Abstract
BACKGROUND
Although higher sodium intake is known to increase blood pressure, its association with cardiovascular mortality is less established. We examined the association of baseline sodium intake in a hypertensive cohort with all-cause and cardiovascular mortality over a mean follow-up of 18.6 years.
METHODS
Three thousand five hundred five subjects were participants in a worksite hypertension program. Sodium intake was estimated by 24-hour urine excretion. Mortality data were obtained from the US National Death Index. Unadjusted and multivariable-adjusted associations between sodium quartiles (quartile I (QI) to quartile IV (QIV)) and mortality were assessed using Cox models.
RESULTS
Estimated mean ± SD sodium intake was 130±69 mmol overall (55±20 mmol in QI; 220±56 mmol in QIV). Baseline systolic blood pressure did not vary significantly between groups. Last available mean systolic blood pressure was highest in QI and lowest in QIV (137±16 vs. 134±14mm Hg; P = 0.009). Overall there were 1,013 deaths (399 cardiovascular). Unadjusted models exhibited significant inverse relationships between sodium and mortality outcomes. In adjusted models, sodium intake was not significantly associated with cardiovascular mortality (QI vs. QIV: hazard ratio (HR) = 1.00; 95% confidence interval (CI) = 0.71–1.42; P = 0.99). A borderline significant direct association with all-cause mortality was observed (QI vs. QIV: HR = 0.81; 95% CI = 0.66–1.00; P = 0.05) driven partly by noncardiovascular deaths.
CONCLUSIONS
Our study found no significant association between sodium intake and cardiovascular outcomes, although a significant association with all-cause mortality was observed. Although these findings suggest that sodium may not have a strong relationship with cardiovascular mortality, the inconsistent results cast doubt on whether a single measurement can reliably predict mortality over a prolonged follow-up period.
Keywords: blood pressure, epidemiology, hypertension, mortality, sodium
Hypertension affects approximately 30% of all US adults1 and is the leading risk factor for cardiovascular morbidity and mortality.2 Although antihypertensive medication is the mainstay of blood pressure (BP) control, dietary sodium restriction has been widely recommended as a public health measure for both prevention and treatment of hypertension.3 Sodium restriction has been found to lower mean BP,4–6 with a recent Cochrane review indicating an average systolic BP (SBP) reduction of 3.5% in hypertensive patients when sodium intake was reduced by 125 mmol/day.7
At the same time, controversy exists on whether this sodium–BP effect actually translates to cardioprevention because intensive sodium restriction may have adverse effects on insulin resistance, triglycerides, and sympathetic nervous system activation.7,8 Studies directly examining the association between sodium intake and cardiovascular outcomes have produced conflicting results, with some finding a direct association, some showing an inverse or J-shaped curve, and others finding no relation to cardiovascular disease mortality. The lack of a consistent association is highlighted by the recent Institute of Medicine report,9 which concluded that the available data preclude a recommendation to restrict sodium to <2,300mg per day, as well as a recent meta-analysis demonstrating no association between reduced sodium intake and cardiovascular mortality despite a significant reduction in BP.10 Average sodium intakes vary widely across geographic locales, and differences among the populations studied may partially account for the seemingly inconsistent results.11–32 This variation underscores the importance of population-specific studies.
In the United States, an adverse association of lower sodium intake with cardiovascular outcomes in a group of healthy hypertensive patients was reported in 1995. During an average of 3.8 years of follow-up, with a mean sodium intake of 115 mmol, an inverse association with myocardial infarction (MI) was found.13 Our study describes the association of sodium intake with long-term all-cause and cardiovascular mortality in this same cohort, now after nearly 20 years of mean follow-up. This represents the longest follow-up to date of baseline sodium excretion with mortality outcomes in hypertensive subjects.
METHODS
Study population
Patient recruitment methods have been described in detail elsewhere.33 Briefly, participants were individuals in a union-sponsored, worksite hypertension program in New York City between 1978 and 1999. Entry criteria were an SBP ≥140mm Hg (≥160mm Hg before Joint National Committee 5), diastolic BP (DBP) ≥90mm Hg (≥95mm Hg before Joint National Committee 5), or being on antihypertensive medication at the time of screening. After a medication washout period of 3–4 weeks, a pretreatment study visit was performed. Three BP readings using a standard sphygmomanometer were taken, the last 2 of which were averaged for the visit reading. Laboratory analyses, including a 24-hour urine collection, were obtained at this visit. Although some laboratory testing was repeated at multiple points throughout follow-up, a urine collection was obtained only at baseline. Antihypertensive medications were prescribed as needed throughout the period of study participation. Medication prescription generally followed Joint National Committee recommendations but was ultimately at the discretion of the physician, and from 1981 to 1988, a subset of participants were randomly assigned to initial monotherapy of a diuretic or β-blocker.34 The last BP available was the last one recorded in the program.
Sodium measurement
A single 24-hour urine collection was obtained at the pretreatment study visit after the medication washout period. Subjects were instructed to follow their usual diet while avoiding “excessively salty foods” for a period of 4–5 days preceding the collection. The first void on the day of collection was discarded, and that of the following day was included in the measurement. Urine sodium was analyzed using a flame photometer (Instrumentation Laboratories, Bedford, MA, through 1985), or ion-selective electrode (Beckman Astra, Brea, CA).13
Endpoints
Mortality data were obtained using National Death Index Plus and the Social Security Administration Death Master File. Mortality data were available through August 2009. Causes of death were categorized as coronary artery disease, including MI, ischemic heart disease, heart failure, and hypertensive heart disease (International Classification of Diseases, Ninth Revision (ICD-9): 402.9, 410–414.9, 427.5, 429.2; International Classification of Diseases, Tenth Revision (ICD-10): I10–11.9, I13–I13.2, I20–I25.9, I46–I46.9); stroke (ICD-9: 434–434.9, 436–438.9; ICD-10: I61–I64.9); other cardiac causes (ICD-9: 390–459.9; ICD-10: I00–I99 excluding those listed above); noncardiovascular causes, of which approximately 50% were cancer deaths (ICD-9: 140–239; ICD-10: C00–D49); and cause of death unknown.35 Unknown causes of death primarily consisted of those after 2007, for which National Death Index ICD codes were not available. For outcome analysis, all cardiovascular mortality included death from coronary artery disease, stroke, and other cardiac causes. Limited cardiovascular mortality included only MI, ischemic or hypertensive heart disease, and heart failure, while excluding stroke and other cardiac causes, which were thought could differ in their relationship to sodium intake.
Statistical analysis
Values of 24-hour urine sodium were divided into sex-specific quartiles, in which men and women were first divided into respective sodium quartiles, which were then combined, such that quartile I (QI) contained the lowest quartile, respectively, of male values and female values, and so on. Units of urinary sodium were expressed in millimoles per 24 hours, with 1 mmol equal to 23mg of sodium. Potential covariables and confounders were identified based on prior literature and biologic plausibility, and bivariable analyses were analyzed using analysis of variance or χ2 testing as appropriate. Estimated glomerular filtration rate was measured using the Chronic Kidney Disease—Epidemiology formula.36 Continuous variables were log-transformed as needed to meet normality assumptions. Unadjusted and adjusted Cox proportional hazards were performed to examine the association of 24-hour urine sodium quartiles with mortality. Quartile IV (QIV) was used as the reference category in all models. A backwards elimination approach was used to arrive at reduced models, and variables found to be independent predictors at P < 0.05 or that showed evidence of confounding the sodium association (change in hazard ratio (HR) for sodium of >10%) were retained in the model. In addition, age, race, sex, and body mass index were forced into all models. A forward stepping procedure was then applied to all variables that remained in the final model to assess for confounding. Interactions were checked using product terms. The proportional hazard assumption was tested using STATA’s goodness-of-fit command, which examines the proportional hazards assumption for the overall model as well as for each individual variable as a time-varying covariable. Models were analyzed using all-cause mortality, all cardiovascular mortality, and limited cardiovascular mortality as outcomes. Subset analyses were performed as described below. All analyses were performed using STATA 12.0 (STATA Corp, College Station, TX).
RESULTS
Baseline characteristics
Three thousand five hundred five study participants completed a 24-hour urine collection. Baseline characteristics of participants are shown in Table 1. The overall mean ± SD urinary sodium in our cohort was 130±69 mmol (equivalent to approximately 3 grams of sodium, or 7.5 grams of salt), with a mean ± SD of 55±20 mmol in the lowest quartile vs. 221±56 mmol in the highest quartile.
Table 1.
Baseline characteristics by sex-specific urinary sodium quartiles
| Characteristic Urine sodium, mmol/24 h | Quartile I (n = 890) 55±20 | Quartile II (n = 876) 102±17 | Quartile III (n = 865) 143±20 | Quartile IV (n = 874) 221±56 | P value |
|---|---|---|---|---|---|
| Male sex | 564 (63.4) | 567 (64.7) | 552 (63.8) | 559 (64.0) | 0.95 |
| Age, y | 53.7±9.8 | 53.1±9.2 | 51.5±9.9 | 51.0±9.2 | <0.0001 |
| Race | |||||
| Black | 269 (30.2) | 257 (30.5) | 264 (30.5) | 250 (28.6) | 0.04 |
| White | 282 (31.7) | 295 (33.7) | 274 (31.7) | 256 (29.3) | |
| Hispanic | 300 (33.7) | 305 (34.8) | 309 (35.7) | 335 (38.3) | |
| Other | 39 (4.4) | 18 (2.1) | 18 (2.1) | 33 (3.8) | |
| History | |||||
| MI | 10 (1.1) | 4 (0.5) | 9 (1.0) | 13 (1.5) | 0.20 |
| Stroke | 8 (0.9) | 5 (0.6) | 8 (0.9) | 6 (0.7) | 0.80 |
| Angina | 30 (3.4) | 29 (3.3) | 32 (3.7) | 27 (3.1) | 0.92 |
| DM | 36 (4.0) | 55 (6.3) | 48 (5.6) | 52 (6.0) | 0.17 |
| CKD | 13 (1.5) | 12 (1.4) | 10 (1.2) | 19 (2.2) | 0.34 |
| Antihypertensive drug use | 329 (37.0) | 349 (39.9) | 347 (40.2) | 307 (35.2) | 0.27 |
| Smoking | 187 (21.0) | 179 (20.4) | 165 (19.1) | 144 (16.5) | 0.08 |
| BMI, kg/m2 | 27.4±4.1 | 27.8±4.1 | 28.9±4.5 | 30.0±4.9 | <0.0001 |
| SBP, mm Hg | 146.4±18.5 | 145.3±17.7 | 145.2±16.5 | 145.8±16.3 | 0.41 |
| DBP, mm Hg | 93.6±10.0 | 93.9±9.7 | 94.1±9.4 | 95.1±9.6 | 0.01 |
| Hematocrit, mg/dl | 43.7±4.0 | 43.5±4.0 | 43.7±4.1 | 43.4±4.0 | 0.37 |
| Cholesterol, mg/dl | 223.2±45.2 | 222.0±43.5 | 219.4±42.4 | 218.7±39.8 | 0.09 |
| Uric acid, mg/dl | 5.9±1.5 | 5.7±1.4 | 5.7±1.4 | 5.7±1.4 | 0.004 |
| Plasma renin activity, ng/ml/h | 1.7 (0.64–3.4) | 1.4 (0.56–2.6) | 1.4 (0.54–2.6) | 1.2 (0.49–2.4) | 0.0001 |
| eGFR, ml/min/1.73 m2 | 76.5±16.5 | 77.9±16.6 | 78.5±16.4 | 80.6±16.1 | <0.0001 |
| Urine potassium, mmol/24 h | 49.5 (25.9) | 58.0 (36.8) | 60.6 (23.5) | 69.9 (27.0) | <0.0001 |
| Urine volume, L | 1.2±0.6 | 1.3±0.6 | 1.5±0.5 | 1.7±0.6 | <0.0001 |
Results are reported as mean ± SD, median (nterquartile range), or frequency (%).
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; SBP, systolic blood pressure.
The study population was 64% men, with a mean ± SD age of 52±10 years. Those in the highest sodium quartile were significantly younger than those in the lowest, although the mean age at baseline differed by only 2 years. Blacks, whites, and Hispanics each accounted for approximately one-third of the study population and were represented fairly evenly across all sodium quartiles. No significant differences were seen across quartiles with regards to baseline history of cardiovascular disease, diabetes, or chronic kidney disease. Baseline body mass index was significantly associated with sodium quartiles. There was no significant difference in baseline SBP, and although differences in baseline DBP were statistically significant (P = 0.008), the mean absolute difference between QI and QIV was small. Both urine potassium and urine volume were higher in the higher urinary sodium quartiles, suggesting higher total daily food intakes.
Follow-up characteristics
The average in-program time was 6.5±4.4 years, with no significant difference between the sodium quartiles (P = 0.63). Weight increased over the course of the study, with an average participant weight gain of 0.9±6.7kg between the first and last visits. Those in QIV were significantly heavier than those in QI at last follow-up (86.5±17.2 vs. 77.7±13.5kg; P < 0.001), however this difference was similar in magnitude to that at the start of the study.
Last observed creatinine, uric acid, hematocrit (Hct), blood sugar, and cholesterol did not differ significantly across sodium quartiles (data not shown). Only baseline measurements of urine sodium were obtained during the study, and no repeat measurements were available.
Mean BP decreased in all sodium quartiles throughout the duration of the study period, with an overall average final SBP of 136±15mm Hg and DBP of 84±9mm Hg. Last available follow-up values revealed the SBP to be highest in QI, with no significant difference in DBP. Table 2 illustrates the changes in BP measurements throughout the study period.
Table 2.
Relationship of blood pressure to sodium quartiles
| Blood pressure parameters | Quartile I | Quartile II | Quartile III | Quartile IV | P value |
|---|---|---|---|---|---|
| Initial | |||||
| Systolic | 146.4±18.5 | 145.2±17.7 | 145.1±16.5 | 145.8±16.3 | 0.41 |
| Diastolic | 93.6±10.0 | 93.9±9.7 | 94.1±9.4 | 95.1±9.6 | 0.008 |
| No. of hypertensives (%) | 745 (84) | 727 (84) | 744 (87) | 756 (87) | 0.31 |
| First follow-up | |||||
| Systolic | 141.6±15.9 | 142.6±16.1 | 141.2±16.9 | 140.9±15.8 | 0.26 |
| Diastolic | 89.6±8.8 | 89.5±9.4 | 90.0±9.4 | 90.0±8.5 | 0.44 |
| Final In-Program | |||||
| Systolic | 136.7±15.8 | 136.2±15.1 | 135.1±16.0 | 134.3±14.5 | 0.006 |
| Diastolic | 83.8±9.7 | 84.1±8.9 | 84.0±9.5 | 84.4±9.1 | 0.57 |
| No. on medication (%) | 689 (77) | 668 (76) | 655 (76) | 682 (78) | 0.65 |
Blood pressure is expressed in mm Hg. Data are mean ± SD unless otherwise noted.
At the time of the last program visit, approximately 80% of study participants were being prescribed at least 1 antihypertensive medication, with 33% having been prescribed ≥3 drugs. There were no significant differences in the number of antihypertensives prescribed at the time of last program visit (mean in Q1 vs. Q4 = 1.65±1.1 vs. 1.72±1.2; P = 0.51).
Sodium and mortality outcomes
The overall mean length of follow-up, measured as the time from initial intake to death or last known alive, was 18.6 years. Overall, there were 1,013 deaths, with 287 total deaths in QI vs. 216 in QIV. Of the total deaths, 399 were due to cardiovascular causes, with 128 cardiovascular deaths in QI vs. 78 in QIV. Most deaths occurred among patients after they were no longer in the worksite program. Approximately half of noncardiovascular deaths for which the cause was known were due to cancer. Figure 1 shows details of noncardiovascular causes of death. In unadjusted analysis, lower sodium intake was consistently associated with a higher mortality across all outcomes measured. Figure 2 shows crude mortality data as well as unadjusted HRs for all-cause and cardiovascular mortality.
Figure 1.
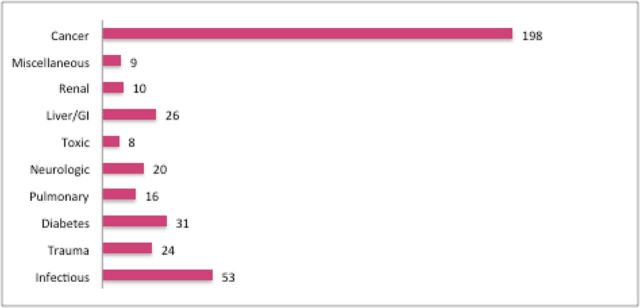
Noncardiovascular causes of death (excluding unknown). Abbreviation: GI, gastrointestinal.
Figure 2.
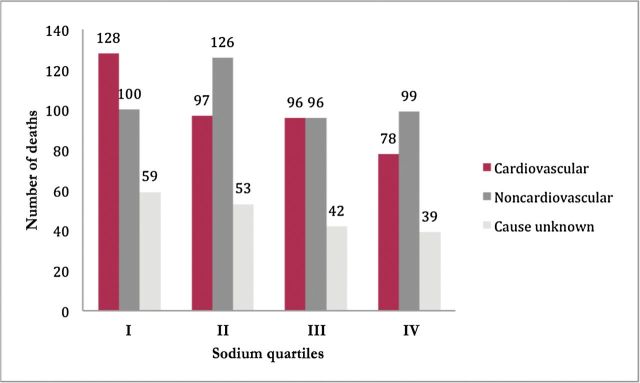
Raw mortality data by sodium quartiles. All-cause mortality (QI vs. QIV: unadjusted hazard ratio (HR) = 1.25; 95% confidence interval (CI) = 1.05–1.49; P = 0.01); all cardiovascular mortality (QI vs. QIV: HR = 1.61; 95% CI = 1.21–2.13; P = 0.001); limited cardiovascular mortality (QI vs. QIV: HR = 1.49; 95% CI = 1.08–2.05; P = 0.01); noncardiovascular mortality (QI vs. QIV: HR = 1.02; 95% CI = 0.77–1.35; P = 0.87).
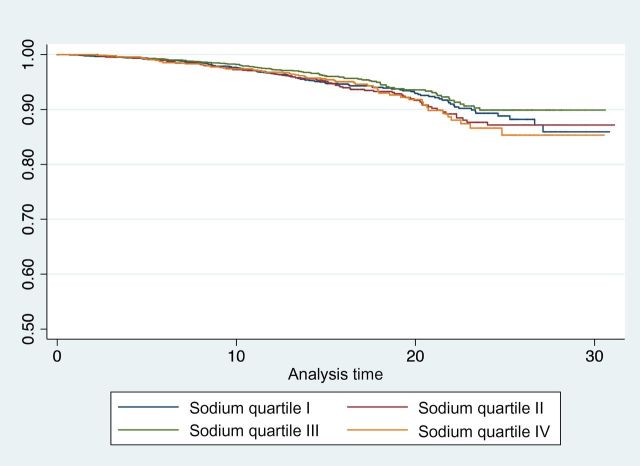
Table 3 shows the results of multivariable Cox proportional hazards modeling for all-cause, cardiovascular, limited cardiovascular, and noncardiovascular mortality, and a survival curve for all cardiovascular mortality is shown in Figure 3. Although a borderline direct association between sodium and all-cause mortality was found, no association between sodium and cardiovascular death was observed. When cardiovascular deaths were excluded from the model, results became highly significant QI vs. QIV: HR = 0.57; P = 0.001), suggesting the overall significance with all-cause mortality was at least partially driven by noncardiovascular events. When looking exclusively at cardiovascular deaths in a noncompeting risk model, urinary sodium was not significantly associated with all cardiovascular or limited cardiovascular mortality (QI vs. QIV: HR = 0.86, P = 0.38; HR = 0.79, P = 0.20, respectively). In a post hoc power analysis, we had >80% power to observe a true, independent population HR for cardiovascular disease mortality of sodium QI to QIV ≤0.85 or ≥1.17 and ≥95% power to detect a true HR ≤0.80 or ≥1.23.
Table 3.
Adjusted hazard ratios for sodium quartiles with mortality outcomes, using quartile IV as reference
| Mortality outcome | Hazard ratio (95% CI) | P value |
|---|---|---|
| All-cause mortalitya(n = 3,052) | ||
| QI | 0.81 (0.66–1.00) | 0.05 |
| QII | 0.90 (0.73–1.10) | 0.31 |
| QIII | 0.88 (0.71–1.09) | 0.23 |
| All cardiovascular mortalityb | ||
| QI | 1.00 (0.71–1.42) | 0.99 |
| QII | 0.96 (0.68–1.36) | 0.82 |
| QIII | 1.06 (0.75–1.49) | 0.74 |
| Limited cardiovascular mortalityc | ||
| QI | 0.93 (0.63–1.39) | 0.73 |
| QII | 0.82 (0.55–1.22) | 0.34 |
| QIII | 1.06 (0.73–1.55) | 0.74 |
| Noncardiovascular mortalityd | ||
| QI | 0.57 (0.41–0.80) | 0.001 |
| QII | 0.85 (0.63–1.16) | 0.31 |
| QIII | 0.80 (0.59–1.10) | 0.18 |
All covariables listed are those at baseline. Backwards elimination approach was used to arrive at all final models.
Abbreviations: QI, quartile I; QII, quartile II; QIII, quartile III.
aAdjusted for age, sex, race, body mass index (BMI), systolic blood pressure (SBP), estimated glomerular filtration rate (eGFR), urine potassium, hematocrit, HxDM, Hx smoking, history of baseline left ventricular hypertrophy (Hx baseline LVH).
bAdjusted for age, sex, race, BMI, SBP, eGFR, urine potassium, hematocrit, plasma renin activity, HxDM, Hx smoking, history of baseline left ventricular hypertrophy.
cAdjusted for age, sex, race, BMI, SBP, urine creatinine, plasma renin activity, HxDM, Hx smoking, history of baseline left ventricular hypertrophy.
dAdjusted for age, sex, race, BMI, SBP, hematocrit, urine potassium, HxDM, Hx smoking. Abbreviations: Hx smoking, history of smoking; HxDM, history of diabetes.
Figure 3.
Kaplan–Meier curve of sodium intake vs. all cardiovascular mortality. Note that the y axis ranges from 0.5 to 1.0 for ease of visualization.
In examining the potential cause for the difference between adjusted and unadjusted analysis, a forward modeling technique was used to assess confounding in all variables that remained in the final models. Baseline age was significantly higher in the lowest sodium quartile (53.7 years in QI vs. 51 years in QIV), and adjusting for this variable alone strongly altered the associations seen in the unadjusted analyses. Other variables tested were not shown to substantially confound this association.
The proportional hazard assumption was tested and was not violated for any of the final models. To assess the possibility of a J- or U-shaped relation, models were retested using quartile II (QII) as a reference quartile. No significant association was found for other quartiles vs. QII for any mortality outcome tested (overall, cardiovascular, limited cardiovascular mortality).
Sensitivity analyses
In addition to quartile analysis, sodium was analyzed as a continuous variable, with similar results. To account for possible problems with 24-hour urine collections, subset analyses excluding the top and bottom 1% of urinary sodium values and excluding those whose calculated creatinine clearance did not fall within ±35% of estimated glomerular filtration rate were performed and did not substantially change our results. In light of the study findings of Geleijnse et al.,22 urine sodium/potassium ratio was also examined and was not found to be a significant independent predictor of mortality. Models excluding SBP (due to its potential mediation) and models excluding participants with a baseline BP <140/90mm Hg after the medication washout period, who may not have been truly hypertensive at baseline, similarly did not affect results.
DISCUSSION
We found a borderline significant association of sodium intake with all-cause mortality, a highly significant association with non–cardiovascular disease mortality, and no significant association with cardiovascular outcomes. These multivariable-adjusted findings were in contrast to unadjusted analyses, in which significant inverse associations were seen for both all-cause and cardiovascular mortality. The degree of discrepancy between adjusted and unadjusted analysis was largely a result of the baseline age difference between the quartiles, even though the difference in mean age was small.
This may also explain the discrepant findings with the earlier report of this cohort,13 which found a significantly greater risk of MI in men with lower sodium intake. It is important to note that the earlier study had many more morbid events than fatalities (221 vs. 61, respectively), and the effect of age on morbidity may be less prominent than that on death. Unfortunately, long-term morbidity data were not available in our study. Furthermore, the follow-up time in the previous study was substantially shorter, with a mean of 3.8 years. On a population level, sodium intake has been shown to be very consistent over prolonged periods of time,37 but it is unclear whether this is also applicable to individuals.
Another possible explanation for the lack of significant association over the long term is the effect of “regression dilution bias,”38 in which, due to a combination of measurement error and within-individual variance, single measurements of a baseline characteristic tend to underestimate the strength of association with an outcome over time. Thus it remains possible that with multiple measurements a significant association could have been seen, although the magnitude or even direction of such a potential association cannot be estimated without making assumptions that cannot be tested.
Finally, during the study the majority of participants were placed on antihypertensives, with similar declines in BP throughout the study period. Although it is possible that attention to BP measurements may have altered participants’ sodium intake, undermining the ability to determine an association, without repeat measurements it is impossible to know whether changes in sodium intake differentially affected those in each quartile.
Despite the nonsignificant cardiovascular findings, a borderline significant direct association with all-cause mortality and a strongly significant association with noncardiovascular mortality remain and must be explained. Because of the diverse causes of noncardiovascular death, of which 50% were cancers, and without a mechanism linking sodium intake to these various causes, it seems more likely that this represents either a chance finding or latent confounding rather than a causative contribution of sodium to mortality. Furthermore, analysis of in-program follow-up data revealed that final in-program SBP was highest in the lowest sodium quartile. This finding contradicts the presumed mechanism that sodium increases risk of mortality by increasing BP. One potential explanation for all of these findings together is that sodium intake, although not the true cause of the association with mortality, is a marker of overall health behaviors, and that those with the lowest sodium intakes may have been practicing healthier habits overall.
Strengths of this study include the long duration of follow-up and the estimation of sodium intake by urinary excretion, as opposed to questionnaire or recall methods, which may be less accurate.39 Other strengths include the use of a medication “washout” period, ensuring that baseline sodium measurements were not affected by medication use, and the worksite program design, in which participants were essentially healthy at entry, reducing concern for reverse causality.
A possible limitation of the study is the instruction to avoid “excessively salty food” in the days before the collection, as patients may have reduced their normal sodium intake, thereby introducing bias. It is important to note that the study period predated the mandated use of nutritional food labeling, therefore attempts to lower sodium intake over a few days may not have been as easily achieved as is currently possible.40 If, however, behavioral changes did occur, this may have similarly affected all quartiles. Although a differential bias affecting primarily those in the higher sodium quartiles is possible, it would be difficult to assess this in the absence of follow-up sodium measurements, which is a major limitation of the study. Although a large study of temporal trends in sodium intake in the United States found no significant change over a 40-year period,37 these studies looked at a population level, and on the individual level, intakes may have varied substantially. Finally, the healthy worker effect, participation in an intensive worksite hypertension program, and lack of knowledge of change in hypertensive treatment after the end of program participation may reduce the generalizability of our findings.
Taken together, our results indicate that the association of sodium intake, measured at baseline, is not significantly associated with long-term cardiovascular outcomes. If such an association does exist in this population, our data suggest it is likely to be modest and muted by intervening factors over the prolonged period of observation. The association with all-cause mortality found in our study is of borderline significance, driven in part by noncardiovascular events, and in the absence of a proposed mechanism of causality may be a chance association. Further studies with repeated assessments of sodium excretion may more clearly define the long-term association between sodium intake and mortality outcomes.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This research was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, components of the National Institutes of Health (NIH), through CTSA grant numbers UL1RR02575, KL2RR025749, TL1RR025748, UL1TR001073-1, TL1TR001072-01, and KL2TR001071-01. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors would like to acknowledge the contribution of Dr. Aileen McGinn for her assistance with statistical review.
REFERENCES
- 1. Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606. [DOI] [PubMed] [Google Scholar]
- 2. Danael G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic factors. PLoS One Med 2009; 6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10. [DOI] [PubMed] [Google Scholar]
- 4. He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet 2011; 378:380–382. [DOI] [PubMed] [Google Scholar]
- 5. He FJ, Macgregor GA. Salt intake, plasma sodium, and worldwide salt reduction. Ann Med 2012; 44:S127–S137. [DOI] [PubMed] [Google Scholar]
- 6. MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double-blind study of three sodium intakes and long-term effects of sodium restriction in essential hypertension. Lancet 1989; 2:1244–1247. [DOI] [PubMed] [Google Scholar]
- 7. Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev 2011; 2011:CD004022. [DOI] [PubMed] [Google Scholar]
- 8. Feldman RD, Schmidt ND. Moderate dietary salt restriction increases vascular and systemic insulin resistance. Am J Hypertens 1999; 12:643–647. [DOI] [PubMed] [Google Scholar]
- 9. Strom BL, Yaktine AL, Oria M; Committee on the Consequences of Sodium Restriction in Populations, Food and Nutrition Board, Board on Population Health and Public Health Practice, Institute of Medicine Washington, D.C: The National Academies Press, 2013. [PubMed] [Google Scholar]
- 10. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kagan A, Popper JS, Rhoads GG, Yano K. Dietary and other risk factors for stroke in Hawaiian Japanese men. Stroke 1985; 16:390–396. [DOI] [PubMed] [Google Scholar]
- 12. Hu HH, Sheng WY, Chu FL, Lan CF, Chiang BN. Incidence of stroke in Taiwan. Stroke 1992; 23:1237–1241. [DOI] [PubMed] [Google Scholar]
- 13. Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 1995; 25:1144–1152. [DOI] [PubMed] [Google Scholar]
- 14. Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997; 315:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alderman MH, Cohen H, Madhavan S. Dietary sodium intake and mortality: the National Health and Nutrition Examination Survey (NHANES I). Lancet 1998; 351:781–785. [DOI] [PubMed] [Google Scholar]
- 16. Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, Willett WC. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 1998; 98:1198–1204. [DOI] [PubMed] [Google Scholar]
- 17. Tuomilehto J, Jousilahti P, Rastenyte D, Moltchanov V, Tanskanen A, Pietinen P, Nissinen A. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet 2001; 357:848–851. [DOI] [PubMed] [Google Scholar]
- 18. Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke 2004; 35:1543–1547. [DOI] [PubMed] [Google Scholar]
- 19. He J, Ogden LG, Vupputuri S, Bazzano LA, Loria C, Whelton PK. Dietary sodium intake and subsequent risk of cardiovascular disease in overweight adults. JAMA 1999; 282:2027–2034. [DOI] [PubMed] [Google Scholar]
- 20. Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III). J Gen Intern Med 2008; 23:1297–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen HW, Hailpern SM, Fang J, Alderman MH. Sodium intake and mortality in the NHANES II follow-up study. Am J Med 2006; 119:275.e7–275.14. [DOI] [PubMed] [Google Scholar]
- 22. Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol 2007; 22:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Kondo T, Inaba Y, Tanabe N, Tamakoshi A. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr 2008; 88:195–202. [DOI] [PubMed] [Google Scholar]
- 24. Larsson SC, Virtanen MJ, Mars M, Mannisto S, Pietinen P, Albanes D, Virtamo J. Magnesium, calcium, potassium, and sodium intakes and risk of stroke in male smokers. Arch Intern Med 2008; 168:459–465. [DOI] [PubMed] [Google Scholar]
- 25. Takachi R, Inoue M, Shimazu T, Sasazuki S, Ishihara J, Sawada N, Yamaji T, Iwasaki M, Iso H, Tsubono Y, Tsugane S. Consumption of sodium and salted foods in relation to cancer and cardiovascular disease: the Japan Public Health Center-based Prospective Study. Am J Clin Nutr 2010; 91:456–464. [DOI] [PubMed] [Google Scholar]
- 26. Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, Jerums G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011; 34:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, Wadén J, Tolonen N, Saraheimo M, Gordin D, Groop PH; FinnDiane Study Group. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011; 34:861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011; 171:1183–1191. [DOI] [PubMed] [Google Scholar]
- 29. Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerova J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA; European Project on Genes in Hypertension (EPOGH) Investigators. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011; 305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 30. O’Donnell MJ, Yusuf S, Mente A, Gao P, Mann JF, Teo K, McQueen M, Sleight P, Sharma AM, Dans A, Probstfield J, Schmieder RE. Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA 2011; 306:2229–2238. [DOI] [PubMed] [Google Scholar]
- 31. Taylor RS, Ashton KE, Moxham T, Hooper L, Ebrahim S. Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review). Am J Hypertens 2011; 24:843–853. [DOI] [PubMed] [Google Scholar]
- 32. Intersalt Cooperative Research Group. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 1988; 297:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH. Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991; 324:1098–1104. [DOI] [PubMed] [Google Scholar]
- 34. Alderman MH, Cohen HW, Sealey JE, Laragh JH. Pressor responses to antihypertensive drug types. Am J Hypertens 2010; 23:1031–1037. [DOI] [PubMed] [Google Scholar]
- 35. Gonzalez MC, Cohen HW, Sealey JE, Laragh JH, Alderman MH. Enduring direct association of baseline plasma renin activity with all-cause and cardiovascular mortality in hypertensive patients. Am J Hypertens 2011; 24:1181–1186. [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro III AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bernstein AM, Willett WC. Trends in 24-h urinary sodium excretion in the United States, 1957–2003: a systematic review. Am J Clin Nutr 2010; 92:1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999; 150:341–353. [DOI] [PubMed] [Google Scholar]
- 39. Barrett-Connor E. Nutrition epidemiology: how do we know what they ate? Am J Clin Nutr 1991; 54:182S–187S. [DOI] [PubMed] [Google Scholar]
- 40. Wartella EA, Lichtenstein AH, Boon CS, eds; National Research Council. Examination of Front of Package Nutrition Rating Systems and Symbols: Phase I Report. National Academies Press: Washington, DC, 2010. [PubMed] [Google Scholar]