Abstract
Introduction:
We previously reported that female smokers evidence greater subjective craving and stress/emotional reactivity to personalized stress cues than males. The present study employed the same dataset to assess whether females in the follicular versus luteal phase of the menstrual cycle accounted for the gender differences.
Methods:
Two objective criteria, onset of menses and luteinizing hormone surge (evaluated via home testing kits), were used to determine whether female smokers were in either the follicular (n = 22) or the luteal (n = 15) phase of their menstrual cycle, respectively. The females and a sample of male smokers (n = 53) were then administered a laboratory-based cue reactivity paradigm that involved assessment of craving, stress, and emotional reactivity in response to counterbalanced presentations of both a personalized stress script and neutral/relaxed script.
Results:
While there were no significant differences between females in the follicular versus luteal phase on any outcome measure, females in the luteal menstrual phase reported greater craving than males whereas females in the follicular phase reported greater stress and arousal than males and perceived the stress cues as more emotionally aversive than males.
Conclusions:
This preliminary investigation suggests that gender differences in craving versus affective responding to stress cues may, in part, be explained variation by menstrual cycle phase. Study limitations and implications of the findings for future research and treatment are briefly discussed.
Introduction
Negative emotion and stress have an important role in the development and maintenance of smoking behavior.1–4 Since emotional and stress responding are known to vary between females and males,5–8 there is a need to elucidate the relationship between gender, emotional/stress reactivity, and smoking behavior. Our research group recently reported gender differences among smokers in response to negative affect/stress cues.9 Specifically, we observed greater subjective craving, stress, arousal, and negatively valenced emotion in female (n = 37) versus male (n = 53) smokers who were administered a laboratory-based cue reactivity (CR) procedure involving aural presentation of personalized stressful life event scripts. While there are a number of factors that potentially contribute to this gender difference, one obvious candidate is the reproductive hormone variation associated with menstrual cycle phase of female smokers. To our knowledge, no existing studies of gender differences in smoker’s reactivity to stress cues have reported on the potential role that menstrual cycle phase might play in observed CR differences. In the present study, we use the sample from our previously reported study to preliminarily address this question. This research is justifiably preliminary and exploratory given the relatively small sample and the absence of any previous studies on which to base specific hypotheses.
Methods
Overview
The present study focused on laboratory data obtained prior to and following the presentation of two cue types; a personalized stressful script and a personalized neutral/relaxed control script. Smoking cues were also presented during the laboratory session but gender differences in smoking CR were not observed (see previous report); consequently, these data are not considered here. Dependent measures consisted of subjective ratings of craving, stress, and other dimensions of emotionality (i.e., valence, arousal, and dominance; described below), which were obtained immediately after each cue presentation; while heart rate and skin conductance measures were obtained, no significant gender × cue type interactions were observed (see previous report); accordingly, these data are not considered here. Thus, the overall study design consisted of the between-subjects factor, gender, and the within-subjects factor corresponding to stressful versus neutral control cue types (the latter of which served as a covariate). Additional details pertaining to the methods of the parent study are described in our previous report.9
Participants
Eligible individuals (a) were nontreatment-seeking male and female smokers between the ages of 18–40, (b) smoked at least 10 cigarettes per day for the previous 3 months, (c) were not dependent on any nonnicotine substance, (d) were not using psychotropic medication. Females were also required to (a) have a regular menstrual cycle, 25–35 days in duration, (b) not meet criteria for premenstrual dysphoric disorder in the last 6 months, (c) not use a hormonal birth control or hormone replacement therapy, and (d) not be pregnant or have had a delivery within the past 3 months, (e) not have been breast feeding within the last 3 months, and (f) not have had a hysterectomy. A total of 37 females and 53 males were enrolled. Of the female participants, 22 versus 15 were randomized to receive laboratory CR procedures during the follicular versus luteal phase of their menstrual cycle, respectively (menstrual phase determination is described in Procedure below).
Cues
Personalized negative affect/stress and neutral/relaxing scripts were 75-s narrative descriptions of a recent very stressful event (not trauma) or personal neutral/relaxing experience, respectively, which were prepared with each participant at assessment. Both scripts were audio-recorded and presented over headphones.
Dependent Measures
All measures were obtained prior to and following the cue presentations. Craving was measured via the Questionnaire of Smoking Urges–Brief (QSU; Cox et al. 10), which has 10 items related to urges to smoke (7-point scales anchored with 1 = strongly disagree and 7 = strongly agree). A single, 10-cm (range 0–10) analog scale was used to evaluate stress (“How much stress do you feel at present?”). The Self-Assessment Manikin (SAM; Bradley and Lang11) employs three, 9-point scales to assess dimensions of emotion. The dimensions were arousal (1 = calm/relaxed; 9 = excited/nervous), valence (1 = sad; 9 = happy), and dominance (1 = no control; 9 = full control). A cartoon manikin that depicted varying degrees of each dimension represented points on each scale.
Procedure
All participants received a general clinical evaluation (collection of demographic data, smoking history data, nicotine dependence assessment via Fagerström Test for Nicotine Dependence (FTND); Heatherton et al. 12, psychiatric assessment via SCID-IV; First et al. 13, and menstrual cycle data). Female participants were randomized (see below) to receive a CR laboratory session in either the follicular or luteal phase of their menstrual cycle. Follicular phase determination was anchored to the onset of menses and corresponded to a noncontiguous 7-day period following menses, specifically 1–3 days following menses onset and 7–10 days following menses onset. By contrast, luteal phase was defined in relation to the onset of ovulation and corresponded to a contiguous 7-day period, specifically 6–13 days following ovulation onset. Ovulation was established via a home testing kit. While the use of ovulations kits was essential only for the luteal phase group, all female participants used the kits so that there would be no group differences with respect to the amount of effort/attention paid to cycle phase transitions.
Although the randomization plan was applied to the full sample, its implementation was not without compromise. Overall, it was unsuccessful in 32% (n = 12) of the participants; 27% (n = 10) consisted of participants randomized to the luteal phase but who received CR assessment in the follicular phase and 5% (n = 2) consisted of those randomized to the follicular phase but received CR in the luteal phase. Thus, the imbalance in the follicular and luteal groups is explained by the five times greater rate of randomization compromise in the luteal phase randomized group. The main reason the investigators compromised randomization was to minimize attrition that might have occurred if participants had to wait another full menstrual cycle (25–30 days) in order to conform to the randomization plan.
CR procedures were administered at the Medical University of South Carolina’s Clinical and Translational Research Center. Prior to the CR session, participants completed a breath alcohol (BA) assessment, urine drug screen (UDS) and urine pregnancy screening (females only). Participants were rescheduled if they evidenced a positive BA and/or UDS whereas a positive pregnancy test resulted in study withdrawal. All participants smoked prior to the CR session to ensure similar time elapsed since last cigarette; breath carbon monoxide (CO) was assessed to corroborate recent smoking.
CR sessions were conducted in a laboratory setting with a wall-mounted surveillance camera connected to a monitor in an adjacent room that enabled study staff to monitor cue presentation. Participants were seated in a reclining chair for the duration of the testing session. Prior to the CR session, participants completed baseline self-report measures. All participants were exposed to counterbalanced cue presentations each lasting 90 s. Immediately following each cue presentation, participants provided subjective ratings (described above) and then were instructed to watch a 10-min nature slideshow (the slideshow was designed to mitigate any carryover effects from the cue presentations). Instructions given to participants during cue presentations were prerecorded and presented on a computer. These instructions (and script cues described above) were delivered to participants via noise-canceling headphones.
Data Analytic Plan
One-way analysis of variance (ANOVA) (continuous measures) and chi-square test of independence (categorical measures) were used to assess group differences on various demographic, smoking-related, and procedural variables. Analysis of covariance (ANCOVA) was adopted to analyze subjective craving and emotional responses. Covariates included in the initial analyses were similar to those included in our previous report9 and consisted of the response during the neutral/relaxed script, FTND score, order of stimulus presentation, CO level at beginning of CR session, and days between study entry (screening) and CR session. Only significant covariates were retained in the final models.
Results
In general, the sample consisted of young (mean age, 29.6 years), employed (70%) smokers, with some postsecondary education (71.1%). Table 1 depicts key demographic and clinical variables for males, follicular phase females, and luteal phase females. Statistical tests (ANOVA and chi square) failed to identify any group differences on these variables (all p’s > .1 except for employment status where p = .09). Overall, the groups were similar demographically and with respect to smoking behavior.
Table 1.
Means (SE) or Percentages (Frequency) on Key Demographic and Clinical Measures
| Variable | Males (n = 53) | Follicular females (n = 22) | Luteal females (n = 15) |
|---|---|---|---|
| Age | 29.1 (0.9) | 31.9 (1.3) | 28.1 (1.6) |
| Race | |||
| White | 84.9 (45) | 81.8 (18) | 73.3 (11) |
| Other | 15.1 (8) | 18.2 (4) | 26.7 (4) |
| Employed | |||
| Yes | 75.5 (40) | 72.7 (16) | 46.7 (7) |
| No | 24.5 (13) | 27.3 (6) | 53.3 (8) |
| Education | |||
| H.S. diploma or less | 26.4 (14) | 31.8 (7) | 33.3 (5) |
| Some college or more | 73.6 (39) | 68.2 (15) | 66.7 (10) |
| FTND score | |||
| Low (1–5) | 39.6 (21) | 45.5 (10) | 41.1 (37) |
| High (6–10) | 60.4 (32) | 54.5 (12) | 58.9 (53) |
| Mean cigarettes smoked per day | 20.2 (1.1) | 18.4 (1.3) | 17.7 (2.3) |
| Mean years of regular smoking | 11.4 (0.8) | 12.4 (1.3) | 9.4 (1.4) |
| Percent wanting to quit at the time of study entry | |||
| Yes | 79.2 (42) | 72.7 (16) | 73.3 (11) |
| No | 20.8 (11) | 27.3 (6) | 26.7 (4) |
| Percent with ≥1 quit attempt in the past | |||
| Yes | 67.9 (36) | 86.4 (19) | 80.0 (12) |
| No | 32.1 (17) | 13.6 (3) | 20.0 (3) |
Note. Means were compared using one-way analysis of variance and proportions were compared using chi-square tests of independence. Groups did not differ on any tabled variable.
The groups did appear to differ on two other potentially relevant procedural variables. First, the mean number of days between study entry (screening) and the CR session was 10.3 (min = 2, max = 27) days for males and 30.4 (min = 2, max = 59) days for follicular females and 26.4 (min = 2, max = 50) days for luteal females. Second, the mean (SE) CO levels obtained at the beginning of the laboratory session for the males, follicular phase females, and luteal phase females were 23.4 (1.8), 22.1 (2.7), and 14.7 (2.2), respectively. ANOVA applied to the group means on these two measures identified a significant difference on the latency to CR session measure, F (2, 87) = 37.6, p < .001, and a marginally significant difference on the CO measure, F (2, 86) = 2.9, p = .06. Tukey’s honest significant difference (HSD) tests revealed that the significant effect on the latency measure was due to the large difference between males and each of the female groups whereas the marginal effect on the CO measure was due to the difference between the males and luteal phase females only. The presence of these differences necessitated that both variables be considered as a covariate in the initial analytic models.
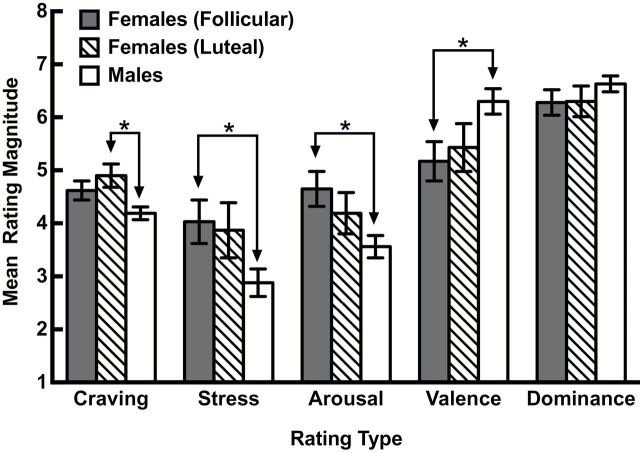
Initial statistical models included the compliment of covariates noted in the Data Analytic Plan. Final models included only those covariates identified as having a significant relationship to the outcome measures; these were the response to neutral/relaxed script, which was significant for all measures, all p’s < .01, and stimulus order, which was significant only for arousal outcome, p < .05. Figure 1 depicts the adjusted mean (± SE) craving, stress, arousal, valence, and dominance rating magnitudes of the follicular females, luteal females, and male smokers in response to negative affect/stress script cue. Examination of the figure shows that luteal but not follicular phase females reported greater stress cue-elicited craving than males, whereas follicular but not luteal phase females evidenced greater cue-elicited stress and arousal and lower valence ratings than males (no differences were apparent on the dominance measure). These apparent differences were confirmed by the ANCOVA’s, all F’s (2, 85/86) ≥ 3.9, all p’s ≤ .02, and the associated Bonferroni corrected pairwise comparisons, all p’s < .05. With respect to effect size, the partial eta squared statistics associated with the significant differences ranged from 0.08 to 0.10, thereby indicating that cycle phase accounted for approximately 10% of the variability in the observed gender differences. Finally, there were no phase (luteal vs. follicular) differences on any outcome.
Figure 1.
Mean (± SE) craving, stress, arousal, valence, and dominance rating magnitudes of follicular phase females, luteal phase females, and male smokers in response to stressful script cues. Means are adjusted for significant covariates. *Indicates p < .05.
Discussion
We,9 and others,14 have previously reported that women smokers are more responsive to stress/negative affect cues than male smokers. The present study is the first to report on the potential impact of menstrual cycle on gender divergent subjective responses to stress/negative affect cues. Specifically, we reanalyzed the data from our previous report and found that females in the luteal phase experienced stronger postcue craving than males, while females in the follicular phase experienced stronger postcue stress and arousal and perceived the cues to be more negatively valenced (i.e., aversive) than males. Thus, this study not only extends findings of two previous studies but it also suggests that the gender difference in craving may be driven by females in the luteal menstrual cycle phase whereas gender differences in emotional responses to the same cues may be driven by females in the follicular phase of their menstrual cycle.
To date, only two previous studies15,16 have reported on the association between menstrual phase and craving. However, one of them16 did not examine differences between female and male smokers and therefore, was not relevant to the present findings. The other study, by Franklin et al. 15, compared the smoking cue-elicited craving of treatment-seeking females (n = 41) and males (n = 69). The females were retrospectively grouped according to whether they received the laboratory CR assessment while they were in their follicular (n = 17) versus luteal (n = 24) menstrual cycle phase. Results indicated that follicular phase females reported lower craving than males, the latter of which did not differ from luteal phase females. By contrast, the present study found greater craving in the luteal phase females versus males and no evidence of a craving differential between follicular phase females versus males. While this apparent conflict in findings may decrease confidence about the significance of menstrual phase effects on smoking-related craving, there are several methodological differences that may account for the outcome disparity. For example, the present study used prospective biological verification of menstrual phase to assign nontreatment-seeking females to the follicular versus luteal phase condition whereas the Franklin et al. 15 employed a retrospective approach with treatment-seeking females and males. If we are to understand the role of menstrual cycle effects on clinically meaningful outcomes, then it may be beneficial for future studies to draw on the methodological strengths in each of these studies. Arguably, such a hybrid study design would likely consist of a prospective, biologically verified phase assignment procedure with a treatment-seeking sample.
The effect of menstrual cycle phase on smoking cessation success has been mixed, with some research-finding females quitting during luteal phase were less likely to be successful,17,18 while other studies have demonstrated that quitting in follicular phase did less well than those quitting in luteal phase.19,20 The findings of the present study suggest that, relative to men, different challenges may be present across the luteal and follicular phases of female smokers. This may, in part, explain some of the mixed findings in research examining the relationship between menstrual phase cycle and quit success.
Several CR studies, especially studies examining craving in response to smoking cues, have failed to find significant differences in craving response across gender.21,22 Future research examining gender differences in responses to smoking and stress cues should account for menstrual phase of female participants to maximize the chance of detecting effects that may be present. Another gainful line of future research could employ imaging methodologies to determine if stress cue-elicited neural activation differs between female and male smokers and if those differences vary by cycle phases (cf., Dagher et al. 23). Lastly, since menstrual cycle phase can be construed as a proxy for ovarian hormone levels, future research that measures these hormone levels directly, either in plasma or saliva, could significantly increase knowledge of nicotine addiction. Related to this point, a recent study by our group24 reported on the relationship between ovarian hormones and topographical features of smoking behavior measured during ad lib smoking in laboratory setting. One of the essential findings of this study was that decreasing estradiol and progesterone were associated with greater puff intensity. Assuming that puff intensity is a proxy for smoking motivation and knowing that decreases in estradiol and progesterone occur in the luteal phase of the menstrual cycle, these findings are consistent with the present observation that craving is elevated in luteal phase females, at least relative to men. Regardless of whether or not this convergence in findings will stand in the face of replication, it seems likely that a greater understanding of the complex relationship between gender and smoking behavior will be achieved by studies that favor the direct assessment of ovarian hormones levels over menstrual phase determination.
Several limitations of the current project should be noted. The study was primarily exploratory and the sample size was small. The observed differences (while significant) were small in magnitude; however, this is to be expected when examining gender differences in stress CR since these responses are likely to be multiply determined. Second, the sample consisted of nontreatment-seeking smokers; as such, it would valuable to replicate this study in a sample of smokers attempting abstinence to determine whether responses to stress cues during different cycle phases are associated with either laboratory measures of ability to resist smoking (i.e., assess at what point participants would choose to smoke vs. earn monetary rewards) or quit status. Third, the randomization process did not proceed as planned for an appreciable minority (32%) of the participants and this could have resulted in some bias in the female samples. This is a difficult issue to address because any attempt to require participants assigned to a certain cycle phase be tested in that phase could increase attrition (i.e., if a scheduled CR session was missed and not done relatively soon thereafter, then it would necessitate females being retained in the study for at least one additional menstrual cycle). Fourth and finally, males had their CR assessment done sooner after study entry and it is possible that this difference in handling of males and females introduced bias or inflated response variability. Future studies could avoid this potential problem by matching males and females on time between study entry and CR assessment.
The findings of the present research point to the possibility that menstrual cycle phase impacts female smokers’ response to stress cues. Further, females, relative to males, may have to overcome obstacles to cessation (craving vs. heightened emotional responsiveness) that vary across phases of the menstrual cycle. While this possibility points to a potential need for cessation interventions that are sensitive to gender and menstrual phase, the exact nature of these interventions will remain speculative until larger N, prospective studies definitively document the role of these factors in smoking-related nicotine addiction.
Funding
This research was supported by NIDA grants P50 DA016511-11 Specialized Center of Research (SCOR) on Sex and Gender Factors Affecting Women’s Health (SCOR PI: K. T. Brady, and SCOR Component 4 PI’s: MES and KMG), South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Numbers UL1 RR029882 and UL1 TR000062. Dr. KMG has received research support from Pfizer, Inc. (medication and placebo supply for NIH-funded research).
Declaration of Interests
There are no conflicts of interest pertaining to the present work. Dr. HPU is now Medical Advisor at Eli Lilly & Company, Drop Code 6112, Indianapolis, IN 46285.
Acknowledgments
The authors thank A. McCullough, J. O. Hinton, D. Paquette, P. Muldrow, C. Horne, E. Klintworth, and L. E. Beech for their invaluable contributions to this research.
References
- 1. Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24:247–255. [DOI] [PubMed] [Google Scholar]
- 2. McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–855. [DOI] [PubMed] [Google Scholar]
- 3. McKee SA, Sinha R, Weinberger AH, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sung HY, Prochaska JJ, Ong MK, Shi Y, Max W. Cigarette smoking and serious psychological distress: a population-based study of California adults. Nicotine Tob Res. 2011;13:1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int J Behav Med. 2004;11:116–121. [DOI] [PubMed] [Google Scholar]
- 6. Labouvie-Vief G, Lumley MA, Jain E, Heinze H. Age and gender differences in cardiac reactivity and subjective emotion responses to emotional autobiographical memories. Emotion. 2003;3:115–126. [DOI] [PubMed] [Google Scholar]
- 7. Matud MP. Gender differences in stress and coping styles. Pers Indiv Differ. 2004;37:1401–1415. [Google Scholar]
- 8. Schmaus BJ, Laubmeier KK, Boquiren VM, Herzer M, Zakowski SG. Gender and stress: differential psychophysiological reactivity to stress reexposure in the laboratory. Int J Psychophysiol. 2008;69:101–106. [DOI] [PubMed] [Google Scholar]
- 9. Saladin ME, Gray KM, Carpenter MJ, LaRowe SD, DeSantis SM, Upadhyaya HP. Gender differences in craving and cue reactivity to smoking and negative affect/stress cues. Am J Addict. 2012;21:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. [DOI] [PubMed] [Google Scholar]
- 11. Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25: 49–59. [DOI] [PubMed] [Google Scholar]
- 12. Heatherton T, Kozlowski L, Frecker R, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 13. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I Disorders, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 14. Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: effects of a family history of smoking. Drug Alcohol Depend. 2007;88:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franklin TR, Napier K, Ehrman R, Gariti P, O’Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6:171–175. [DOI] [PubMed] [Google Scholar]
- 16. Gray KM, DeSantis SM, Carpenter MJ, Saladin ME, LaRowe SD, Upadhyaya HP. Menstrual cycle and cue reactivity in women smokers. Nicotine Tob Res. 2010;12:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carpenter MJ, Saladin ME, Leinbach AS, Larowe SD, Upadhyaya HP. Menstrual phase effects on smoking cessation: a pilot feasibility study. J Womens Health (Larchmt). 2008;17:293–301. [DOI] [PubMed] [Google Scholar]
- 18. Franklin TR, Ehrman R, Lynch KG, et al. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt). 2008;17:287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allen SS, Bade T, Hatsukami DK, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10:35–45. [DOI] [PubMed] [Google Scholar]
- 20. Mazure CM, Toll B, McKee SA, Wu R, O’Malley SS. Menstrual cycle phase at quit date and smoking abstinence at 6 weeks in an open label trial of bupropion. Drug Alcohol Depend. 2011;114:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Niaura R, Shadel WG, Abrams DB, Monti PM, Rohsenow DJ, Sirota A. Individual differences in cue reactivity among smokers trying to quit: effects of gender and cue type. Addict Behav. 1998;23:209–224. [DOI] [PubMed] [Google Scholar]
- 22. Rohsenow DJ, Monti PM, Hutchison KE, Swift RM, MacKinnon SV, Sirota AD, Kaplan GB. High-dose transdermal nicotine and naltrexone: effects on nicotine withdrawal, urges, smoking, and effects of smoking. Exp Clin Psychopharmacol. 2007;15, 81–92. 2007-01684-007 [pii] 10.1037/1064-1297.15.1.81 [DOI] [PubMed] [Google Scholar]
- 23. Dagher A, Tannenbaum B, Hayashi T, Pruessner JC, McBride D. An acute psychosocial stress enhances the neural response to smoking cues. Brain Res. 2009;1293:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schiller CE, Saladin ME, Gray KM, Hartwell KJ, Carpenter MJ. Association between ovarian hormones and smoking behavior in women. Exp Clin Psychopharmacol. 2012;20:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]