relationship between the 2 entities, or some combination therein?
In general, bacterial enterocolitis is described at rates ranging from 3.3% to 10% within the first 72 hours of diarrheal admissions [28–30] to 0%–0.7% thereafter [28–31]. Our findings (0.9% and 0%, respectively) appear concordant. As such, we posit that SOT recipients are not at an increased risk for bacterial enterocolitis compared with the general population, although this may reflect an overall low prevalence in light of the local epidemiology.
Protozoan causes of diarrhea were rare: 1 case of Giardia identified in combination with C. difficile and norovirus detection, and 1 case of Blastocystis hominis, which is of unclear pathogenicity [32]. Giardia and Cryptosporidium occur at low rates [33, 34]. Microsporidia has been limited to case reports [35, 36]. To our knowledge, Cyclospora, Cystoisospora, and Entamoeba histolytica have not been reported among transplant recipients in the United States, and are otherwise epidemiologically rare; incidences differ elsewhere [37–40].
In renal transplant recipients, we observed that self-limited diarrhea with etiology NOS was often accompanied by pyelonephritis. Attributing diarrhea to pyelonephritis would have accounted for 59 (11.0%) diarrhea admissions overall, and 18.0% in renal allograft recipients specifically. Although not captured by our data, the presence of diarrhea on admission likely precludes a component of antibiotic-associated diarrhea, but more investigation is needed to affirm this observation. Conversely, the incidence of pyelonephritis with admissions that lacked diarrhea would be a necessary comparator.
Numerous factors in clinical care and treatment, including the sequence of testing, were not captured by this retrospective analysis, limiting its generalizability. We did not assess for presence or absence of hypervirulent C. difficile (BI/NAP1/027). Our institution's infection control rates did not identify an increased or abnormal burden of disease over the study period, nor were there nosocomial outbreaks among study patients. Our study does not address if SOT recipients are necessarily at an overall greater risk of infectious diarrhea. Our findings may be most applicable to the SOT types most frequently encountered at our institution, particularly kidney, liver, and heart recipients. Although we have compared disease-specific incidences with those of other researchers, we cannot account for inter- and intrainstitutional variance. Local epidemiology may account for some distributions, and we caution against generalizing from our findings pending further study. Although a protocol for the evaluation of diarrhea exists at our institution, it is heterogeneously applied. Only 71 (16.8%) community-onset diarrheal admissions had all 5 diagnostic measures performed, with a median of 2 tests (IQR, 1–4); C. difficile PCR, blood CMV viral load, and stool culture were performed most often. Finally, the extent and burden of chronic diarrhea among outpatients remains incompletely characterized, and the minority of patients captured by our study may not be reflective of this overall group.
In some instances, advanced molecular diagnostics may represent a superior testing modality with potential to reduce the diagnostic deficit. Qualification and quantification of such a benefit amid additional cost remains nebulous. For example, nucleic acid amplification test identification of viral enteritis might not necessarily dictate antimicrobial therapy, but knowledge of the etiology of diarrhea may be sufficient to mitigate further testing, empiric medication changes, and invasive procedures such as endoscopies. We believe our current study has provided a modest basis on which to measure clinical practices. The next evaluation should assess best practices and how to best deploy these in a modern schema.
CONCLUSIONS
At a single center, the majority of SOT recipients admitted with diarrhea did not have an identified etiology—infectious or otherwise—but nonetheless had a self-limited course. Despite a variety of available testing modalities, the greatest yields were derived from stool norovirus PCR, C. difficile PCR, blood CMV viral load, and stool culture. It is not yet established if testing should be performed simultaneously, sequentially, tiered, or otherwise stratified. Based on our results, a reasonable up-front strategy may include the aforementioned tests. Likewise, in areas of low epidemiologic prevalence, it may be wise to reevaluate routine testing for uncommon pathogens (eg, ova and parasites, Giardia) as well as fecal leukocytes. It will be critical to evaluate the cost of consequences of testing strategies. Amid advances in molecular diagnostics, the evidence upon which testing strategies are based should continue to be evaluated and reassessed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. This study utilized data derived from the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program, supported by the National Center for Research Resources (grant 5UL1RR025741), now at the National Center for Advancing Translational Sciences (grant 8UL1TR000150).
Author contributions. I. A. E. and M. P. A. conceived and designed the experiments. I. A. E. and S. P. performed the experiments. I. A. E., S. P., V. S., M. G. I., and M. P. A. analyzed the data. I. A. E. and S. P. contributed reagents/materials/analysis tools. I. A. E., S. P., V. S., M. G. I., and M. P. A. drafted the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Maes B, Hadaya K, de Moor B, et al. Severe diarrhea in renal transplant patients: results of the DIDACT study. Am J Transplant. 2006;6:1466–72. doi: 10.1111/j.1600-6143.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 2.Bunnapradist S, Neri L, Wong W, et al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis. 2008;51:478–86. doi: 10.1053/j.ajkd.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Herrero JI, Benlloch S, Bernardos A, et al. Gastrointestinal complications in liver transplant recipients: MITOS study. Transplant Proc. 2007;39:2311–3. doi: 10.1016/j.transproceed.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Gil-Vernet S, Amado A, Ortega F, et al. Gastrointestinal complications in renal transplant recipients: MITOS study. Transplant Proc. 2007;39:2190–3. doi: 10.1016/j.transproceed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Unterman S, Zimmerman M, Tyo C, et al. A descriptive analysis of 1251 solid organ transplant visits to the emergency department. West J Emerg Med. 2009;10:48–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Nagaraj N, Kahan B, Adler DG. Gastrointestinal complications in renal transplant patients: a large, single-center experience. Dig Dis Sci. 2007;52:3394–5. doi: 10.1007/s10620-006-9581-7. [DOI] [PubMed] [Google Scholar]
- 7.Altiparmak MR, Trablus S, Pamuk ON, et al. Diarrhoea following renal transplantation. Clin Transplant. 2002;16:212–6. doi: 10.1034/j.1399-0012.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- 8.van Boekel GA, Aarnoutse RE, van der Heijden JJ, Hoogtanders KE, Hilbrands LB. Effect of mild diarrhea on tacrolimus exposure. Transplantation. 2012;94:763–7. doi: 10.1097/TP.0b013e3182629e13. [DOI] [PubMed] [Google Scholar]
- 9.Bunnapradist S, Lentine KL, Burroughs TE, et al. Mycophenolate mofetil dose reductions and discontinuations after gastrointestinal complications are associated with renal transplant graft failure. Transplantation. 2006;82:102–7. doi: 10.1097/01.tp.0000225760.09969.1f. [DOI] [PubMed] [Google Scholar]
- 10.Vanhove T, Kuypers D, Claes KJ, et al. Reasons for dose reduction of mycophenolate mofetil during the first year after renal transplantation and its impact on graft outcome. Transpl Int. 2013;26:813–21. doi: 10.1111/tri.12133. [DOI] [PubMed] [Google Scholar]
- 11.Langone A, Doria C, Greenstein S, et al. Does reduction in mycophenolic acid dose compromise efficacy regardless of tacrolimus exposure level? An analysis of prospective data from the Mycophenolic Renal Transplant (MORE) Registry. Clin Transplant. 2013;27:15–24. doi: 10.1111/j.1399-0012.2012.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah SA, Tsapepas DS, Kubin CJ, et al. Risk factors associated with Clostridium difficile infection after kidney and pancreas transplantation. Transpl Infect Dis. 2013;15:502–9. doi: 10.1111/tid.12113. [DOI] [PubMed] [Google Scholar]
- 13.Pant C, Anderson MP, O'Connor JA, Marshall CM, Deshpande A, Sferra TJ. Association of Clostridium difficile infection with outcomes of hospitalized solid organ transplant recipients: results from the 2009 Nationwide Inpatient Sample database. Transpl Infect Dis. 2012;14:540–7. doi: 10.1111/j.1399-3062.2012.00761.x. [DOI] [PubMed] [Google Scholar]
- 14.Len O, Rodriguez-Pardo D, Gavalda J, et al. Outcome of Clostridium difficile-associated disease in solid organ transplant recipients: a prospective and multicentre cohort study. Transpl Int. 2012;25:1275–81. doi: 10.1111/j.1432-2277.2012.01568.x. [DOI] [PubMed] [Google Scholar]
- 15.Boutros M, Al-Shaibi M, Chan G, et al. Clostridium difficile colitis: increasing incidence, risk factors, and outcomes in solid organ transplant recipients. Transplantation. 2012;93:1051–7. doi: 10.1097/TP.0b013e31824d34de. [DOI] [PubMed] [Google Scholar]
- 16.Rice JP, Spier BJ, Cornett DD, Walker AJ, Richie K, Pfau PR. Utility of colonoscopy in the evaluation of diarrhea in solid organ transplant recipients. Transplantation. 2009;88:374–9. doi: 10.1097/TP.0b013e3181ae98ab. [DOI] [PubMed] [Google Scholar]
- 17.Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13(suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 18.Guerrant RL, Van Gilder T, Steiner TS, et al. Practice guidelines for the management of infectious diarrhea. Clin Infect Dis. 2001;32:331–51. doi: 10.1086/318514. [DOI] [PubMed] [Google Scholar]
- 19.Garcia LS. Clinical microbiology procedures handbook. 3rd ed. Washington, DC: ASM Press; 2010. [Google Scholar]
- 20.Versalovic J. Manual of clinical microbiology. 10th ed. Washington, DC: ASM Press; 2011. [Google Scholar]
- 21.Kufelnicka AM, Kirn TJ. Effective utilization of evolving methods for the laboratory diagnosis of Clostridium difficile infection. Clin Infect Dis. 2011;52:1451–7. doi: 10.1093/cid/cir201. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–55. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 23.Deshpande A, Pasupuleti V, Rolston DD, et al. Diagnostic accuracy of real-time polymerase chain reaction in detection of Clostridium difficile in the stool samples of patients with suspected Clostridium difficile infection: a meta-analysis. Clin Infect Dis. 2011;53:e81–90. doi: 10.1093/cid/cir505. [DOI] [PubMed] [Google Scholar]
- 24.Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the National Outbreak Reporting System, United States. Emerg Infect Dis. 2013;19:1305–9. doi: 10.3201/eid1908.130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bok K, Green KY. Norovirus gastroenteritis in immunocompromised patients. N Engl J Med. 2012;367:2126–32. doi: 10.1056/NEJMra1207742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roos-Weil D, Ambert-Balay K, Lanternier F, et al. Impact of norovirus/sapovirus-related diarrhea in renal transplant recipients hospitalized for diarrhea. Transplantation. 2011;92:61–9. doi: 10.1097/TP.0b013e31821c9392. [DOI] [PubMed] [Google Scholar]
- 27.Westhoff TH, Vergoulidou M, Loddenkemper C, et al. Chronic norovirus infection in renal transplant recipients. Nephrol Dial Transplant. 2009;24:1051–3. doi: 10.1093/ndt/gfn693. [DOI] [PubMed] [Google Scholar]
- 28.Bauer TM, Lalvani A, Fehrenbach J, et al. Derivation and validation of guidelines for stool cultures for enteropathogenic bacteria other than Clostridium difficile in hospitalized adults. JAMA. 2001;285:313–9. doi: 10.1001/jama.285.3.313. [DOI] [PubMed] [Google Scholar]
- 29.Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med. 1996;120:206–11. [PubMed] [Google Scholar]
- 30.Chitkara YK, McCasland KA, Kenefic L. Development and implementation of cost-effective guidelines in the laboratory investigation of diarrhea in a community hospital. Arch Intern Med. 1996;156:1445–8. [PubMed] [Google Scholar]
- 31.Yannelli B, Gurevich I, Schoch PE, Cunha BA. Yield of stool cultures, ova and parasite tests, and Clostridium difficile determinations in nosocomial diarrheas. Am J Infect Control. 1988;16:246–9. doi: 10.1016/s0196-6553(88)80003-8. [DOI] [PubMed] [Google Scholar]
- 32.Kuo HY, Chiang DH, Wang CC, et al. Clinical significance of Blastocystis hominis: experience from a medical center in northern Taiwan. J Microbiol Immunol Infect. 2008;41:222–6. [PubMed] [Google Scholar]
- 33.Ziring D, Tran R, Edelstein S, et al. Infectious enteritis after intestinal transplantation: incidence, timing, and outcome. Transplant Proc. 2004;36:379–80. doi: 10.1016/j.transproceed.2004.01.093. [DOI] [PubMed] [Google Scholar]
- 34.Bonatti H, Barroso LF, 2nd, Sawyer RG, Kotton CN, Sifri CD. Cryptosporidium enteritis in solid organ transplant recipients: multicenter retrospective evaluation of 10 cases reveals an association with elevated tacrolimus concentrations. Transpl Infect Dis. 2012;14:635–48. doi: 10.1111/j.1399-3062.2012.00719.x. [DOI] [PubMed] [Google Scholar]
- 35.Sax PE, Rich JD, Pieciak WS, Trnka YM. Intestinal microsporidiosis occurring in a liver transplant recipient. Transplantation. 1995;60:617–8. doi: 10.1097/00007890-199509270-00018. [DOI] [PubMed] [Google Scholar]
- 36.Goetz M, Eichenlaub S, Pape GR, Hoffmann RM. Chronic diarrhea as a result of intestinal microsposidiosis in a liver transplant recipient. Transplantation. 2001;71:334–7. doi: 10.1097/00007890-200101270-00029. [DOI] [PubMed] [Google Scholar]
- 37.Arslan H, Inci EK, Azap OK, Karakayali H, Torgay A, Haberal M. Etiologic agents of diarrhea in solid organ recipients. Transpl Infect Dis. 2007;9:270–5. doi: 10.1111/j.1399-3062.2007.00237.x. [DOI] [PubMed] [Google Scholar]
- 38.Azami M, Sharifi M, Hejazi SH, Tazhibi M. Intestinal parasitic infections in renal transplant recipients. Braz J Infect Dis. 2010;14:15–8. doi: 10.1590/s1413-86702010000100004. [DOI] [PubMed] [Google Scholar]
- 39.Naeini AE, Sharifi M, Shahidi S, et al. Intestinal fungal and parasitic infections in kidney transplant recipients: a multi-center study. Saudi J Kidney Dis Transpl. 2012;23:677–83. doi: 10.4103/1319-2442.98110. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher SM, Stark D, Harkness J, Ellis J. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev. 2012;25:420–49. doi: 10.1128/CMR.05038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.