Abstract
Background:
Chronic inflammation is involved in the development of colorectal cancer (CRC) and microsatellite instability (MSI), a distinct phenotype of CRC. Experimental evidence indicates an anti-inflammatory and antineoplastic effect of marine ω-3 polyunsaturated fatty acids (PUFAs). However, epidemiologic data remain inconclusive.
Methods:
We investigated whether the association between marine ω-3 PUFAs and CRC varies by MSI-defined subtypes of tumors in the Nurses’ Health Study and Health Professionals Follow-up Study. We documented and classified 1125 CRC cases into either MSI-high tumors, in which 30% or more of the 10 microsatellite markers demonstrated instability, or microsatellite-stable (MSS) tumors. Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of MSS tumors and MSI-high tumors in relation to marine ω-3 PUFA intake. All statistical tests were two-sided.
Results:
Marine ω-3 PUFA intake was not associated with overall incidence of CRC. However, a statistically significant difference was detected by MSI status (P heterogeneity = .02): High marine ω-3 PUFA intake was associated with a lower risk for MSI-high tumors (comparing ≥0.30g/d with <0.10g/d: multivariable HR = 0.54, 95% CI = 0.35 to 0.83, P linearity = .03) but not MSS tumors (HR = 0.97, 95% CI = 0.78 to 1.20, P linearity = .28). This differential association appeared to be independent of CpG island methylator phenotype and BRAF mutation status.
Conclusions:
High marine ω-3 PUFA intake is associated with lower risk for MSI-high CRC but not MSS tumors, suggesting a potential role of ω-3 PUFAs in protection against CRC through DNA mismatch repair. Further research is needed to confirm our findings and elucidate potential underlying mechanisms.
Chronic inflammation plays an important role in the development of colorectal cancer (CRC), a leading cause of cancer-related death worldwide (1,2). Accumulating evidence from animal and in vitro studies indicates that long-chain marine ω-3 polyunsaturated fatty acids (PUFAs) (ie, eicosapentaenoic acid [EPA], docosahexaenoic acid [DHA], and docosapentaenoic acid [DPA]) have potent anti-inflammatory activity and inhibit colorectal carcinogenesis (3,4). However, epidemiologic findings on the association between marine ω-3 PUFAs and CRC are inconsistent, with an inverse association reported in some prospective studies (5,6) but not in others (7–11). Recent evidence suggests that the association may vary by subsite of tumors. High ω-3 PUFA intake appeared to be associated with a lower risk of proximal colon cancer and with an unaltered or even increased risk of distal colon cancer (12,13).
The potential differential role of ω-3 PUFA intake according to anatomic site may be because of variation in the prevalence of specific molecular subtypes that result from various genetic and epigenetic alterations in the proximal compared with distal colon (14,15). Specifically, 10% to 15% of CRCs display microsatellite instability (MSI) with predominance in the proximal colon (16–18). MSI is caused by the loss of DNA mismatch repair (MMR) activity (19). Experimental studies have demonstrated that inflammation inactivates MMR function and increases mutation rates (20–22). Prostaglandin E2 (PGE2), an arachidonic acid-derived proinflammatory product, has been shown to silence DNA repair genes by enhancing DNA methylation to promote colonic tumor growth (23). Based on these findings and prior data showing a stronger inverse association of ω-3 PUFA with proximal colon cancer, we hypothesized that marine ω-3 PUFAs were more likely to inhibit inflammatory pathways associated with the development of tumors that arise from defective MMR.
To test this hypothesis and extend our previous analysis of ω-3 PUFA (12), we investigated the association of marine ω-3 PUFA intake with incidence of CRC according to MSI status of the tumors using two large US cohorts, the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS), in which detailed dietary and lifestyle data and tumor specimens have been collected during 24 to 26 years of follow-up. These two cohort studies offered us a unique opportunity to integrate data on long-term marine ω-3 PUFA intake and molecular features in CRC tissue over longitudinal follow-up. This integrative approach enabled us to provide novel evidence for possible roles of ω-3 PUFAs in prevention of certain CRC subsets that possess distinct molecular features.
Because most CRCs with MSI demonstrate widespread methylation of CpG islands, ie, the so-called CpG island methylator phenotype (CIMP), and many harbor V600E BRAF mutations, we also classified tumors by CIMP and BRAF mutation status and examined the association between marine ω-3 PUFA intake and incident CRC according to MSI in strata of CIMP and BRAF mutation status.
Methods
Study Population
The NHS enrolled 121 701 registered female nurses who were age 30 to 55 years in 1976 in the United States (24). The HPFS included 51 529 US male professionals who were age 40 to 75 years in 1986 (25). In both cohorts, follow-up questionnaires were administered biennially, with response rates of approximately 90% for each of the questionnaires, to collect and update lifestyle and disease information. We collected updated dietary data using the validated food frequency questionnaires (FFQs). We obtained written, informed consent from all participants. The study protocol was approved by the institutional review boards of Brigham and Women’s Hospital and Harvard School of Public Health. Details about the two cohorts are provided in the Supplementary Materials (available online).
Dietary Assessment
Details on assessment of ω-3 PUFA intake have been described in previous publications (12,26). Briefly, in each FFQ we asked participants their average frequency of consuming each food of a standardized portion size during the previous year. Nine response options were provided, ranging from “never or less than once per month” to “6 or more times per day.” The average daily intake of nutrients was calculated by multiplying the reported frequency of consumption of each item by its nutrient content and then summing across from all foods. Use and dosage of fish oil supplements were assessed in the two cohorts since 1990. We included supplement consumption in calculation of marine ω-3 PUFA intake. We adjusted nutrient intake for total caloric intake using the nutrient residual method (27). Marine ω-3 PUFA intake was calculated by summing EPA, DHA, and DPA consumption.
The reproducibility and validity of FFQs in assessing PUFA intake have been assessed previously (28). In a random sample of 118 Boston-area HPFS participants age 45 to 70 years who completed two consecutive FFQs (in 1986 and 1987), two one-week dietary records approximately seven months apart, and provided subcutaneous fat aspirate samples, the mean PUFA intake from the two FFQs were similar (14.0g/d vs 13.4g/d, intraclass correlation coefficient r = 0.59), and the Spearman correlation coefficient for estimates of PUFA intake from the FFQ was 0.60 with dietary records and 0.50 with subcutaneous fat aspirates. Similar findings were observed in a validation study in the NHS cohort (29,30).
End Point Ascertainment
In both cohorts, participants who reported a diagnosis of CRC in the biennial questionnaires were requested for permission to acquire their medical records and pathologic reports. We identified deaths with over 96% sensitivity through the National Death Index and next of kin. For all CRC deaths, we asked for permission from next of kin to review medical records. Medical records were reviewed by a study physician, who was blinded to exposure information, to confirm CRC diagnosis and to extract information on anatomic location, stage, and histologic type of cancer (31). Through 2010, we documented 1488 cases of CRC in the NHS and 1013 cases of CRC in the HPFS. Among these cases, we successfully collected sufficient paraffin-embedded archival tumor tissue blocks for MSI testing from 1125 patients (606 in the NHS and 519 in the HPFS). To assess any potential ascertainment bias resulting from tissue specimen availability, we compared the basic characteristics of cases with and without MSI data. As shown in Supplementary Table 1 (available online), we did not observe substantial differences, except that compared with cases without MSI data cases with available MSI data were somewhat older, had a higher proportion of regular aspirin or non-steroidal anti-inflammatory drug (NSAID) use, and consumed more ω-6 PUFAs and vegetables.
MSI Assessment
DNA was extracted from paraffin-embedded archival tumor and normal tissues. We determined MSI status using 10 microsatellite markers (D2S123, D5S346, D17S250, BAT25, BAT26, BAT40, D18S55, D18S56, D18S67, and D18S487), as previously described (32). Tumors were classified as MSI-high if at least 30% of the markers demonstrated instability and as MSI-low or MSS if less than 30% of the markers demonstrated instability. We combined MSI-low tumors into MSS tumors because of the small number of MSI-low cases. Details on CIMP and BRAF mutation assessment are provided in the Supplementary Materials (available online).
Statistical Analysis
For each participant, we calculated follow-up time (in months) from the age at which the baseline questionnaire was returned until the age at the date of death, CRC diagnosis, loss to follow-up, or end of follow-up (June 1, 2010 for the NHS, January 31, 2010 for the HPFS), whichever came first. To capture long-term habitual consumption, we calculated the cumulative average of marine ω-3 PUFA intake. We used time-varying Cox proportional hazards regression model with age as the time scale to estimate the hazard ratio (HR) and 95% confidence interval (CI) of CRCs associated with marine ω-3 PUFA intake. We also stratified the model by calendar time to account for any period effect. In the multivariable analysis, we adjusted for several risk factors for CRC (see footnote of Table 2). Details regarding the assessment of covariables are provided in the Supplementary Materials (available online). Tests for trend were conducted using the median value for each category as a continuous variable in the regression model. We first performed the analyses in each cohort separately, and because no appreciable difference was detected by cohort (P heterogeneity = .13 for overall CRC), we then conducted the pooling analysis using the sex-stratified Cox regression model in the combined dataset.
Table 2.
Hazard ratio and 95% confidence interval of incident colorectal cancer, overall and by MSI status, according to intake of marine ω-3 polyunsaturated fatty acids in the pooled cohorts of Nurses’ Health Study (1984–2010) and Health Professionals Follow-up Study (1986–2010)*
| Analysis | <0.10g/d | 0.10–0.19g/d | 0.20–0.29g/d | ≥0.30g/d | P trend |
|---|---|---|---|---|---|
| Overall CRC† | |||||
| Person-years | 450 660 | 917 634 | 668 272 | 861 194 | |
| No. of cases (n = 1125) | 177 | 353 | 256 | 339 | |
| Age-adjusted HR (95% CI)‡ | 1.00 (referent) | 0.96 (0.80 to 1.15) | 0.88 (0.73 to 1.07) | 0.83 (0.69 to 1.00) | .01 |
| Multivariable HR (95% CI)§ | 1.00 (referent) | 0.95 (0.79 to 1.15) | 0.89 (0.73 to 1.09) | 0.87 (0.72 to 1.05) | .09 |
| MSS CRC | |||||
| No. of cases (n = 941) | 135 | 300 | 214 | 292 | |
| Age-adjusted HR (95% CI)‡ | 1.00 (referent) | 1.08 (0.88 to 1.33) | 0.97 (0.78 to 1.21) | 0.93 (0.75 to 1.14) | .09 |
| Multivariable HR (95% CI)§ | 1.00 (referent) | 1.08 (0.87 to 1.32) | 0.98 (0.79 to 1.22) | 0.97 (0.78 to 1.20) | .31 |
| MSI-high CRC | |||||
| No. of cases (n = 184) | 42 | 53 | 42 | 47 | |
| Age-adjusted HR (95% CI)‡ | 1.00 (referent) | 0.57 (0.38 to 0.86) | 0.60 (0.39 to 0.93) | 0.52 (0.34 to 0.79) | .02 |
| Multivariable HR (95% CI)§ | 1.00 (referent) | 0.57 (0.38 to 0.86) | 0.61 (0.40 to 0.94) | 0.54 (0.35 to 0.83) | .05 |
| P heterogeneity|| | .007 | .05 | .02 | ||
* All P values are two-sided. CI = confidence interval; CRC = colorectal cancer; HR = hazard ratio; MSI = microsatellite instability; MSS = microsatellite stable.
† Among patients with available MSI data.
‡ Sex-stratified Cox proportional hazards model was used.
§ Additionally adjusted for family history of colorectal cancer, history of endoscopy, pack-years of smoking before age 30 years (in women: 0, 0 to <5, and ≥5; in men: 0, >0 to <10, and ≥10), current smoking status, body mass index (continuous), physical activity (in women: 0 to <5, 5 to <11.5, 11.5 to <22, and ≥22 metabolic equivalents [METs]/week; in men: 0 to <10, 10 to <22.5, 22.5 to <41.5, and ≥41.5 METs/week), multivitamin use, regular use of aspirin or nonsteroidal anti-inflammatory drugs (≥2 tablets/week), alcohol consumption (in women: 0 to <0.15, 0.15 to <2.0, 2.0 to <7.5, and ≥7.5g/d; in men: 0 to <5, 5 to <10, 10 to <15, 15 to <30, and ≥30g/d) and Dietary Approaches to Stop Hypertension score (in quartiles).
|| Likelihood ratio test was used to compare the model that allows for separate associations for MSS and MSI-high tumors to the model that assumes a common association across subtypes.
We assessed the proportional hazards assumption by including the product term between age and each covariable (including the exposure of interest, marine ω-3 PUFA intake) into the Cox model, and testing the statistical significance of the term by Wald test. No deviation from proportional hazards assumption was detected at the α of 0.05 level. We also tested the assumption using age in a categorical form (<45, 45–49, 50–59, 60–69, and ≥70 years) instead of in a continuous form and did not find any statistically significant interaction either between age and each covariable.
To examine whether the associations vary by tumor subtypes, we fitted the subtype-stratified Cox proportional cause-specific hazards regression model using the duplication method (33), and performed a heterogeneity test using the likelihood ratio test, by comparing the model in which the association with exposures was allowed to vary by tumor subtypes to a model in which all the associations were held constant. We assessed the potential nonlinear relationship between exposure and subtypes of CRC using stepwise restricted cubic spline analysis (34), with a P value of .05 as the criteria for both inclusion and retention in the model. We used the likelihood ratio test to determine the overall statistical significance and the significance of the nonlinearity.
We corrected for measurement error in marine ω-3 PUFA intake using two one-week dietary records completed six months apart in the validation studies of the two cohorts (28,30,35). We used a risk set regression calibration method, which recalibrates the measurement error model for time-varying exposures within each risk set of the Cox regression model (36). Both the point and interval estimates were corrected for multivariable HRs of CRC subtypes associated with cumulative average of marine ω-3 PUFA intake.
We used SAS 9.3 for all analyses (SAS Institute Inc., Cary, NC). All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
Table 1 presents the basic characteristics of study participants in the two cohorts. Individuals with higher intake of marine ω-3 PUFA were more likely to be active, undergo lower gastrointestinal endoscopy, and regularly take multivitamins and fish oil supplements. They also had more frequent consumption of poultry, fruits, and vegetables, and less frequent consumption of unprocessed and processed red meat.
Table 1.
Age-standardized characteristics of person-years according to intake of marine ω-3 PUFAs in the Nurses’ Health Study (women, 1984–2010) and Health Professionals Follow-up Study (men, 1986–2010)*
| Characteristic | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|
| <0.10g/d | 0.10–0.19g/d | 0.20–0.29g/d | ≥0.30g/d | <0.10g/d | 0.10–0.19g/d | 0.20–0.29g/d | ≥0.30g/d | |
| Person-years | 230,847 | 229,354 | 146,130 | 201,784 | 75,740 | 81,886 | 76,234 | 172,820 |
| Intake of marine ω-3 PUFAs, g/d | 0.06 | 0.14 | 0.24 | 0.55 | 0.06 | 0.14 | 0.25 | 0.60 |
| Age, y | 63.6 | 59.7 | 60.8 | 61.8 | 62.3 | 61.2 | 62.2 | 63.5 |
| Body mass index, kg/m2 | 26.1 | 26.2 | 26.0 | 26.1 | 25.7 | 25.8 | 25.6 | 25.6 |
| Physical activity, MET-hours/wk† | 14.9 | 16.3 | 18.4 | 20.5 | 31.9 | 31.5 | 35.1 | 36.8 |
| Pack-years of smoking before age 30 y | 7.0 | 6.9 | 6.9 | 7.1 | 11.4 | 11.0 | 10.9 | 10.9 |
| Current smoking, % | 14 | 14 | 12 | 11 | 7 | 7 | 6 | 4 |
| Colorectal cancer in a parent or sibling, % | 17 | 18 | 17 | 17 | 14 | 14 | 14 | 14 |
| History of colonoscopy/sigmoidoscopy, % | 21 | 20 | 22 | 24 | 23 | 24 | 26 | 29 |
| Current multivitamin use, % | 53 | 51 | 55 | 59 | 48 | 48 | 50 | 55 |
| Current use of fish oil supplements, % | 0.1 | 0.2 | 0.3 | 18 | 2 | 2 | 3 | 13 |
| Regular aspirin or NSAID use, %‡ | 53 | 52 | 52 | 52 | 49 | 50 | 50 | 51 |
| Postmenopausal, % | 81 | 81 | 81 | 82 | - | - | - | - |
| Current postmenopausal hormone use, %§ | 51 | 52 | 51 | 47 | - | - | - | - |
| Dietary intake | ||||||||
| ω-3 PUFAs, g/d | 1.1 | 1.1 | 1.3 | 1.6 | 1.2 | 1.3 | 1.4 | 1.8 |
| 18:3ω-3 (ALA), g/d | 1.0 | 1.0 | 1.0 | 1.1 | 1.1 | 1.1 | 1.1 | 1.2 |
| 20:5ω-3 (EPA), mg/d | 15.0 | 38.3 | 76.2 | 203 | 15.0 | 37.9 | 80.3 | 225 |
| 22:5ω-3 (DPA), mg/d | 11.6 | 15.5 | 21.1 | 31.3 | 12.2 | 16.8 | 22.7 | 37.2 |
| 22:6ω-3 (DHA), mg/d | 34.1 | 86.9 | 146 | 312 | 33.3 | 88.0 | 147 | 341 |
| ω-6 PUFAs, g/d | 9.1 | 9.0 | 8.9 | 9.0 | 12.2 | 12.1 | 11.9 | 11.8 |
| Fish, servings/wk | 0.6 | 1.4 | 2.1 | 3.4 | 0.4 | 1.3 | 1.8 | 3.6 |
| Unprocessed red meat, servings/wk | 3.7 | 3.7 | 3.5 | 2.9 | 4.5 | 4.4 | 4.3 | 3.3 |
| Processed red meat, servings/wk | 1.7 | 1.8 | 1.6 | 1.3 | 2.4 | 2.6 | 2.4 | 1.7 |
| Poultry, servings/wk | 2.2 | 2.6 | 2.8 | 3.0 | 2.3 | 2.8 | 3.0 | 3.4 |
| Total fruit, servings/d | 2.2 | 2.3 | 2.6 | 2.5 | 2.3 | 2.4 | 2.7 | 2.8 |
| Total vegetable, servings/d | 2.7 | 3.1 | 3.4 | 3.6 | 2.8 | 3.1 | 3.5 | 3.8 |
| Alcohol, g/d | 4.6 | 5.6 | 6.7 | 6.2 | 10.1 | 11.3 | 12.9 | 11.4 |
| DASH diet score | 22.6 | 23.4 | 24.4 | 25.4 | 22.5 | 23.0 | 24.2 | 25.2 |
* All variables are age standardized except age. Continuous variables are described as mean. ALA = α-linolenic acid; DASH = Dietary Approaches to Stop Hypertension; DHA = docosahexaenoic acid; DPA = docosapentaenoic acid; EPA = eicosapentaenoic acid; MET = metabolic equivalent; NSAID = nonsteroidal anti-inflammatory drug; PUFA = polyunsaturated fatty acid.
† Physical activity is represented by the product sum of the MET of each specific recreational activity and hours spent on that activity per week.
‡ Regular users are defined as two or more standard (325mg) tablets of aspirin or two or more tablets of NSAIDs per week.
§ Proportion of current postmenopausal hormone use is calculated among postmenopausal women only.
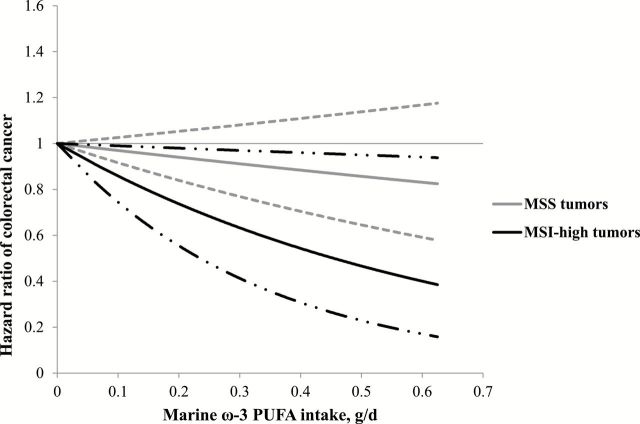
We then examined the association of marine ω-3 PUFA intake with both overall and MSI-defined CRC in the two cohorts. As previously reported, marine ω-3 PUFA was not associated with overall incidence of CRC (Table 2). Notably, we found that the association between marine ω-3 PUFA intake and CRC risk differed by MSI status, with an inverse association restricted to MSI-high tumors. In the pooled cohorts, the multivariable hazard ratio comparing marine ω-3 PUFA intake of 0.30g/d or higher to that of less than 0.10g/d was 0.54 (95% CI = 0.35 to 0.83) for MSI-high tumors and was 0.97 (95% CI = 0.78 to 1.20) for MSS tumors. The P value for heterogeneity was .02. Similar results were found in women and men (Supplementary Table 2, available online). In the spline analysis, we did not detect a nonlinear relationship (Figure 1); high marine ω-3 PUFA intake demonstrated a linear association with lower risk of MSI-high tumors (P linearity = .03) but not MSS tumors (P linearity = .28). Among individuals with marine ω-3 PUFA intake of 0.30g/d or more, the incidence rates of MSI-high and MSS tumors were 5.5 and 33.9 per 100 000 persons per year, respectively.
Figure 1.
Restricted cubic spline plot for marine ω-3 PUFA intake in relation to risk of MSS and MSI-high colorectal cancers in the pooled cohorts of the Nurses’ Health Study (1984–2010) and Health Professionals Follow-up Study (1986–2010). Dash and hatched lines represent 95% confidence intervals. Multivariable model was adjusted for the same set of covariables as in Table 2. No spline variable was selected. P linearity = .03 for MSI-high tumors and .28 for MSS tumors. All P values are two-sided. MSI = microsatellite instability; MSS = microsatellite-stable; PUFA = polyunsaturated fatty acids.
We corrected for measurement error in marine ω-3 PUFA intake across the follow-up. The results did not appreciably change. In the multivariable-adjusted model, the hazard ratio of MSI-high CRC associated with 0.15g/day increase of marine ω-3 PUFA intake changed from 0.88 (95% CI = 0.76 to 1.00) to 0.86 (95% CI = 0.71 to 1.04). The corresponding hazard ratios for MSS CRC were 0.99 (95% CI = 0.94 to 1.04) before correction and 0.99 (95% CI = 0.93 to 1.05) after correction (data not shown).
For tumors subclassified by CIMP and BRAF mutation status (Supplementary Table 3, available online), the heterogeneity test for the association with marine ω-3 PUFA did not reach statistical significance (P heterogeneity > .15), although marine ω-3 PUFA appeared to be somewhat more strongly associated with lower risk of CIMP-high and BRAF-mutated tumors than with CIMP-low/negative and BRAF–wild-type tumors.
We jointly classified tumors by MSI and CIMP/BRAF status to assess whether the differential associations of marine ω-3 PUFA with MSI-defined CRC subtypes are independent of CIMP and BRAF mutation. In addition, MSS tumors have been shown to be highly heterogeneous and include some CRCs with molecular features that characterize the sporadic MSI-high subset, notably CIMP and BRAF mutation (37–39). Therefore, such joint classification also allows us to explore whether there is a subset of MSS tumors that is associated with ω-3 PUFAs. As shown in Table 3, marine ω-3 PUFA demonstrated an inverse association with risk of MSI-high CRC, but not MSS CRC, irrespective of CIMP or BRAF mutation.
Table 3.
Hazard ratio and 95% confidence interval of incident colorectal cancer, jointly classified by MSI and BRAF/CIMP status, according to intake of marine ω-3 polyunsaturated fatty acids in the Nurses’ Health Study (1984–2010) and Health Professionals Follow-up Study (1986–2010)*
| CIMP or BRAF mutation status of CRC | MSI status of CRC | <0.10g/d | 0.10–0.19g/d | 0.20–0.29g/d | ≥0.30g/d | P trend |
|---|---|---|---|---|---|---|
| CIMP-high | MSS CRC | |||||
| No. of cases (n = 58) | 8 | 15 | 13 | 22 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 0.93 (0.39 to 2.19) | 1.02 (0.42 to 2.47) | 1.41 (0.62 to 3.23) | .25 | |
| MSI-high CRC | ||||||
| No. of cases (n = 130) | 28 | 38 | 31 | 33 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 0.62 (0.38 to 1.01) | 0.69 (0.41 to 1.16) | 0.62 (0.37 to 1.04) | .23 | |
| P heterogeneity‡ | .42 | .46 | .09 | |||
| CIMP-low/negative | MSS CRC | |||||
| No. of cases (n = 818) | 121 | 268 | 183 | 246 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 1.08 (0.87 to 1.34) | 0.95 (0.75 to 1.21) | 0.93 (0.74 to 1.17) | .16 | |
| MSI-high CRC | ||||||
| No. of cases (n=45) | 14 | 13 | 9 | 9 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 0.43 (0.20 to 0.91) | 0.39 (0.17 to 0.91) | 0.28 (0.12 to 0.66) | .02 | |
| P heterogeneity‡ | .02 | .04 | .007 | |||
| BRAF-mutated | MSS CRC | |||||
| No. of cases (n = 64) | 8 | 20 | 17 | 19 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 1.24 (0.54 to 2.84) | 1.45 (0.62 to 3.40) | 1.37 (0.59 to 3.17) | .52 | |
| MSI-high CRC | ||||||
| No. of cases (n = 82) | 19 | 26 | 20 | 17 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 0.61 (0.34 to 1.11) | 0.64 (0.34 to 1.21) | 0.47 (0.24 to 0.93) | .06 | |
| P heterogeneity‡ | .17 | .13 | .05 | |||
| BRAF-wild-type | MSS CRC | |||||
| No. of cases (n = 815) | 121 | 262 | 181 | 251 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 1.05 (0.84 to 1.31) | 0.92 (0.73 to 1.17) | 0.90 (0.72 to 1.13) | .10 | |
| MSI-high CRC | ||||||
| No. of cases (n = 85) | 20 | 21 | 16 | 28 | ||
| Multivariable HR (95% CI)† | 1.00 (referent) | 0.48 (0.26 to 0.89) | 0.48 (0.25 to 0.93) | 0.60 (0.33 to 1.08) | .47 | |
| P heterogeneity‡ | .02 | .07 | .20 |
* All P values are two-sided. CI = confidence interval; CIMP = CpG island methylator phenotype; CRC = colorectal cancer; HR = hazard ratio; MSI = microsatellite instability; MSS = microsatellite stable.
† Adjusted for the same set of covariables as in Table 2.
‡Likelihood ratio test was used to compare the model that allows for separate associations for MSS and MSI-high tumors to the model that assumes a common association across subtypes.
Discussion
In two large US cohorts, we observed that marine ω-3 PUFA intake was associated with lower risk of MSI-high colorectal tumors but not MSS tumors. This association appeared independent of CIMP and BRAF mutation. Given the predominance of MSI-high tumors in the proximal colon, our molecular analyses extend the previous findings of a stronger inverse association of marine ω-3 PUFA intake with proximal colon than with distal colon cancer (12,13) and generate hypotheses for the potential mechanistic role of ω-3 PUFAs in colorectal carcinogenesis.
Our findings are supported by substantial in vitro and animal evidence and by the chemopreventative efficacy of ω-3 PUFAs that has been demonstrated in a randomized controlled trial (RCT) of patients with history of adenomatous polyps, the precursor to the vast majority of CRCs (4). In RCTs, supplementation of ω-3 PUFAs also reduced mucosal epithelial proliferation index and polyp number and size (40–42). One of the primary mechanisms whereby ω-3 PUFAs exert their antineoplastic activity has been hypothesized to be through abrogation of several proinflammatory pathways, including biosynthesis of arachidonic acid-derived eicosanoids (eg, PGE2), activity of transcription factors (eg, peroxisome proliferator-activated receptor [PPAR]-α and PPAR-γ), and expression and signal transduction of inflammation-related genes (eg, prostaglandin-endoperoxide synthase 2 [PTGS2, also known as cyclooxygenase-2]) (3). Recently, lipid mediators derived from EPA and DHA, including resolvins, protectins and maresins, have been shown to possess potent anti-inflammatory effects, which may also contribute to the antineoplastic activity of ω-3 PUFAs (43,44).
Despite these compelling data, epidemiologic studies associating ω-3 PUFAs with CRC risk have been inconsistent. A recent meta-analysis of seven prospective studies reported a relative risk of 0.98 (95% CI = 0.88 to 1.09) for CRC comparing the highest to the lowest category of ω-3 PUFA intake (45). Nevertheless, more recent studies have found that the relationship between marine ω-3 PUFA intake and CRC may vary according to anatomic subsite. In a large Japanese cohort, the inverse association of marine ω-3 PUFAs with CRC was limited to tumors in the proximal site of the large bowel (13). Using the NHS and HPFS data, we previously reported that high marine ω-3 PUFA intake was associated with an increased risk of distal colon cancer, whereas a suggestive inverse association with proximal colon cancer and rectal cancer was observed for dietary intake assessed about 10 years before CRC diagnosis in men (12). Thus, the inability to account for such variation by subsite, in addition to factors related to study design and population (eg, variation in the length of follow-up and intake level) may contribute to the mixed results of previous studies.
Accumulating evidence supports that CRC is a heterogeneous disease associated with various genetic and epigenetic alterations (14). Although examining anatomic associations of tumors can capture some of this heterogeneity, the recent recognition that molecular features of CRC vary along bowel subsites in a linear pattern, rather than changing abruptly at specific locations, highlights the importance of integrating molecular-level information into etiologic investigation of CRC (15,46). Therefore, we undertook this integrative approach and investigated whether variation in molecular features of CRC can explain the subsite-dependent associations between ω-3 PUFA intake and CRC risk. Our results suggest that ω-3 PUFAs may preferentially inhibit the development of colorectal tumors that arise from defective MMR.
Several lines of evidence support the involvement of inflammation in the MMR deficiency associated with MSI-high CRC. A substantial proportion of patients with colonic inflammation–associated neoplasms demonstrated hypermethylation of hMLH1, the predominant mechanism for sporadic MSI-high CRC (47). Moreover, MSI has been observed in premalignant inflamed tissues without dysplasia among patients with ulcerative colitis (48,49). Experimental evidence has shown that chronic inflammation triggered by oxidative stress or hypoxia inactivates MMR gene expression by mutation (21,50) or epigenetic silencing (51) and may directly damage MMR proteins (20). In vitro studies have demonstrated that proinflammatory cytokines upregulate hypoxia inducible factor 1, which can inhibit expression of MMR proteins in human CRC tissues (52,53).
Therefore, considering the well-established anti-inflammatory role of ω-3 PUFAs, it is biologically plausible that ω-3 PUFAs preferably protect against the development of MSI-high CRC. However, further investigation is needed to confirm these findings and decipher underlying mechanisms. In particular, the VITamin D and OmegA-3 TriaL, an ongoing randomized placebo-controlled trial of supplementation of marine ω-3 PUFA (EPA+DHA, 1g/d) in 20 000 participants, may provide important information at a higher end of intake in this regard, as the collection of biospecimens has been planned (54).
Our study has several strengths. First, unlike the retrospective collection of data after cancer diagnosis employed in most previous studies on nutritional exposure and molecular subtypes of cancer, the dietary data used in our study were collected before diagnosis, which minimizes the possibility of any differential measurement error between cases and noncases. Second, the availability of repeated dietary data over prolonged follow-up allowed us to evaluate cumulative average consumption of marine ω-3 PUFAs with dampened measurement errors. In addition, we corrected for measurement error using dietary record data collected in the validation studies within the two cohorts and our results did not appreciably change, although there may still be remaining errors related to between-person variation in absorption and metabolism of ω-3 PUFA that is not captured by FFQ or dietary record. Finally, detailed data on a wide range of exposures provided the ability to adjust for multiple confounding factors, although the possibility of residual confounding cannot be excluded.
The limitations of our study should also be noted. First, tissue marker data were not available among all patients diagnosed with CRC in our cohorts. However, comparison of cases with and without tumor tissue data did not reveal any substantial difference. Second, despite the large cohort size, the number of MSI-high cases was relatively small. Nevertheless, the consistent findings in the two cohorts are reassuring. Third, the health professional background of our cohorts and the relatively modest consumption of marine ω-3 PUFAs may limit the generalizability our findings to other populations with different patterns of dietary intake. Lastly, multiple comparisons were performed in our analyses, and therefore our results should be interpreted with caution.
In conclusion, the results of our prospective study have provided support that the association between marine ω-3 PUFAs and CRC may vary according to specific molecularly defined subtypes that develop through microsatellite instability. Our findings generate hypotheses for the potential anticancer activity of ω-3 PUFAs and may have clinical implications for the potential of using marine ω-3 PUFAs in prevention of CRC.
Funding
This work was supported by the National Institutes of Health (P01 CA87969, UM1 CA167552, P50 CA127003, R01 CA137178, R01 CA151993, K24 DK 098311, K07 CA190673, and 1U54CA155626) and by a grant from the Agrusa Fund for Colorectal Cancer Research.
Supplementary Material
The funders had no role in design and conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review, or approval of the manuscript, nor the decision to submit the manuscript for publication.
A. T. Chan previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pfizer Inc., and Pozen Inc. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, Pfizer Inc., or Pozen Inc. No other conflict of interest exists.
M. Song is a trainee of the Harvard Transdisciplinary Research Center on Energetics and Cancer (TREC). We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions, as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
References
- 1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2. Terzic J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology. 2010;138(6):2101–2114. [DOI] [PubMed] [Google Scholar]
- 3. Larsson SC, Kumlin M, Ingelman-Sundberg M, et al. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79(6):935–945. [DOI] [PubMed] [Google Scholar]
- 4. Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2012;61(1):135–149. [DOI] [PubMed] [Google Scholar]
- 5. Norat T, Bingham S, Ferrari P, et al. Meat, fish, and colorectal cancer risk: the European Prospective Investigation into cancer and nutrition. J Natl Cancer Inst. 2005;97(12):906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hall MN, Chavarro JE, Lee IM, et al. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniel CR, McCullough ML, Patel RC, et al. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev. 2009;18(2):516–525. [DOI] [PubMed] [Google Scholar]
- 8. Lin J, Zhang SM, Cook NR, et al. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004;160(10):1011–1022. [DOI] [PubMed] [Google Scholar]
- 9. Terry P, Bergkvist L, Holmberg L, et al. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(8):913–914. [PubMed] [Google Scholar]
- 10. Butler LM, Wang R, Koh WP, et al. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009;124(3):678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kantor ED, Lampe JW, Peters U, et al. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer. 2014;66(4):716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song M, Chan AT, Fuchs CS, et al. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int J Cancer. 2014;135(10):2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sasazuki S, Inoue M, Iwasaki M, et al. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer. 2011;129(7):1718–1729. [DOI] [PubMed] [Google Scholar]
- 14. Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375(9719):1030–1047. [DOI] [PubMed] [Google Scholar]
- 15. Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. [DOI] [PubMed] [Google Scholar]
- 17. Ward R, Meagher A, Tomlinson I, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001;48(6):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–618. [DOI] [PubMed] [Google Scholar]
- 19. Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(6):2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang CL, Marra G, Chauhan DP, et al. Oxidative stress inactivates the human DNA mismatch repair system. Am J Physiol Cell Physiol. 2002;283(1):C148–154. [DOI] [PubMed] [Google Scholar]
- 21. Gasche C, Chang CL, Rhees J, et al. Oxidative stress increases frameshift mutations in human colorectal cancer cells. Cancer Res. 2001;61(20):7444–7448. [PubMed] [Google Scholar]
- 22. Lee SH, Chang DK, Goel A, et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J Immunol. 2003;170(4):2214–2220. [DOI] [PubMed] [Google Scholar]
- 23. Xia D, Wang D, Kim SH, et al. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nat Med. 2012;18(2):224–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 25. Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464–468. [DOI] [PubMed] [Google Scholar]
- 26. Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA. 2001;285(3):304–312. [DOI] [PubMed] [Google Scholar]
- 27. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4 Suppl):1220S–1228S; discussion 1229S-1231S. [DOI] [PubMed] [Google Scholar]
- 28. Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol. 1992;135(4):418–427. [DOI] [PubMed] [Google Scholar]
- 29. Garland M, Sacks FM, Colditz GA, et al. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67(1):25–30. [DOI] [PubMed] [Google Scholar]
- 30. Willett WC. Reproducibility and Validity of Food-Frequency Questionnaires. In. Nutritional Epidemiology. Second ed. New York: Oxford University Press; 1998. [Google Scholar]
- 31. Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Handbook (6th Edition). In: Cancer AJCo, (ed). 6 ed: Springer; 2002. [Google Scholar]
- 32. Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19(1):59–68. [DOI] [PubMed] [Google Scholar]
- 33. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51(2):524–532. [PubMed] [Google Scholar]
- 34. Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. [DOI] [PubMed] [Google Scholar]
- 35. Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–1126; discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 36. Liao X, Zucker DM, Li Y, et al. Survival analysis with error-prone time-varying covariates: a risk set calibration approach. Biometrics. 2011;67(1):50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitehall VL, Wynter CV, Walsh MD, et al. Morphological and molecular heterogeneity within nonmicrosatellite instability-high colorectal cancer. Cancer Res. 2002;62(21):6011–6014. [PubMed] [Google Scholar]
- 38. Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53(8):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jass JR. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathol. 2007;50(1):113–130. [DOI] [PubMed] [Google Scholar]
- 40. Courtney ED, Matthews S, Finlayson C, et al. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22(7):765–776. [DOI] [PubMed] [Google Scholar]
- 41. Huang YC, Jessup JM, Forse RA, et al. n-3 fatty acids decrease colonic epithelial cell proliferation in high-risk bowel mucosa. Lipids. 1996;31 Suppl:S313–317. [DOI] [PubMed] [Google Scholar]
- 42. West NJ, Clark SK, Phillips RK, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59(7):918–925. [DOI] [PubMed] [Google Scholar]
- 43. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campbell EL, MacManus CF, Kominsky DJ, et al. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc Natl Acad Sci U S A. 2010;107(32):14298–14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen XJ, Zhou JD, Dong JY, et al. Dietary intake of n-3 fatty acids and colorectal cancer risk: a meta-analysis of data from 489 000 individuals. Br J Nutr. 2012;108(9):1550–1556. [DOI] [PubMed] [Google Scholar]
- 46. Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: the evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102(6):365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fleisher AS, Esteller M, Harpaz N, et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLHI. Cancer Res. 2000;60(17):4864–4868. [PubMed] [Google Scholar]
- 48. Brentnall TA, Crispin DA, Bronner MP, et al. Microsatellite instability in nonneoplastic mucosa from patients with chronic ulcerative colitis. Cancer Res. 1996;56(6):1237–1240. [PubMed] [Google Scholar]
- 49. Ishitsuka T, Kashiwagi H, Konishi F. Microsatellite instability in inflamed and neoplastic epithelium in ulcerative colitis. J Clin Pathol. 2001;54(7):526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jackson AL, Chen R, Loeb LA. Induction of microsatellite instability by oxidative DNA damage. Proc Natl Acad Sci U S A. 1998;95(21):12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edwards RA, Witherspoon M, Wang K, et al. Epigenetic repression of DNA mismatch repair by inflammation and hypoxia in inflammatory bowel disease-associated colorectal cancer. Cancer Res. 2009;69(16):6423–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koshiji M, To KKW, Hammer S, et al. HIF-1 alpha induces genetic instability by transcriptionally downregulating MutS alpha expression. Mol Cell. 2005;17(6):793–803. [DOI] [PubMed] [Google Scholar]
- 53. Jung YJ, Isaacs JS, Lee S, et al. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. Faseb J. 2003;17(14):2115–2117. [DOI] [PubMed] [Google Scholar]
- 54. Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.