Abstract
BACKGROUND
Short-term clinical trials suggest that dietary protein lowers blood pressure (BP); however, long-term effects of total, animal, and plant proteins are less clear. Our goal was to evaluate effects of these dietary proteins on mean systolic BP (SBP) and diastolic BP (DBP) and incident high BP (HBP) risk among middle-aged adults in the Framingham Offspring Study.
METHODS
Men and women (aged 30–54 years) without prevalent HBP, cardiovascular disease, or diabetes with 3-day dietary records from exams 3 or 5 (n = 1,361) were included and followed for a mean of 11.3 years for development of HBP. Protein intakes adjusted for body size were derived using the residual method. Analysis of covariance and Cox proportional hazard’s models were used to adjust for age, sex, education, height, activity, smoking, fat calories, diet quality, and body mass index.
RESULTS
Higher protein intakes were associated with lower mean SBP and DBP. Both animal and plant proteins lowered BP and led to statistically significant reductions in HBP risk (hazard ratios of 0.68 and 0.51, respectively). Participants in the highest tertile of total protein intake had 40% less risk (95% confidence interval [CI], 0.45–0.78) of developing HBP. Beneficial effects of protein were apparent for men and women and for normal–weight and overweight individuals. Higher protein diets also characterized by higher fiber intakes led to a 59% reduction (95% CI, 0.37–0.66) in HBP risk.
CONCLUSIONS
Adults consuming more dietary protein from either plant or animal sources had lower long-term risks of HBP.
Keywords: blood pressure, diet patterns, hypertension, protein.
High blood pressure (HBP) is a major cause of cardiovascular disease and renal failure.1 Evidence from recent reviews2,3 and meta-analyses4,5 suggests that dietary protein consumption may benefit BP. Both of these meta-analyses of short-term clinical trials concluded that compared with carbohydrates, higher dietary protein led to modest BP reductions. In the Rebholtz meta-analysis, both animal and plant proteins were found to have similar short-term effects on BP.5 Observational studies, which have been largely cross-sectional, have shown weak beneficial effects of plant proteins, in particular on BP.2 Few observational studies (and no clinical trials) have addressed the long-term effects of animal and plant proteins on BP, and results are conflicting.6–8
The Dietary Approaches to Stop Hypertension (DASH) studies have documented the importance of diet patterns to BP.9 The greatest reductions in BP in the DASH trials have been seen with higher intakes of fruits and vegetables (FV) plus higher intakes of low-fat dairy products (an important source of dietary protein). In general, the effects of FV alone are modest.10 Higher dietary fiber intakes have also been associated with a BP-lowering effect, particularly among hypertensive individuals.11 Combined effects of dietary protein and fiber intakes on BP are less clear, although at least 1 review suggests that they may be additive.12
Our goal in this study was to evaluate the longitudinal effects of the amount and type of dietary protein on mean BP and the risk of incident HBP among middle-aged adults in the Framingham Offspring Study (FOS). The interactive effects of dietary protein with FV and dietary fiber were also examined.
METHODS
Study population
The FOS began in 1971 with enrollment of 5,124 offspring (and spouses) from the original Framingham Heart Study cohort. Participants were evaluated at roughly 4-year intervals following the baseline visit, and BP was measured at each exam. Diet was assessed using 3-day diet records at the third (starting in 1984) and fifth examination visits.13
Participants (n = 3,284) who had complete dietary data at examination 3, 5, or both were included. For those with complete data at both visits, mean dietary protein intake was estimated. For those with dietary data only at exam 3 or 5, protein intake from that exam was used. Exam 3 served as the baseline visit for eligible participants with dietary data at that visit; exam 5 served as the baseline for those with missing dietary data at exam 3.
Additional exclusions included 1,284 who were outside of the age range (30–54 years) at the time of dietary assessment; 294 with extreme values for total energy (<1,200 or >4,000 kcal/day for men; <1,000 or >3,500 kcal/day for women), alcohol (>20% of calories) or food consumed (e.g., >35 eggs/wk), a body mass index (BMI) <18.5 (weight (in kilograms) divided by average height (in meters squared)), missing data on potential confounders, or lacking all follow-up data; and 345 participants with prevalent type 2 diabetes, cardiovascular disease, or hypertension at baseline, leaving 1,361 participants available for proportional hazard’s modeling. For calculation of mean BP at the first follow-up exam after baseline, an additional 26 participants were excluded who were missing that data.
Dietary assessment
Approximately 16,000 days of diet records were collected during exams 3 and 5. A trained nutritionist instructed families in the completion of diet records (on 2 weekdays and 1 weekend day) and the use of 2-dimensional food models for estimating portion sizes. Diet records were entered into the Nutrition Data System (NDS), developed at the University of Minnesota.14 The NDS program calculated each participant’s daily intake of protein (grams) in addition to other macro- and micronutrients, including dietary fiber. The NDS provided estimated daily intakes for total, animal, and plant protein. FV intake per day (quantified in cup equivalents) was calculated by linking food codes output from the NDS system with US Department of Agriculture pyramid food codes derived from the Continuing Survey of Food Intake of Individuals.15 FV and dietary fiber were combined with dietary protein in selected analyses.
Blood pressure outcomes
A Framingham physician measured BP using a standard mercury sphygmomanometer. Two measurements were taken after participants sat quietly for 5 minutes. Mean baseline systolic BP (SBP) and diastolic BP (DBP) values were estimated during the same examination cycle in which baseline dietary assessment was completed. Follow-up SBP and DBP were measured at 4-year intervals at the routine examination visits. Incident HBP was defined as any of the following: mean SBP ≥140mm Hg or DBP ≥ 90mm Hg at 2 consecutive exams, mean SBP ≥160mm Hg or DBP ≥95mm Hg on a single exam, or use of antihypertensive medication for BP-lowering purposes. Follow-up for incident HBP started at the time of the last dietary protein assessment and continued until the first of the following: incident HBP, loss to follow-up, death, or end of follow-up (through the end of exam 7).
Potential confounders
The following were considered as potential confounding factors: age, sex, education level (high school or less vs. beyond high school), physical activity, cigarette smoking, Healthy Eating Index (HEI) score, baseline BP, height, baseline BMI, and dietary fat intake (% of calories from fat and saturated fat). A physical activity index, a modification of the original method by Kannel,16 was calculated as the number of self-reported hours per day spent doing moderate or vigorous activities multiplied by a numeric weight derived from the oxygen consumption required (liters per minute) for that activity. Cigarette smoking was assessed at every exam visit. Participants were considered current smokers if they smoked at any time during the baseline exam period. Mean cigarettes smoked per day during the same period were estimated. Body weight was measured at baseline using a calibrated spring balance scale, and height was measured at each exam with a standard stadiometer. To reduce the effect of measurement error, average adult height from all available measures for those between ages 30 and 54 years was used to calculate baseline BMI. To measure overall diet quality, HEI scores were derived from the 2005 MyPyramid Food Guidance System, which incorporates key recommendations in the 2005 Dietary Guidelines for Americans.17 Other specific dietary factors such as total energy, dietary sodium, monounsaturated fatty acids, alcohol intake, FV, and fiber were assessed as potential confounders and dropped from the final models as they led to less than a 5% change in the effect estimates.
Statistical analysis
To derive estimates of protein intake (total, animal, plant) that were adjusted for body size, mean intakes were computed using the residual method, regressing each participant’s protein intake on body weight. This method was modeled from Willett’s energy-adjustment method,18 with resulting protein residuals being uncorrelated with (and therefore not confounded by) body weight.
For the initial analyses, each protein variable was classified into tertiles using the weight-adjusted residual variables. To assess the effects of protein combined with intakes of FV and fiber, weight-adjusted protein residuals were dichotomized (< median vs. ≥ median), as were intakes of total FV and fiber, using sex-specific medians (FV, 2.92 cup-equivalents/day for men and 2.65 cup-equivalents/day for women; fiber, 16.83g/day for men and 13.38g/day for women). Diet combinations were created by classifying each participant into 1 of 4 dietary intake categories (e.g., low intakes of both protein and FV, low protein and high FV intakes, high protein and low FV intakes, and high intakes of both).
Analysis of covariance modeling was used to compare adjusted mean SBP and DBP levels after 4 years of follow-up across tertiles of protein consumption as well as protein combined with FV and fiber intakes. In this prospective analysis, it was necessary to consider participants who developed HBP during the follow-up period (since hypertension treatment would impact follow-up BP levels). For those who developed HBP but were not on drug treatment, no adjustment to the follow-up SBP or DBP measures was needed. However, for new cases of treated HBP, mean baseline BP levels were substituted for follow-up BP.
Cox proportional hazard’s models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for long-term risk of developing HBP associated with dietary protein intake independently and combined with FV and fiber intakes. Final multivariable models included the following potential confounders: age, sex, education level, height, physical activity, smoking status, HEI score, and percent of energy from fat. Since BMI could be a causal intermediate in these analyses, the final models were run with and without follow-up BMI. In addition, stratified analyses were completed by protein type (animal vs. plant), baseline BMI (<25 vs. ≥25kg/m2), and sex. All analyses were performed using Statistical Analysis Systems software, version 9.1 (SAS Institute, Cary, NC ).
RESULTS
Baseline characteristics of FOS participants in each tertile of total protein intake are shown in Table 1. Those with higher protein intakes were taller, more educated, leaner, and more likely to be male (P < 0.001, for all). Those with lower protein intakes consumed fewer calories, FV, and fiber (P < 0.001, for all). Baseline BP was slightly lower among individuals in the highest tertile of baseline protein intake (P = 0.02 for SBP; P = 0.06 for DBP).
Table 1.
Baseline characteristics by tertile of total protein intake in the Framingham Offspring Study
| Tertiles of estimated protein intake | ||||
|---|---|---|---|---|
| Baseline characteristics | 1 (low) | 2 | 3 (high) | P value |
| n | 444 | 445 | 446 | |
| Age, y | 44.4 (6.2) | 43.2 (6.4) | 43.4 (6.5) | 0.01 |
| Height, cm | 167.3 (8.9) | 168.1 (8.6) | 171.3 (9.3) | <0.001 |
| Body mass index, kg/m2 | 26.3 (4.7) | 24.8 (3.6) | 25.0 (3.5) | <0.001 |
| Male, % | 27.7 | 37.8 | 60.0 | <0.001 |
| Current smoker, % | 28.4 | 24.5 | 24.7 | 0.21 |
| Attained high school education, % | 58.2 | 70.1 | 70.8 | <0.001 |
| Physical activity index | 12.2 (7.3) | 12.3 (8.2) | 12.8 (8.1) | 0.42 |
| Systolic blood pressure, mm Hg | 117.7 (13.0) | 115.7 (11.6) | 114.6 (11.9) | 0.02 |
| Diastolic blood pressure, mm Hg | 76.3 (8.4) | 75.2 (8.5) | 75.1 (8.0) | 0.06 |
| Energy, kcal/d | 1,555 (364) | 1,876 (414) | 2,393 (535) | <0.001 |
| Total protein,a g/d | 58.0 (8.5) | 77.7 (4.8) | 102.6 (13.8) | <0.001 |
| Animal protein,a g/d | 39.3 (8.5) | 54.8 (7.6) | 75.1 (13.5) | <0.001 |
| Plant protein,a g/d | 17.7 (5.0) | 21.7 (6.7) | 26.2 (8.9) | <0.001 |
| Calories from carbohydrates, % | 47.6 (8.3) | 45.1 (7.6) | 43.4 (7.7) | <0.001 |
| Calories from fat, % | 35.4 (6.3) | 36.2 (6.4) | 36.4 (6.5) | 0.049 |
| Calories from saturated fat, % | 12.2 (2.9) | 12.3 (2.9) | 12.6 (3.0) | 0.06 |
| Fruits/vegetables, cup eq/d | 2.6 (1.2) | 3.0 (1.3) | 3.5 (1.7) | <0.001 |
| Fiber, g/d | 13.1 (4.5) | 15.5 (5.4) | 18.9 (7.3) | <0.001 |
| Whole grains, oz eq/d | 0.5 (0.6) | 0.6 (0.8) | 0.7 (0.8) | <0.001 |
Values are mean (standard deviation) for continuous variables and % for categorical variables. P denotes the significance of the linear trend across tertiles of protein intake.
aProtein intakes expressed as weight-adjusted residuals (g/d).
Table 2 shows that protein consumption (total, animal, plant) was inversely associated with both SBP and DBP after 4 years of follow-up. Overweight participants (BMI ≥25kg/m2) had generally higher BP levels than leaner individuals (BMI <25kg/m2); however, protein intake was linked with lower BPs for both groups. Among those who were overweight, both animal and plant proteins led to lower DBP levels, while leaner participants who consumed more animal and plant proteins had lower SBP levels.
Table 2.
Multivariable-adjusted blood pressure levels by tertiles of total, animal, and plant protein intake among adults
| Baseline intake | Alla | Baseline BMIb < 25kg/m2 | Baseline BMIb ≥ 25kg/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total proteinc | n | SBP | DBP | n | SBP | DBP | n | SBP | DBP |
| T1 (low) | 444 | 120.5 (0.6) | 77.1 (0.4) | 195 | 116.5 (0.9) | 73.7 (0.6) | 249 | 124.4 (0.9) | 80.4 (0.5) |
| T2 | 445 | 119.1 (0.6) | 76.5 (0.4) | 251 | 115.8 (0.8) | 74.4 (0.5) | 194 | 122.7 (0.9) | 79.0 (0.6) |
| T3 (high) | 446 | 117.7 (0.6) | 75.6 (0.4) | 237 | 113.1 (0.8) | 72.9 (0.6) | 209 | 122.6 (0.9) | 78.3 (0.6) |
| P for trend | 0.001 | 0.02 | 0.006 | 0.29 | 0.17 | 0.02 | |||
| Animal proteinc | |||||||||
| T1 (low) | 445 | 120.0 (0.6) | 77.0 (0.4) | 207 | 116.2 (0.9) | 73.8 (0.6) | 238 | 124.1 (0.9) | 80.2 (0.6) |
| T2 | 442 | 119.8 (0.6) | 76.7 (0.4) | 238 | 115.7 (0.8) | 74.2 (0.6) | 204 | 124.2 (0.9) | 79.5 (0.6) |
| T3 (high) | 448 | 117.6 (0.6) | 75.6 (0.4) | 238 | 113.5 (0.8) | 73.1 (0.6) | 210 | 121.7 (0.9) | 78.2 (0.6) |
| P for trend | 0.02 | 0.049 | 0.03 | 0.41 | 0.08 | 0.02 | |||
| Plant proteinc | |||||||||
| T1 (low) | 445 | 120.7 (0.6) | 77.5 (0.4) | 192 | 116.4 (1.0) | 74.4 (0.6) | 253 | 125.1 (0.8) | 80.7 (0.5) |
| T2 | 446 | 118.4 (0.6) | 76.5 (0.4) | 247 | 115.4 (0.8) | 73.7 (0.5) | 199 | 121.3 (0.9) | 79.3 (0.6) |
| T3 (high) | 444 | 118.3 (0.6) | 75.3 (0.4) | 244 | 113.8 (0.9) | 73.0 (0.6) | 200 | 123.1 (1.0) | 77.6 (0.6) |
| P for trend | 0.03 | 0.001 | 0.04 | 0.11 | 0.06 | 0.0002 | |||
Values are mean (standard error).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; T, tertile.
aAdjusted for age, sex, education, height, activity, smoking status, % energy from fat, BMI status (normal, overweight, or obese).
bAdjusted for age, sex, education, height, activity, smoking status, % energy from fat.
cProtein intakes expressed as weight-adjusted residuals in grams per day.
The effects of dietary protein combined with total FV or fiber consumption on adjusted mean BP levels are shown in Table 3. First, we explored independent effects of the sex-specific tertiles of FV and fiber intake on mean BP levels (data not shown) and found that those in the highest tertile of FV intake had a mean SBP that was 2.1mm Hg lower (P = 0.02) and a DBP that was 0.8mm Hg lower (P = 0.16) than those with the lowest intakes. The highest dietary fiber intakes were associated with stronger BP-lowering effects (P = 0.001 for SBP; P = 0.002 for DBP).
Table 3.
Multivariable-adjusted blood pressure levels according to combined intakes of total protein with fruits and vegetables or fiber
| Mean blood pressures after 4 years of follow-up | |||||
|---|---|---|---|---|---|
| Baseline diet patternb | N | SBPb | P value | DBPb | P value |
| All | |||||
| Protein + FV | |||||
| Low protein, low FV (reference) | 387 | 120.3 (0.7) | – | 76.8 (0.4) | – |
| Low protein, high FV | 279 | 120.4 (0.8) | 0.88 | 77.4 (0.5) | 0.35 |
| High protein, low FV | 280 | 118.6 (0.8) | 0.10 | 76.2 (0.5) | 0.31 |
| High protein, high FV | 389 | 117.4 (0.7) | 0.002 | 75.5 (0.4) | 0.02 |
| Protein + fiber | |||||
| Low protein, low fiber (reference) | 413 | 121.1 (0.6) | – | 77.3 (0.4) | – |
| Low protein, high fiber | 253 | 119.0 (0.8) | 0.04 | 76.7 (0.5) | 0.32 |
| High protein, low fiber | 256 | 119.2 (0.8) | 0.07 | 77.0 (0.5) | 0.63 |
| High protein, high fiber | 413 | 117.1 (0.6) | <0.001 | 75.0 (0.4) | <0.001 |
| Men | |||||
| Protein + FV | |||||
| Low protein, low FV (reference) | 113 | 124.2 (1.1) | – | 80.5 (0.8) | – |
| Low protein, high FV | 80 | 122.7 (1.4) | 0.40 | 80.2 (0.9) | 0.75 |
| High protein, low FV | 165 | 120.0 (1.0) | 0.13 | 79.5 (0.6) | 0.31 |
| High protein, high FV | 200 | 120.2 (0.9) | 0.006 | 78.3 (0.6) | 0.02 |
| Protein + fiber | |||||
| Low protein, low fiber (reference) | 128 | 123.2 (1.1) | – | 80.4 (0.7) | – |
| Low protein, high fiber | 65 | 124.5 (1.5) | 0.49 | 80.2 (1.0) | 0.88 |
| High protein, low fiber | 150 | 121.7 (1.0) | 0.30 | 79.7 (0.7) | 0.46 |
| High protein, high fiber | 215 | 120.5 (0.8) | 0.055 | 78.2 (0.6) | 0.02 |
| Women | |||||
| Protein + FV | |||||
| Low protein, low FV (reference) | 274 | 117.8 (0.8) | – | 74.4 (0.5) | – |
| Low protein, high FV | 199 | 118.5 (1.0) | 0.55 | 75.4 (0.6) | 0.21 |
| High protein, low FV | 115 | 116.1 (1.2) | 0.24 | 73.6 (0.8) | 0.39 |
| High protein, high FV | 189 | 115.2 (1.0) | 0.04 | 73.4 (0.6) | 0.22 |
| Protein + fiber | |||||
| Low protein, low fiber (reference) | 285 | 119.5 (0.8) | – | 75.2 (0.5) | – |
| Low protein, high fiber | 188 | 115.9 (1.0) | 0.004 | 74.2 (0.6) | 0.24 |
| High protein, low fiber | 106 | 117.8 (1.3) | 0.25 | 75.3 (0.8) | 0.94 |
| High protein, high fiber | 198 | 114.4 (0.9) | <0.001 | 72.6 (0.6) | 0.001 |
Values are mean (standard error).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FV, fruits and vegetables; SBP, systolic blood pressure.
aCutpoint for dichotomous baseline intakes was set at the median (<median vs. ≥median) for protein intake (as weight-adjusted residuals, g/d), FV (cup equivalents/d), and fiber (g/d).
bAdjusted for age, sex (only for all subjects model), education, height, activity, smoking status, % energy from fat, baseline BMI.
Table 3 shows that participants who consumed more protein combined with either higher FV or fiber intakes had the lowest mean SBP and DBP levels. For example, adults with higher protein and fiber intakes had SBP levels that were 4.0mm Hg lower (and DBP levels that were 2.3mm Hg lower) than those with lower intakes of both (P < 0.001 for both SBP and DBP). In general, the effects of dietary fiber on BP were stronger than those for FV. Finally, more protein consumption led to beneficial effects on BP for both men and women, while the benefits of dietary fiber seemed stronger for women than for men.
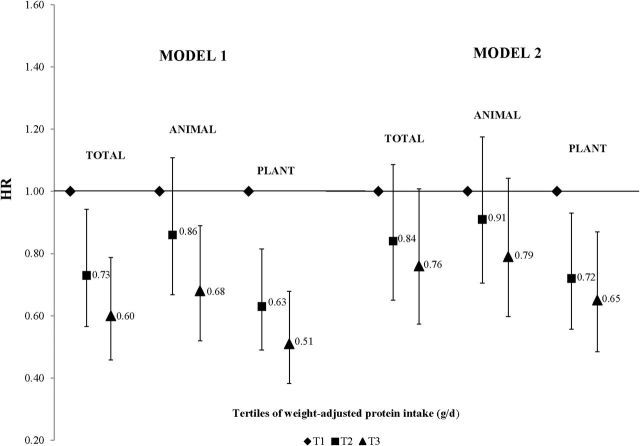
There were 346 cases of incident HBP that occurred during the follow-up period (mean time = 11.3 years). Figure 1 illustrates the HRs for incident HBP associated with total, animal, and plant protein intakes. After adjusting for age, sex, education, height, physical activity, smoking, HEI score, and percent of calories from fat, participants in the highest tertile of total protein intake had a 40% lower risk of incident HBP (HR, 0.60; 95% CI, 0.45–0.78) compared with those in the lowest tertile. Both animal (HR, 0.68; 95% CI, 0.52–0.89) and plant (HR, 0.51; 95% CI, 0.38–0.68) protein consumption led to statistically significant reductions in HBP risk. To determine whether BMI might explain any of the effects of protein on HBP risk, baseline BMI was added to model 2; statistically significant reductions in HBP risk remained for high plant protein intakes but were somewhat attenuated for total and animal proteins.
Figure 1.
Adjusted hazard ratios for incident high blood pressure (HBP) by tertile of total, animal, and plant protein intake. Model 1 is adjusted for age, sex, education level, height, physical activity, smoking status, Healthy Eating Index score, and percent of calories from fat. Model 2 is additionally adjusted for body mass index. Abbreviation: HR, hazard ratio.
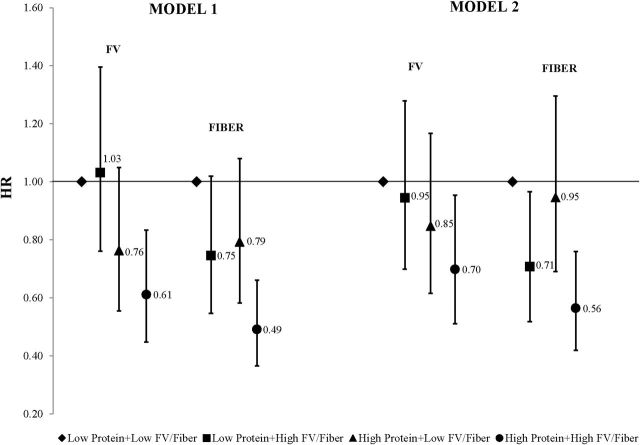
Adjusted HRs for incident HBP associated with protein combined with either FV or fiber are shown in Figure 2. Overall, participants who consumed more protein combined with more FV intakes had a statistically significant 39% reduction in risk of HBP compared with those in the referent group (model 1). Those who consumed more protein with a diet higher in fiber had a 51% lower risk (95% CI, 0.37–0.66) of incident HBP than those with lower intakes of both. These effects were slightly weakened by the addition of BMI to model 2.
Figure 2.
Adjusted hazard ratios for incident high blood pressure (HBP) by category of total protein combined with fruits and vegetables (FV) or fiber. Four patterns of dietary intake include the following: low protein/low FV (or fiber; referent category); low protein/high FV (or fiber); high protein/low FV (or fiber); and high protein/high FV (or fiber). High vs. low protein: < median vs. ≥ median. High vs. low FV (and fiber): < sex-specific vs. ≥ sex-specific median. Model 1 is adjusted for age, sex, education level, height, physical activity, smoking status, Healthy Eating Index score, and percent of calories from fat. Model 2 is additionally adjusted for body mass index. Abbreviations: FV, fruits and vegetables; HR, hazard ratio.
DISCUSSION
In this study, adults who consumed more protein, whether from animal or plant sources, had statistically significantly lower SBP and DBP levels after 4 years of follow-up. In general, these beneficial effects were evident for both overweight (BMI ≥25kg/m2) and normal weight (BMI <25kg/m2) individuals. Consuming more dietary protein was also associated with lower long-term risks of incident HBP. When the diet was also characterized by higher intakes of fiber, higher protein intakes led to 40%–60% reductions in risk of HBP.
Our results add to a very limited number of longer-term prospective studies of protein intake and BP in adults.2 Our results contrast with those of both the Western Electric Study (WES) and the Seguimiento Universidad de Navarra (SUN) study that found only vegetable protein consumption was inversely correlated with BP.6,8 In the Coronary Artery Risk Development in Young Adults (CARDIA) study, total protein intake among 4,100 young adults, aged 18–30 years, was inversely associated with both SBP and DBP, but the effects were strongest for DBP.7 In the Framingham Study with a somewhat older study population, the protein effects were generally similar for SBP and DBP.
Short-term clinical trials of protein biomarkers also suggest beneficial effects on BP.2,19,20 These clinical trials have typically compared higher-protein diets with higher-carbohydrate diets, making it difficult to separate beneficial effects of higher protein intakes from those of a lower-carbohydrate diet. The OmniHeart Trial compared the following 3 “healthy dietary” interventions among adults with prehypertension or stage 1 hypertension: a diet similar to that in the DASH with 58% of calories from carbohydrates (vs. 48% in the 2 other arms); a diet higher in unsaturated fats, particularly monounsaturated fats (21% vs. 13% from monounsaturated fats); and a diet higher in protein (25% of calories from protein vs. 15%).21 Partial substitution of carbohydrates with either protein or unsaturated fats led to greater BP reductions than the higher carbohydrate diet alone. The current long-term data from the FOS offer important evidence to suggest that both animal and plant proteins have BP-lowering effects in nonhypertensive adults.
Dietary proteins may affect BP through a number of pathways, and those pathways may differ according to the amino acid composition of the food source.3 Arginine, an amino acid found in many plant and animal sources including eggs, acts as a vasodilator through nitric oxide pathways, contributing to lower BP.22 Also, the increased plasma amino acid levels from a higher protein diet may affect proximal sodium reabsorption or lead to alterations in cell permeability, thereby enhancing BP-related renal dynamics.23 Dairy products are common sources of animal protein in the American diet. Some studies including at least 1 meta-analysis24 suggest that biologically-active peptides from milk protein, including 2 casein-derived tripeptides (isoleucine-proline-proline and valine-proline-proline), may directly impact BP by inhibiting the angiotensin-converting enzyme pathway.25
It has been suggested in a number of studies including those of the landmark DASH diet trials that FV intakes are linked with a lower risk of hypertension.9,26 Mechanisms could involve antioxidant defense capacity and the ability to combat oxidative stress.27 FV contain phytochemicals including flavonols, phytosterols, and polyphenols that are thought to have BP-lowering effects.28 They are also important sources of other nutrients such as magnesium and potassium, which have known BP-lowering effects.13 Despite these purported mechanisms, the overall effects of FV on BP are generally modest.10
We found a strong beneficial effect of dietary fiber on BP when combined with a high-protein diet. Several meta-analyses of randomized controlled trials provide strong support for BP-lowering effects of fiber, particularly among hypertensive participants.11,29 While exact underlying mechanisms are unclear, it has been hypothesized that dietary fiber enhances insulin sensitivity and improves vascular endothelial function, which may, in turn, benefit BP.30,31
It is also possible that the observed benefits of FV or dietary fiber on BP could be a consequence of intermediate effects on BMI, as was seen in a recent study.32 Our analyses suggest that while there is modest attenuation of the results by the inclusion of BMI in the models, independent beneficial effects of these diet patterns characterized by higher intakes of protein and higher intakes of FV or fiber remain.
Epidemiologic studies of diet–disease relations share a number of limitations. Of necessity, dietary data for adults are obtained by self-report and are thus subject to both random error and potentially biased reporting. In addition, of the 5,124 participants enrolled in the FOS, only 3,284 (64%) provided dietary data. Further, we had no protein biomarker information in this study to validate reported intakes.
The FOS has a number of important strengths as well. The available diet record data were collected in a standardized fashion and, of those included in these analyses, most participants (62%) contributed 6 days of diet records. Our study is one of very few long-term studies that have separated the independent effects of animal and plant proteins on BP. Further, in this study, we extensively and systematically collected information on potential confounders, enhancing both the precision and validity of the results.
CONCLUSIONS
The longitudinal data from this study suggest that higher intakes of dietary protein from both animal and plant sources may be linked with significantly lower risks for developing HBP during the middle adult years. The observed beneficial effects on BP were partially attenuated by controlling for intermediate effects of protein on body fat. These results provide no evidence to suggest that individuals concerned about the development of HBP should avoid dietary protein. Rather, protein intake may play a role in the long-term prevention of HBP.
DISCLOSURE
The author declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (NHLBI/NIH Contract N01-HC-25195), the Boston University School of Medicine, and a grant from the American Egg Board/US Department of Agriculture.
REFERENCES
- 1. Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, Roccella EJ, Stout R, Vallbona C, Winston MC, Karimbakas J. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288: 1882–1888. [DOI] [PubMed] [Google Scholar]
- 2. Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, van’t V, Geleijnse JM. Dietary protein and blood pressure: a systematic review. PLoS One 2010;5: e12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teunissen-Beekman KF, van Baak MA. The role of dietary protein in blood pressure regulation. Curr Opin Lipidol 2013;24: 65–70. [DOI] [PubMed] [Google Scholar]
- 4. Tielemans SM, Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Geleijnse JM. Intake of total protein, plant protein and animal protein in relation to blood pressure: a meta-analysis of observational and intervention studies. J Hum Hypertens 2013;27: 564–571. [DOI] [PubMed] [Google Scholar]
- 5. Rebholz CM, Friedman EE, Powers LJ, Arroyave WD, He J, Kelly TN. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am J Epidemiol 2012;176 Suppl 7: S27––S43. [DOI] [PubMed] [Google Scholar]
- 6. Alonso A, Beunza JJ, Bes-Rastrollo M, Pajares RM, Martinez-Gonzalez MA. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Arch Med Res 2006;37: 778–786. [DOI] [PubMed] [Google Scholar]
- 7. Liu K, Ruth KJ, Flack JM, Jones-Webb R, Burke G, Savage PJ, Hulley SB. Blood pressure in young blacks and whites: relevance of obesity and lifestyle factors in determining differences. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Circulation 1996;93: 60–66. [DOI] [PubMed] [Google Scholar]
- 8. Stamler J, Liu K, Ruth KJ, Pryer J, Greenland P. Eight-year blood pressure change in middle-aged men: relationship to multiple nutrients. Hypertension 2002;39: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 9. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47: 296–308. [DOI] [PubMed] [Google Scholar]
- 10. Hartley L, Igbinedion E, Holmes J, Flowers N, Thorogood M, Clarke A, Stranges S, Hooper L, Rees K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst Rev 2013;6: CD009874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Whelton SP, Hyre AD, Pedersen B, Yi Y, Whelton PK, He J. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J Hypertens 2005;23: 475–481. [DOI] [PubMed] [Google Scholar]
- 12. Lee YP, Puddey IB, Hodgson JM. Protein, fibre and blood pressure: potential benefit of legumes. Clin Exp Pharmacol Physiol 2008;35: 473–476. [DOI] [PubMed] [Google Scholar]
- 13. Posner BM, Martin-Munley SS, Smigelski C, Cupples LA, Cobb JL, Schaefer E, Miller DR, D’Agostino RB. Comparison of techniques for estimating nutrient intake: the Framingham Study. Epidemiology 1992;3: 171–177. [DOI] [PubMed] [Google Scholar]
- 14. Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc 1988;88: 1268–1271. [PubMed] [Google Scholar]
- 15. Bowman SA, Friday JE, Moshfegh A. MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003–2004 [Online]. Beltsville, MD: Food Surveys Research Group. Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture. Available at: http://www.ars.usda.gov/ba/bhnrc/fsrgc/fsrg. Accessed 11 July 2014.
- 16. Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med 1979;139: 857–861. [PubMed] [Google Scholar]
- 17. Guenther PM, Reedy J, Krebs-Smith SM, Reeve BB, Basiotis PP. Development and Evaluation of the Healthy Eating Index-2005: Technical Report. Center for Nutrition Policy and Promotion, US Department of Agriculture, 2007. Available at: http://www.cnpp.usda.gov/HealthyEatingIndex.htm. Accessed 11 July 2014.
- 18. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65: 1220S–1228S. [DOI] [PubMed] [Google Scholar]
- 19. Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 2006;166: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stamler J, Elliott P, Kesteloot H, Nichols R, Claeys G, Dyer AR, Stamler R. Inverse relation of dietary protein markers with blood pressure. Findings for 10,020 men and women in the INTERSALT Study. INTERSALT Cooperative Research Group. INTERnational study of SALT and blood pressure. Circulation 1996;94: 1629–1634. [DOI] [PubMed] [Google Scholar]
- 21. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, III, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005;294: 2455–2464. [DOI] [PubMed] [Google Scholar]
- 22. Palloshi A, Fragasso G, Piatti P, Monti LD, Setola E, Valsecchi G, Galluccio E, Chierchia SL, Margonato A. Effect of oral L-arginine on blood pressure and symptoms and endothelial function in patients with systemic hypertension, positive exercise tests, and normal coronary arteries. Am J Cardiol 2004;93: 933–935. [DOI] [PubMed] [Google Scholar]
- 23. Woods LL. Mechanisms of renal hemodynamic regulation in response to protein feeding. Kidney Int 1993;44: 659–675. [DOI] [PubMed] [Google Scholar]
- 24. Xu JY, Qin LQ, Wang PY, Li W, Chang C. Effect of milk tripeptides on blood pressure: a meta-analysis of randomized controlled trials. Nutrition 2008;24: 933–940. [DOI] [PubMed] [Google Scholar]
- 25. Nakamura Y, Yamamoto N, Sakai K, Okubo A, Yamazaki S, Takano T. Purification and characterization of angiotensin I-converting enzyme inhibitors from sour milk. J Dairy Sci 1995;78: 777–783. [DOI] [PubMed] [Google Scholar]
- 26. Boeing H, Bechthold A, Bub A, Ellinger S, Haller D, Kroke A, Leschik-Bonnet E, Muller MJ, Oberritter H, Schulze M, Stehle P, Watzl B. Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr 2012;51: 637–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gallistl S, Sudi KM, Cvirn G, Muntean W, Borkenstein M. Effects of short-term energy restriction and physical training on haemostatic risk factors for coronary heart disease in obese children and adolescents. Int J Obes Relat Metab Disord 2001;25: 529–532. [DOI] [PubMed] [Google Scholar]
- 28. Most MM. Estimated phytochemical content of the dietary approaches to stop hypertension (DASH) diet is higher than in the Control Study Diet. J Am Diet Assoc 2004;104: 1725–1727. [DOI] [PubMed] [Google Scholar]
- 29. Streppel MT, Arends LR, Veer Pv, Grobbee DE, Geleijnse JM. Dietary fiber and blood pressure: a meta-analysis of randomized placebo-controlled trials. Arch Intern Med 2005;165: 150–156. [DOI] [PubMed] [Google Scholar]
- 30. Bessesen DH. The role of carbohydrates in insulin resistance. J Nutr 2001;131: 2782S–2786S. [DOI] [PubMed] [Google Scholar]
- 31. Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why). J Hypertens 2001;19: 523–528. [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Manson JE, Gaziano JM, Buring JE, Sesso HD. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am J Hypertens 2012;25: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]