Abstract
Background:
Prior cancer is a common exclusion criterion in lung cancer trials. This practice reflects concerns that prior cancer may affect trial conduct or outcomes. However, the impact of prior cancer on survival in lung cancer is not known.
Methods:
We identified patients older than age 65 years with stage IV lung cancer diagnosed between 1992 and 2009 in the Surveillance, Epidemiology, and End Results–Medicare linked registry. Prior cancer was characterized by type, stage, and timing. All-cause and lung cancer–specific survival were compared between patients with and without prior cancer using propensity score–adjusted Cox regression.
Results:
Overall, 102 929 patients with stage IV lung cancer were identified, of whom 14.7% had a history of prior cancer. More than two-thirds (76.0%) of prior cancers were localized or regional stage; most were diagnosed five or fewer years prior to the lung cancer diagnosis. In propensity score–adjusted analysis, patients with prior cancer had better all-cause (hazard ratio [HR] = 0.93, 95% confidence interval [CI] = 0.91 to 0.94) and lung cancer–specific (HR = 0.81, 95% CI = 0.79 to 0.82) survival. In a simulated clinical trial–eligible population (age <75 years, no comorbidity, treated with chemotherapy), similar trends were noted. In subset analyses according to stage, type, and timing of prior cancer, no group of patients with prior cancer had inferior survival compared with patients without prior cancer.
Conclusion:
Among patients with stage IV lung cancer, prior cancer does not convey an adverse effect on clinical outcomes, regardless of prior cancer stage, type, or timing. Broader inclusion in clinical trials of advanced lung cancer patients with a history of prior cancer should be considered.
Fewer than 5% of adults with cancer in the United States participate in clinical trials (1–4). Low accrual rates prolong study duration, limit generalizability, lead to premature study termination, limit the number of patients exposed to potentially beneficial experimental therapies, and leave important clinical questions unanswered (5,6). Barriers to clinical trial accrual include patient, clinician, trial, and system factors (1,3,7–15). Among these, clinical trial eligibility criteria present a major barrier to study enrollment and represent one of the few accrual factors directly controlled by investigators and sponsors (6,13,1 16–19).
In cancer clinical trials, a history of prior cancer is a common exclusion criterion. For example, over 80% of lung cancer trials sponsored or endorsed by the Eastern Cooperative Oncology Group (ECOG) exclude prior malignancy (20). Across trials, this restriction is variably defined, but is most commonly applied to patients with a prior cancer diagnosed within five years of the current cancer. For this reason alone, it is estimated that up to 18% of lung cancer patients are excluded from clinical trials (20). Given the near four-fold increase in the number of US cancer survivors over the past 30 years (21), the extent to which this practice limits trial accrual will likely increase.
The reflexive exclusion of patients with prior cancer from clinical trials presumably reflects concerns that a prior cancer diagnosis could interfere with study conduct or outcomes. However, studies evaluating the impact of prior cancer on lung cancer outcomes yield conflicting results, are predominantly small, single-center series, and primarily focus on resected, early-stage tumors (22–27). We therefore determined the prevalence and prognostic impact of prior cancers among patients with advanced lung cancer using a large, representative, population-based, clinically detailed dataset.
Methods
Data Sources
This study was approved by the University of Texas (UT) Southwestern Institutional Review Board. Data were obtained from the 1992–2009 National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program linked with 1991–2010 Medicare claims files from the Centers for Medicare and Medicaid. SEER is a nationally representative collection of population-based cancer registries (28). Linked SEER-Medicare data provide treatment and outcome information on SEER patients with Medicare. Data for this study were available from 17 registries, broadly representing approximately 28% of the US population (29).
Study Population
The study population included patients older than age 65 years with stage IV lung cancer diagnosed between 1992 and 2009. We used SEER historic stage to identify stage IV patients (30). We included only those older than age 65 years to allow for one year of complete Medicare claims data prediagnosis to determine comorbidity. We used data of patients diagnosed between 1992 and 2009 because Medicare claims were available as of 1991, and 2009 was the most recent year of data available at the time the present study was conducted. All patients had full coverage of Medicare Parts A and B from one year before and one year after the lung cancer diagnosis. We included only patients with non–small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) histology. To ensure complete claims data, we excluded members of health maintenance organizations and patients with only autopsy or death certificate records. We further excluded patients if they had incomplete diagnosis or death dates, or had a discrepancy between SEER and Medicare birth dates of a year or more.
Measures
We measured two primary outcomes: overall (all-cause) and cause-specific (lung cancer) mortality. Survival was measured as the interval in months between date of diagnosis (defined as the 15th of the month because SEER provides only month and year of diagnosis) and date of death provided by SEER. Patients were followed until respective dates of death (all-cause or lung cancer–specific) or censored at the end of 2009 (last date of death in 2011 SEER submission).
A history of prior cancer was determined as described in our previous study (20). In brief, the index lung cancer was defined as the first of any primary lung cancers diagnosed while the patient lived in a SEER registry area (see the Supplementary Methods, available online, for further details). Characteristics and number of of prior cancer(s) were recorded, including type, stage, and timing in relation to the index lung cancer.
We did not include patients with a prior primary lung cancer diagnosis in our analyses for a number of reasons. First, it is challenging to accurately identify same-site second primary cancers using registry data (31). Indeed, unless there is a clear difference in histology between the two lung cancers, it is difficult in clinical practice to determine if a patient has a second primary or recurrent disease. Second, in clinical trials of advanced lung cancer, a history of prior early-stage lung cancer that has subsequently recurred is generally permitted. This group appears to represent approximately 10% of trial populations (32).
We examined multiple SEER covariables associated with lung cancer prognosis as endorsed by earlier studies (33). For a full account of these covariables see the Supplementary Methods (available online). Surgery within 120 days of diagnosis, use of chemotherapy within 120 days, and use of radiotherapy within one year of diagnosis were identified using Medicare inpatient, outpatient, and physician claims (coded yes/no) following prior research. ICD-9 and CPT codes were used to identify these measures are listed in Supplementary Table 1 (available online) (34). Comorbidity was measured by searching inpatient, outpatient, or carrier claims for multiple chronic conditions occurring between 12 months prediagnosis using the Charlson Comorbidity Index–Klabunde adaptation (35,36). We classified comorbidity as none, one, or two or more following common practice. Patients with Medicaid were identified following common practice using the state buy-in variable (37).
Statistical Analysis
Using descriptive statistics, we reported the prevalence and correlates of stage IV lung cancer patients with prior cancer diagnosis. We quantified the type and stage of prior cancer in addition to time elapsed between the prior cancer diagnosis and index lung cancer diagnosis. We used unadjusted Kaplan-Meier analysis to compare survival functions for both all-cause and lung cancer–specific survival of no prior cancer diagnosis vs any prior cancer. Kaplan-Meier curves were also constructed according to characteristics of the most recent prior cancer, including timing, stage, and type of prior cancer. Associated P values were calculated. Propensity scores were constructed to adjust for observable differences (or potential confounders) between patients with and without a prior cancer diagnosis (see the Supplementary Methods, available online, for further details). Univariate, multivariable covariable-adjusted, and multivariable propensity score-adjusted Cox proportional hazards models were used to compare lung cancer–specific and all-cause mortality across patients with and without a prior cancer. We assessed the proportional hazard assumption using the SAS proportionality test (38). The assumption was not met, which is not surprising because in large datasets such as ours, very small departures from proportional hazards can be detected. To assess the impact of this violation of the assumption, we generated time by covariate interactions for all covariates in the model. In each model, the size, direction, and significance of hazard ratio demonstrating the impact of prior cancer was virtually unchanged. We further examined outcomes by charactersitics representative of common clinical trial eligbility criteria (20) as follows: 1) patients with no prior cancer, 2) patients with in situ/localized cancers diagnosed five or more years ago and who are likely to be eligible for clinical trials, and 3) all other patients with prior cancer and who are likely to be ineligible for clinical trials. In a sensitivity analysis, we compared multiple methods of propensity score adjustment (Supplementary Methods, available online). We also examined the robustness of our findings among a subsample of patients simulated to more closely resemble a clinical trial population: younger than age 75 years, no documented comorbidities, and received chemotherapy for the stage IV lung cancer diagnosis. Analyses were performed using SAS statistical software 9.3 (SAS Institute Inc., Cary, NC) and Stata 13.1 (StataCorp. LP., College Station, TX). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
In all, 102 929 eligible stage IV lung cancer patients were identified, of whom 15 170 (14.7%) had a documented prior cancer. Baseline characteristics are listed in Table 1. Prior cancer differed across all measured covariables and was more common among older, male, non-Medicaid patients. After adjustment for propensity scores, all covariables were balanced among patients with and without a prior cancer.
Table 1.
Baseline patient characteristics of the stage IV lung cancer SEER-Medicare cohort (n = 102 929)
| Patient characteristics | All patients No. | Prior malignancy No. (%) | P* | Adjusted P† |
|---|---|---|---|---|
| Age, y | <.001 | .74 | ||
| <75 | 46 258 | 5622 (12.2) | ||
| 75–85 | 44 745 | 7496 (16.8) | ||
| ≥85 | 11 926 | 2052 (17.2) | ||
| Sex | <.001 | .95 | ||
| Female | 46 706 | 5749 (12.3) | ||
| Male | 56 223 | 9421 (16.8) | ||
| Race | <.001 | .95 | ||
| White | 87 800 | 13 219 (15.1) | ||
| Black | 8896 | 1295 (14.6) | ||
| Other | 5025 | 517 (10.3) | ||
| Hispanic | 1208 | 139 (11.5) | ||
| Marital status | <.001 | .93 | ||
| Married | 51 186 | 8224 (16.1) | ||
| Sep/div/wid‡ | 40 390 | 5526 (13.7) | ||
| Single | 7970 | 954 (12.0) | ||
| Unknown | 3383 | 466 (13.8) | ||
| Histology | <.001 | .89 | ||
| Adenocarcinoma | 32 346 | 5031 (15.6) | ||
| Squamous | 14 952 | 2342 (15.7) | ||
| Small cell | 17 977 | 2410 (13.4) | ||
| NSCLC§ | 37 654 | 5387 (14.3) | ||
| Charlson Comorbidity Score | <.001 | .94 | ||
| 0 | 36 485 | 5345 (14.6) | ||
| 1 | 31 651 | 4643 (14.7) | ||
| 2+ | 31 477 | 4866 (15.5) | ||
| Not available | 3316 | 316 (9.5) | ||
| Medicaid | <.001 | .10 | ||
| Yes | 18 678 | 2026 (10.8) | ||
| No | 84 251 | 13 144 (15.6) | ||
| Lung cancer treatment | <.001 | .33 | ||
| Surgery only | 1353 | 235 (17.4) | ||
| Chemotherapy only | 13 949 | 2257 (16.2) | ||
| Radiation only | 21 573 | 3251 (15.1) | ||
| ≥2 treatments | 22 231 | 3227 (14.5) | ||
| No surg/chemo/rad|| | 43 823 | 6200 (14.1) | ||
| Cause of death# | <.001 | |||
| Alive | 4743 | 789 (16.6) | ||
| Lung cancer specific | 82 007 | 10 650 (13.0) | ||
| All other causes | 16 179 | 3731 (23.1) | ||
| Total | ||||
| 102 929 | 15 170 (14.7) | |||
* Two-sided P value was calculated from logistic model Wald Chi-square. NSCLC = non–small cell lung cancer; SEER = Surveillance, Epidemiology, and Ends Results.
† Two-sided propensity score–adjusted P value: Groups are well balanced for covariables of interest if P values are not statistically significant.
‡ Separated/divorced/widowed.
§ Non–small cell lung carcinoma.
|| No surgery/chemotherapy/radiation.
# Dependent variable, no adjusted P value necessary.
Figure 1 depicts the type, stage, and timing of the most recent prior cancer. Prostate (27.9%), gastrointestinal (15.1%), other genitourinary (14.4%), and breast (14.2%) were the most common cancer types. Among women, the most common prior cancers were breast (37.0%), gastrointestinal (16.8%), and gynecologic (16.0%). For men, the most common prior cancers were prostate (44.8%), other genitourinary (17.8%), and gastrointestinal (14.0%). Localized and regional stages were most frequently observed, together accounting for 76% of cases. Only 6.3% of prior cancers were distant stage. The median times of diagnosis for prior cancers (measured from the index lung cancer date) were as follows: most recent, 4.7 years; second most recent, 8.3 years; third most recent, 10.9 years. Additional characteristics of second and third most recent prior cancers are provided in Supplementary Figure 1 (available online).
Figure 1.
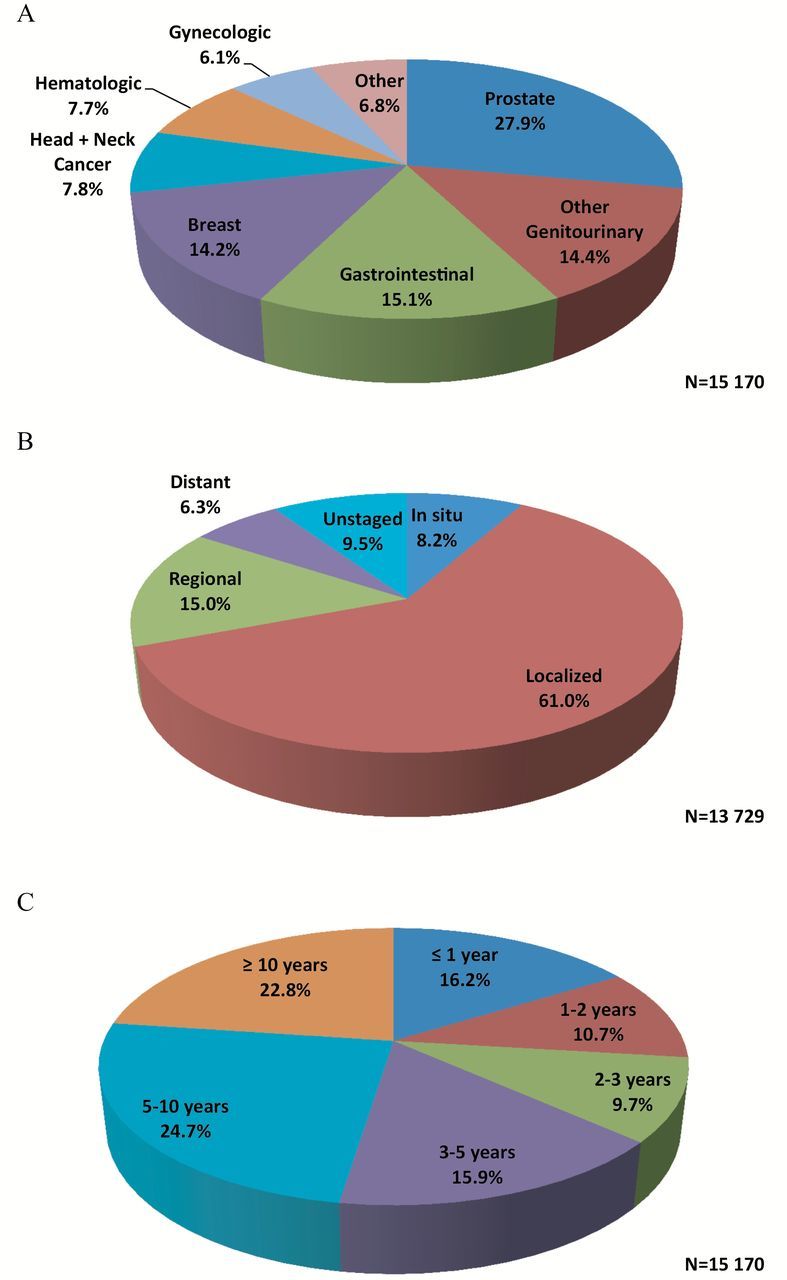
Type (A), stage (B), and timing (C) of the most recent prior cancers. Cell sizes less than 11 are suppressed per the Surveillance, Epidemiology, and End Results–Medicare data use agreement; Denominators are not equal because of missing data. All statistical tests were two-sided.
The Kaplan-Meier curves demonstrated statistically significantly different survival (P < .001) between patients with and without a prior cancer, demonstrating a favorable effect of prior cancer on all-cause and lung cancer–specific survival (Figure 2). Overall survival according to timing, stage, and type of prior cancer diagnosis is shown in Figure 3. In general, no subgroup of patients with prior cancer had inferior survival compared with patients with no prior cancer.
Figure 2.
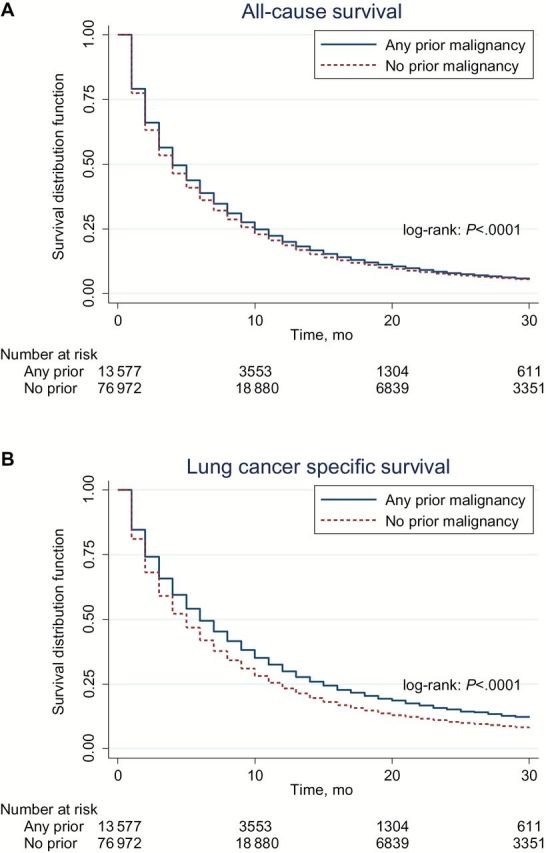
All-cause (A) and lung cancer–specific (B) survival for patients with and without any prior malignancy. All statistical tests were two-sided.
Figure 3.
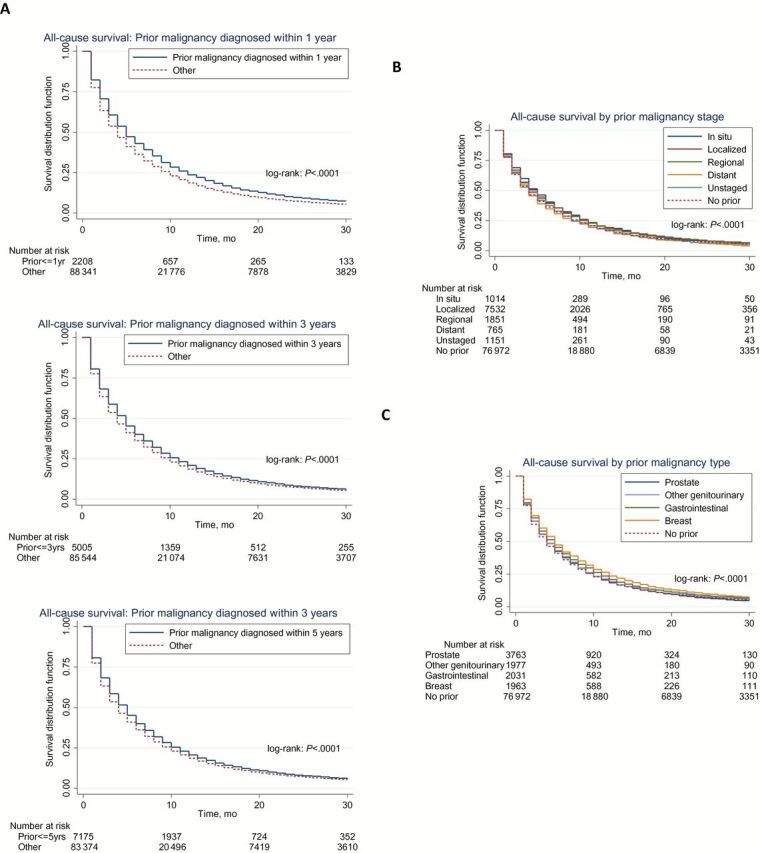
Overall survival according to type (A), stage (B), and timing (C) of prior cancer diagnosis. “Other” denotes patients with no prior malignancy or a history of prior malignancy diagnosed outside the referenced time frame. All statistical tests were two-sided.
To analyze outcomes according to characteristics representative of common clinical trial eligibility criteria (20), we grouped patients according to both stage and timing of prior cancer. All-cause survival was similar among the groups, but again slightly favored those with prior cancer. The three groups and their hazard ratios for all-cause mortality are as follows: 1) patients with no prior cancer (referent), 2) patients with in situ/localized cancers diagnosed five or more years ago and who are likely to be eligible for clinical trials (hazard ratio [HR] = 0.96, 95% confidence interval [CI] = 0.93 to 0.99), and 3) all other patients with prior cancer and who are likely to be ineligible for clinical trials (HR = 0.94, 95% CI = 0.92 to 0.96).
In multivariable covariate-adjusted Cox models, prior cancer was associated with favorable all-cause (HR = 0.93, 95% CI = 0.91 to 0.94, P < .001) and lung cancer–specific (HR = 0.81, 95% CI = 0.79 to 0.82, P < .001) survival (Table 2). Non–small cell histology, female sex, younger age, lower comorbidity burden, and receipt of cancer-specific treatment were also associated with improved survival. In sensitivity analyses, effect size, direction, and statistical significance of the prior cancer effect on both outcomes was consistent across all three analyzed methods of propensity score adjustment (data not shown) and furthermore was consistent with the multivariable covariable-adjusted model. Thus for simplicity, we opted to present the multivariable covariable-adjusted model in Table 2. Univariate modeling recapitulated these results as well (data not shown).
Table 2.
Multivariable covariate-adjusted hazard ratios for all-cause and lung cancer–specific mortality
| Patient characteristics | All-cause HR* (95% CI†) | All-cause P‡ | Lung cancer–specific HR* (95% CI†) | Lung cancer–specific P‡ |
|---|---|---|---|---|
| Prior cancer diagnosis (vs none) | ||||
| Yes (any) | 0.93 (0.91 to 0.94) | <.001 | 0.81 (0.79 to 0.82) | <.001 |
| Age (vs 66–74), y | ||||
| 75–85 | 1.09 (1.08 to 1.11) | <.001 | 1.08 (1.06 to 1.10) | <.001 |
| >85 | 1.15 (1.12 to 1.17) | <.001 | 1.09 (1.07 to 1.12) | <.001 |
| Sex (vs female) | ||||
| Male | 1.13 (1.11 to 1.14) | <.001 | 1.13 (1.11 to 1.14) | <.001 |
| Race (vs white) | ||||
| Black | 0.94 (0.92 to 0.96) | <.001 | 0.92 (0.90 to 0.94) | <.001 |
| Hispanic | 0.84 (0.82 to 0.87) | <.001 | 0.85 (0.82 to 0.88) | <.001 |
| Other | 0.96 (0.91 to 1.02) | .19 | 0.94 (0.88 to 1.00) | .05 |
| Marital status (vs married) | ||||
| Sep/div/wid§ | 1.06 (1.05 to 1.08) | <.001 | 1.06 (1.04 to 1.08) | <.001 |
| Single | 1.11 (1.08 to 1.13) | <.001 | 1.09 (1.06 to 1.12) | <.001 |
| Unknown | 1.04 (1.01 to 1.08) | .02 | 1.02 (0.99 to 1.07) | .22 |
| Histology (vs other NSCLC) | ||||
| Small cell | 1.04 (1.02 to 1.06) | <.001 | 1.10 (1.08 to 1.12) | <.001 |
| Adenocarcinoma | 0.87 (0.85 to 0.88) | <.001 | 0.89 (0.88 to 0.91) | <.001 |
| Squamous | 0.88 (0.87 to 0.90) | <.001 | 0.92 (0.90 to 0.94) | <.001 |
| Comorbidity | ||||
| Comorbidities | 1.16 (1.14 to 1.17) | <.001 | 1.11 (1.09 to 1.13) | <.001 |
| Payer status | ||||
| Medicaid | 0.99 (0.97 to 1.00) | .13 | 0.96 (0.94 to 0.98) | <.001 |
| Treatment status (vs any treatment) | ||||
| No treatment | 2.02 (1.99 to 2.05) | <.001 | 1.89 (1.86 to 1.92) | <.001 |
* HR denotes hazard ratio of all-cause and lung cancer–specific death for the above covariables. All statistical tests were-two sided. CI = confidence interval; HR = hazard ratio; NSCLC = non–small cell lung cancer.
† CI denotes confidence interval.
‡ P values were calculated from the Cox proportional hazard models.
§ Separated/divorced/widowed.
Finally, we examined the effect of prior cancer among a subsample of patients who more closely resembled a clinical trial population. Among this subsample of patients who were younger than age 75 years, had no documented comorbidities, and received chemotherapy for the stage IV lung cancer diagnosis (n = 9024), prior cancer occurred in 13.0% of patients. Similar to the overall population, prior cancer did not convey an adverse effect on all-cause (HR = 0.95, 95% CI = 0.89 to 1.01, P = .11) or lung cancer–specific (HR = 0.82, 95% CI = 0.76 to 0.88, P < .001) mortality in propensity-score adjusted models (data not shown).
Discussion
The assumption that a prior cancer diagnosis may interfere with study conduct or outcomes has resulted in widespread and reflexive exclusion of patients with prior cancer from clinical trials across cancer types. In the present study of more than 100 000 stage IV lung cancer cases from the SEER-Medicare dataset, we found that 14.7% of patients with stage IV lung cancer had a history of prior malignancy. More than two-thirds of these prior cancers were in situ, localized, or regional stage. Prior cancers were diagnosed relatively close to the lung cancer diagnosis (median 4.7 years), and in the overall study population and every subgroup analyzed prior cancer did not adversely impact all-cause or lung cancer–specific survival.
Earlier studies investigating the impact of a prior cancer diagnosis on lung cancer outcomes yielded conflicting findings and primarily focused on resected, early-stage lung cancer (22–27,39). In a single-center series of 1914 lung cancer patients, of whom 228 (12%) had a history of at least one previous malignancy, no survival detriment was observed in the group with prior cancer (HR = 0.84, 95% CI = 0.70 to 1.01) (22). A number of smaller studies reported similar trends (24,26,27,39). Conversely, in a study of 2991 patients with resected stage I NSCLC, the subset of 192 patients with prior cancer (6%) had statistically significantly inferior survival (HR = 1.44, 95% CI = 1.16 to 1.78) (23).
The 14.7% rate of prior cancer among patients with stage IV lung cancer suggests that prior cancer-related eligibility criteria may restrict clinical trial enrollment of a substantial proportion of patients. Indeed, in an earlier study, we determined that for some lung cancer clinical trials, up to 18% of patients may be excluded for this reason alone (20). As cancer treatments improve and the number of survivors grows, this proportion will likely increase (22,49–42). Compared with other common exclusion criteria, prior cancer may be one of the most substantial factors limiting accrual. In a recent study of medical comorbidities among 460 patients with lung cancer, the proportion of patients with prior cancer was three times the proportion with renal disease, seven times the proportion with liver disease, and 14 times the proportion with human immunodeficiency virus (33). Furthermore, the observation that the majority of prior cancer cases are diagnosed within five years of the lung cancer diagnosis suggests that the common practice of excluding prior cancer within five years of enrollment still results in exclusion of a substantial proportion of patients (20).
The most common types of prior cancer in our cohort were prostate, gastrointestinal, other genitourinary, and breast. This histologic distribution resembles that reported in other series of prior malignancy among lung cancer patients (22–25,39). The high prevalence of prostate cancer in the cohort reflects its overall prevalence in the general population (43). Like lung cancer, other genitourinary malignancies such as bladder cancer are associated with smoking. Notably, bladder cancer has the highest rate of subsequent primary cancers of any malignancy (16%) (44). Given a predilection for early stage of diagnosis, indolent course, and excellent response to local treatments, it seems unlikely that antecedent bladder or prostate cancers would substantially impact outcomes in advanced lung cancer (45). Furthermore, across cancer types, more than two-thirds of prior cancers in our cohort were localized or regional stage, suggesting that these earlier diagnoses may not adversely impact advanced lung cancer outcomes.
In the overall study population and every subgroup analyzed, prior cancer diagnosis was not associated with inferior survival. By contrast, prior cancer diagnosis was generally associated with improved clinical outcomes, regardless of timing in relation to the lung cancer diagnosis. Similar trends have been identified in lymphoma and gastrointestinal malignancy populations (46–48). There are many potential explanations for these trends. Possibilities include differing patient biology or treatment responsiveness, more frequent engagement in healthcare systems, or a healthy survivor effect. Another possibility is lead-time bias: Although all cases in this study were stage IV, those occurring after a prior cancer may have been diagnosed earlier in the disease course because of more frequent and intensive clinical care related to the prior cancer. This hypothesis echoes earlier findings that increased comorbidity burden is associated with lung cancer diagnosis at an earlier stage (33).
Given what appears to be a slightly favorable prognostic effect of prior cancer, how might these cases be handled in lung cancer clinical trials? In general, clinical trials in lung cancer and other diseases have not excluded patients expected to have good outcomes. Such an approach, we believe, runs counter to goals of fair and equitable access to clinical research and cutting-edge therapies. Furthermore, to apply this approach in lung cancer trials, one would need to exclude women, Asians, and patients with the best functional status (ie, ECOG 0 vs 1), as each of these characteristics has been associated with improved clinical outcomes (32,49,50). Alternatively, one could consider stratifying patients according to prior cancer diagnosis, or including prior cancer in final Cox modeling.
One potential reason for excluding prior cancer from clinical trials is that exposure to prior cancer treatment may render patients less likely to tolerate experimental therapies. Although we are not able to determine treatment toxicities in our current analysis, we believe this concern can be addressed in other ways. First, other eligibility criteria (such as organ function, blood counts, and functional status) may adequately screen for treatment intolerance. Second, prior cancer treatment (distinct from a prior cancer diagnosis) can be employed as an exclusion criterion. This practice is already employed in numerous lung cancer trials (20) and is likely to exclude far fewer patients than does prior cancer diagnosis exclusion.
This study has a number of limitations. Compared with individuals with other common malignancies, individuals with lung cancer are generally older and have greater smoking history. Both factors may influence the likelihood and nature of a prior cancer diagnosis, thereby limiting the generalizability of our findings to other cancer populations (43). Additionally, use of SEER-Medicare data limits our analysis to patients older than age 65 years. However, because of the advanced age at diagnosis of lung cancer (average 71 years), this restriction may exclude a smaller proportion of patients (approximately 30%) compared with other common cancers (51), Furthermore, in an analysis of the youngest and presumably healthiest patients in our cohort (simulated clinical trial-eligible population: age <75 years, no documented comorbidities, and received chemotherapy for the advanced lung cancer diagnosis), our findings were similar to those in the overall study population. Nevertheless, future research may be warranted on the prevalence and impact of prior cancers among younger patients with lung cancer.
In summary, among patients with advanced lung cancer, 14.7% have a history of prior cancer. Most of these cases occur within five years of the lung cancer diagnosis. More than two-thirds are in situ, localized, or regional stage (so presumably cured or at least not life-limiting in the context of advanced lung cancer). In general, prior cancer does not adversely impact all-cause or lung cancer–specific survival. If anything, patients with prior cancer do a little better. Together, these findings suggest that broader inclusion in clinical trials of advanced lung cancer patients with prior cancer could be considered without impacting study outcomes. Such policy modifications could lead to faster accrual, higher trial completion rates, and more generalizable results, ultimately providing better treatments to more patients sooner.
Funding
This work was supported by a National Cancer Institute (NCI) Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to DEG), Cancer Prevention Research Institute of Texas (CPRIT) R1208 (to SLP), and by the University of Texas (UT) Southwestern Center for Patient-Centered Outcomes Research (PCOR), the Agency for Healthcare Research and Quality (1R24HS022418-01) (to EAH, SLP, LX). Funding was also provided by the National Center for Advancing Translational Sciences UT Southwestern Center for Translational Medicine (U54 RFA-TR-12-006) (to EAH, SLP).
Supplementary Material
The authors thank Helen Mayo, MLS, from the University of Texas Southwestern Medical Library for assistance performing literature searches. The authors also acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, Centers for Medicare & Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. Contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
The study funders had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
References
- 1. Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109(3):465–476. [DOI] [PubMed] [Google Scholar]
- 2. Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990;65(10 Suppl):2376–2382. [DOI] [PubMed] [Google Scholar]
- 3. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 4. Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733. [DOI] [PubMed] [Google Scholar]
- 5. Lemieux J, Goodwin PJ, Pritchard KI, et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol. 2008;26(27):4458–4465. [DOI] [PubMed] [Google Scholar]
- 6. Filion M, Forget G, Brochu O, et al. Eligibility criteria in randomized phase II and III adjuvant and neoadjuvant breast cancer trials: not a significant barrier to enrollment. Clin Trials. 2012;9(5):652–659. [DOI] [PubMed] [Google Scholar]
- 7. Rasco DW, Xie Y, Yan J, et al. The impact of consenter characteristics and experience on patient interest in clinical research. Oncologist. 2009;14(5):468–475. [DOI] [PubMed] [Google Scholar]
- 8. Tournoux C, Katsahian S, Chevret S, et al. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006;106(2):258–270. [DOI] [PubMed] [Google Scholar]
- 9. Gerber DE, Rasco DW, Skinner CS, et al. Consent timing and experience: modifiable factors that may influence interest in clinical research. J Oncol Pract. 2012;8(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hietanen PS, Aro AR, Holli KA, et al. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncol. 2007;46(1):42–48. [DOI] [PubMed] [Google Scholar]
- 11. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–4631. [DOI] [PubMed] [Google Scholar]
- 12. Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–1156. [DOI] [PubMed] [Google Scholar]
- 13. Lee JY, Breaux SR. Accrual of radiotherapy patients to clinical trials. Cancer. 1983;52(6):1014–1016. [DOI] [PubMed] [Google Scholar]
- 14. Scoggins JF, Ramsey SD. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. J Natl Cancer Inst. 2010;102(17):1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute-American Society of Clinical Oncology cancer trial accrual symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemieux J, Amireault C, Camden S, et al. Evaluation of factors associated with recruitment in hematological clinical trials: a retrospective cohort study. Hematology. 2010;15(6):373–377. [DOI] [PubMed] [Google Scholar]
- 17. Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297(11):1233–1240. [DOI] [PubMed] [Google Scholar]
- 18. McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials: a pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982;5(2):227–236. [DOI] [PubMed] [Google Scholar]
- 19. Kotwall CA, Mahoney LJ, Myers RE, et al. Reasons for non-entry in randomized clinical trials for breast cancer: a single institutional study. J Surg Oncol. 1992;50(2):125–129. [DOI] [PubMed] [Google Scholar]
- 20. Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. 2014;106(11)dju302 10.1093/jnci/dju302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguilo R, Macia F, Porta M, et al. Multiple independent primary cancers do not adversely affect survival of the lung cancer patient. Eur J Cardiothorac Surg. 2008;34(5):1075–1080. [DOI] [PubMed] [Google Scholar]
- 23. Lopez-Encuentra A, Gomez de la Camara A, Rami-Porta R, et al. Previous tumour as a prognostic factor in stage I non-small cell lung cancer. Thorax. 2007;62(5):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duchateau CS, Stokkel MP. Second primary tumors involving non-small cell lung cancer: prevalence and its influence on survival. Chest. 2005;127(4):1152–1158. [DOI] [PubMed] [Google Scholar]
- 25. Liu YY, Chen YM, Yen SH, et al. Multiple primary malignancies involving lung cancer-clinical characteristics and prognosis. Lung Cancer. 2002;35(2):189–194. [DOI] [PubMed] [Google Scholar]
- 26. Massard G, Ducrocq X, Beaufigeau M, et al. Lung cancer following previous extrapulmonary malignancy. Eur J Cardiothorac Surg. 2000;18(5):524–528. [DOI] [PubMed] [Google Scholar]
- 27. Utsumi T, Fujii Y, Takeda S, et al. Clinical study on lung cancer as a second primary cancer. Surg Today 1998;28(5):487–491. [DOI] [PubMed] [Google Scholar]
- 28. Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3–18. [DOI] [PubMed] [Google Scholar]
- 29. Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010. [Google Scholar]
- 30. Attachment A for the SEER-Medicare PEDSF File SEER Research Data Record Description Cases Diagnosed in 1973-2009. SEER-Medicare Technical Support, Information Management Services, Inc. SEER historic stage A, November 2011:76. [Google Scholar]
- 31. Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer 2014;14:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 33. Ahn DH, Mehta N, Yorio JT, et al. Influence of Medical Comorbidities on the Presentation and Outcomes of Stage I-III Non-Small-Cell Lung Cancer. Clin Lung Cancer. 2013;14(6):644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Warren JL, Yabroff KR, Meekins A, et al. Evaluation of trends in the cost of initial cancer treatment. J Natl Cancer Inst. 2008;100(12):888–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 36. National Cancer Institute. SEER-Medicare: Calculation of comorbidity weights. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html. Accessed March 20, 2014. [Google Scholar]
- 37. Koroukian SM, Dahman B, Copeland G, et al. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010;45(1):265–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hosmer DW, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Hoboken, NJ: John Wiley & Sons; 2008.
- 39. Koppe MJ, Zoetmulder FA, van Zandwijk N, et al. The prognostic significance of a previous malignancy in operable non-small cell lung cancer. Lung Cancer. 2001;32(1):47–53. [DOI] [PubMed] [Google Scholar]
- 40. Nandy N, Dasanu CA. Incidence of second primary malignancies in patients with esophageal cancer: a comprehensive review. Curr Med Res Opin. 2013;29(9):1055–1065. [DOI] [PubMed] [Google Scholar]
- 41. VanderWalde AM, Hurria A. Second malignancies among elderly survivors of cancer. Oncologist. 2011;16(11):1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2020–2026. [DOI] [PubMed] [Google Scholar]
- 43. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 44. Hayat MJ, Howlader N, Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. [DOI] [PubMed] [Google Scholar]
- 45. Smith A, Balar AV, Milowsky MI, Chen RC. Chapter 83 - Bladder Cancer. In: Niederhuber JE, (ed). Abeloff’s Clinical Oncology. 5th ed. Philadelphia, PA: Elsevier Saunders; 2014. [Google Scholar]
- 46. Pulte D, Gondos A, Brenner H. Long-term survival of patients diagnosed with non-Hodgkin lymphoma after a previous malignancy. Leuk Lymphoma. 2009;50(2):179–186. [DOI] [PubMed] [Google Scholar]
- 47. Pandurengan RK, Dumont AG, Araujo DM, et al. Survival of patients with multiple primary malignancies: a study of 783 patients with gastrointestinal stromal tumor. Ann Oncol. 2010;21(10):2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Varty PP, Delrio P, Boulos PB. Survival in colorectal carcinoma associated with previous extracolonic cancer. Ann R Coll Surg Engl. 1994;76(3):180–184. [PMC free article] [PubMed] [Google Scholar]
- 49. Gandara DR, Kawaguchi T, Crowley J, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27(21):3540–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stanley KE. Prognostic factors for survival in patients with inoperable lung cancer. J Natl Cancer Inst. 1980;65(1):25–32. [PubMed] [Google Scholar]
- 51. Rasco DW, Yan J, Xie Y, et al. Looking beyond surveillance, epidemiology, and end results: patterns of chemotherapy administration for advanced non-small cell lung cancer in a contemporary, diverse population. J Thorac Oncol. 2010;5(10):1529–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


