Abstract
OBJECTIVES
Obesity has been thought to predispose patients to excess morbidity after lung resection because of decreased diaphragm excursion, reduced lung volumes and relative immobility. We assessed the relationship of body mass index (BMI) to acute outcomes after major lung resection.
METHODS
Information from our database of lung resections was evaluated for the period 1980–2011. Univariate analysis for adverse events (pulmonary, cardiovascular, other and overall) was used to select variables for inclusion in multivariate logistic regression analyses. Missing values were imputed. BMI was categorized as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), obese (30–34.9) and very obese (≥35).
RESULTS
Among 1369 patients, there were 703 males (51%) and the mean age was 62 ± 11 years. Complications included the following: pulmonary 12%, cardiovascular 15%, other 16%, mortality 5% and any 29%. The incidence of complications decreased during each decade of study (40, 30, 26, 20%; P < 0.0001) and the incidence of obese/very obese increased during the same intervals (11, 22, 30, 25%; P = 0.0007). Adjusting for age, performance status, coronary artery disease, smoking status, diffusing capacity of the lung for carbon monoxide, forced expiratory volume in 1 s and operation year, being overweight/obese/very obese did not increase the risk of postoperative complications in any category. In fact, patients in this group showed a lower rate of cardiovascular complications than those with BMI ≤ 25 (odds ratio (OR): 0.72; 95% confidence interval (CI): 0.51–1.00; P = 0.048). However, being underweight was importantly associated with an increased risk of pulmonary complications (OR: 2.5; 95% CI: 1.3–4.9; P = 0.0087) and of operative mortality (OR: 2.96; 95% CI: 1.28–6.86; P = 0.011).
CONCLUSION
Being overweight or obese does not increase the risk of complications after major lung resection. In contrast, patients who are underweight are at significantly increased risk of pulmonary complications and mortality. Knowledge of the relationship of BMI to perioperative risk for major lung resection is essential in proper risk stratification.
Keywords: Lung resection, Outcomes, Obesity, Body mass index
INTRODUCTION
Body mass index (BMI) intuitively influences the technical aspects of lung cancer surgery and its outcomes. Surgeons traditionally welcome the easily discerned internal anatomy of thin patients, while reluctantly facing challenges that obese patients present. The greater technical and physical demands engendered by substantial girth and excess mediastinal fat are associated with increased operating time for major lung resection [1]. Obesity intuitively increases the perioperative risks of lung surgery owing to associated comorbidities such as diabetes, hypertension and coronary artery disease, and physiological impairment of ventilation. Results of recent reports on acute outcomes of major lung resection in obese patients are mixed: some reports substantiate this increased risk [2, 3], while others demonstrate no increase in risk [4, 5].
Being underweight, despite the perceived intraoperative advantages for the surgeon, is theoretically associated with increased perioperative risks owing to nutritional depletion, muscle weakness and altered metabolism that affect responses to inflammation and wound-healing processes. In contrast to the growing interest in the relationship of increased BMI and surgical outcomes after lung resection, there are no substantial reports on the effects of underweight status specific to lung surgery. A recent report from the Society of Thoracic Surgeons Database indicated that nearly 26% of patients undergoing lung resection were obese, but did not mention the percentage of patients who were underweight [6].
No clear consensus exists regarding the relationship of obesity to outcomes after major lung resection, and there is little information about the influence of being underweight on lung surgery. Our aim in this study was to explore the relationship of BMI, particularly obese and underweight status, to perioperative outcomes after major lung resection.
METHODS
We performed a retrospective review of an Institutional Review Board-approved lung resection database for patients undergoing major lung resection (lobectomy, bilobectomy and pneumonectomy) from 1980 to 2011. Demographic, physiological, operative and outcomes variables were collected. For patients undergoing more than one major lung resection, only the first operation was used in the analyses. Staging was performed according to the American Joint Committee on Cancer seventh edition manual [7]. Operative mortality was defined as death during hospitalization for lung resection or within 30 days of resection. Complications were classified as pulmonary (respiratory failure, pneumonia, lobar collapse requiring intervention and prolonged air leak), cardiovascular (myocardial infarction, pulmonary embolism, arrhythmia requiring therapy, cerebrovascular accident and transient ischaemic attack), other (recurrent nerve injury, wound infection, empyema, bronchopleural fistula and miscellaneous), mortality and overall (pulmonary, cardiovascular, other and mortality).
BMI (kg/m2) was categorized as underweight (<18.5), normal (18.5–24.9), overweight (25–29.9), obese (30–34.9) and very obese (≥35). Univariate analysis for adverse events (pulmonary, cardiovascular, other and overall) was performed for the following variables: BMI, sex, age, diabetes mellitus, hypertension, preoperative radiation therapy, preoperative chemotherapy, coronary artery disease (CAD), cerebrovascular disease, Eastern Cooperative Oncology Group performance status (PS), cigarette smoking status (current vs prior), prior lung resection, diagnosis (cancer or benign) and year of operation. Continuous variables were categorized and χ2 analysis was performed except in cases in which there were less than 10 events in any category, in which case the Fisher exact test was performed. To handle missing data, we generated five imputation datasets using the multiple imputation by chained equations algorithm [8] with predictive mean matching option to ensure that imputed values fell within the range of plausible values. Significant variables on univariate analysis were used to fit a multivariate logistic regression. A three-knot restricted cubic spline function for BMI and a four-knot restricted cubic spline function for year of operation were used to account for nonlinearities on these variables. Different shapes of functions for males and females were deemed not necessary. Results in the five imputations were combined using Rubin's rules for pooling analyses of imputed data [9, 10]. Imputation was performed using Stata version SE12. All remaining analyses were performed using the R Statistical software [11] and the rms package in R [12].
RESULTS
Patient demographics and clinical characteristics are listed in Table 1. There was a significant upward trend in the incidence of obese/very obese during the decades of study (11, 22, 30, 25%; P = 0.0007). There was a similar significant downward trend in the incidence of underweight (8, 5, 3, 3%; P = 0.011). Surgical details and outcomes are listed in Table 2. This data set contains a relatively high number of surgical deaths, which is largely attributable to the earlier decades of the study and the large proportion of patients undergoing pneumonectomy during that time period. Rates of complications decreased during the study period, both as unadjusted rates and when adjusted for BMI, sex, age, coronary artery disease, PS and smoking status (Table 3). The adjusted trend line for pulmonary complications demonstrates a very steep reduction in the rate of such complications in more recent years (Fig. 1).
Table 1:
Clinical characteristics of patients undergoing major lung resection
| Category | Evaluable patients | Value (±standard deviation) or number affected (percent) |
|---|---|---|
| Male gender | 1369 | 703 (51) |
| Age at operation (years) | 1369 | 62 ± 11 |
| Serum creatinine (mg/dl) | 1127 | 1 ± 1 |
| Hypertension | 1367 | 568 (42) |
| Coronary artery disease | 1368 | 281 (21) |
| Diabetes mellitus | 1368 | 197 (14) |
| Any tobacco use | 1367 | 1184 (87) |
| PS 0–1 | 1351 | 1183 (88) |
| BMI | 1366 | |
| Underweight (<18.5) | 64 (5) | |
| Normal (18.5–24.9) | 489 (36) | |
| Overweight (25–29.9) | 489 (36) | |
| Obese (30–34.9) | 193 (14) | |
| Very obese (≥35) | 131 (10) | |
| FEV1% | 1318 | 84 ± 22 |
| ppoFEV1% | 1318 | 64 ± 20 |
| DLCO% | 1240 | 85 ± 22 |
| ppoDLCO% | 1240 | 64 ± 19 |
| Pretreatment clinical stage | 1145 | |
| I | 587 (51) | |
| II | 286 (25) | |
| III | 250 (22) | |
| IV | 22 (2) | |
| Induction chemotherapy | 1302 | 125 (10) |
| Induction radiation therapy | 1307 | 131 (10) |
FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity of the lung for carbon monoxide; ppo: predicted postoperative value
Table 2:
Outcomes of patients undergoing major lung resection
| Category | Evaluable patients | Number affected (percent) |
|---|---|---|
| Operation | 1367 | |
| Lobectomy | 1088 (80) | |
| Bilobectomy | 96 (7) | |
| Pneumonectomy | 183 (13) | |
| Surgical outcomes | 1369 | |
| Mortality | 69 (5) | |
| Pulmonary morbidity | 161 (12) | |
| Cardiovascular morbidity | 202 (15) | |
| Other morbidity | 214 (16) | |
| Overall major complications | 401 (29) | |
| Final pathological stage | 1145 | |
| 0,I | 660 (58) | |
| II | 271 (24) | |
| III | 196 (17) | |
| IV | 18 (2) |
Table 3:
Rates of complications by time period (percentages)
| Category | 1980–1989 | 1990–1999 | 2000–2010 | 2010–2011 | P-value for unadjusted trend | P-value for adjusted trenda |
|---|---|---|---|---|---|---|
| Pulmonary | 17 | 14 | 10 | 4 | 1.56e-06 | 4.73e-06 |
| Cardiovascular | 22 | 14 | 13 | 9 | 0.000358 | 0.00182 |
| Other | 20 | 16 | 14 | 11 | 0.00218 | 0.013 |
| Mortality | 9 | 7 | 3 | 3 | 3.18e-05 | 0.0012 |
| Overall | 40 | 30 | 25 | 20 | 3.63e-07 | 1.28e-05 |
aAdjusted for BMI, age, sex, coronary artery disease, performance status, year of operation, smoking status, diffusing capacity (DLCO), FEV1.
Figure 1:
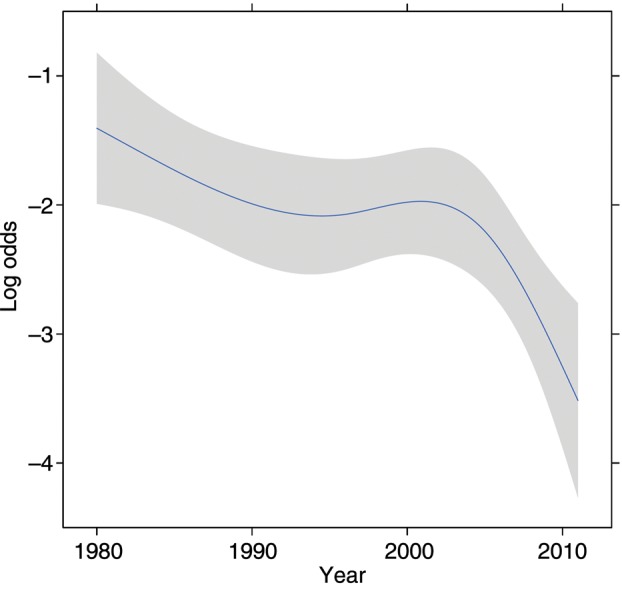
Trend line for postoperative pulmonary complications over time (adjusted for BMI, sex, age, coronary artery disease, PS and smoking status). The grey band represents 95% CIs.
Outcomes according to BMI category are listed in Table 4. Being overweight/obese/very obese did not increase the risk of postoperative complications in any category. In fact, patients in this group showed a lower rate of cardiovascular complications than those with BMI ≤ 25 (OR: 0.72; 95% confidence interval (CI): 0.51–1.00; P = 0.048). Being underweight was significantly associated with an increased risk of pulmonary complications (OR: 2.5; 95% CI: 1.3–4.9; P = 0.0087) and of operative mortality (OR: 2.96; 95% CI: 1.28–6.86; P = 0.011).
Table 4:
Association of BMI and complications after major lung resection
| Complication | BMI category | OR | 95% CI | P-value |
|---|---|---|---|---|
| Pulmonary | Underweight | 2.48 | 1.26–4.88 | 0.0087 |
| Normal | 1.00 | – | – | |
| Overweight | 0.84 | 0.55–1.29 | 0.42 | |
| Obese | 1.14 | 0.65–1.98 | 0.65 | |
| Very obese | 0.75 | 0.36–1.57 | 0.44 | |
| Cardiovascular | Underweight | 1.45 | 0.72–2.95 | 0.30 |
| Normal | 1.00 | – | – | |
| Overweight | 0.72 | 0.49–1.05 | 0.085 | |
| Obese | 0.83 | 0.50–1.39 | 0.49 | |
| Very obese | 0.75 | 0.40–1.41 | 0.37 | |
| Other | Underweight | 1.21 | 0.61–2.39 | 0.59 |
| Normal | 1.00 | – | – | |
| Overweight | 0.91 | 0.64–1.30 | 0.62 | |
| Obese | 0.76 | 0.45–1.27 | 0.30 | |
| Very obese | 0.65 | 0.35–1.22 | 0.18 | |
| Mortality | Underweight | 2.96 | 1.28–6.86 | 0.011 |
| Normal | 1.00 | – | – | |
| Overweight | 0.45 | 0.23–0.89 | 0.022 | |
| Obese | 1.11 | 0.50–2.44 | 0.80 | |
| Very obese | 0.71 | 0.23–2.14 | 0.55 | |
| Overall | Underweight | 1.60 | 0.90–2.86 | 0.11 |
| Normal | 1.00 | – | – | |
| Overweight | 0.89 | 0.67–1.20 | 0.45 | |
| Obese | 0.97 | 0.65–1.44 | 0.87 | |
| Very obese | 0.64 | 0.39–1.05 | 0.077 |
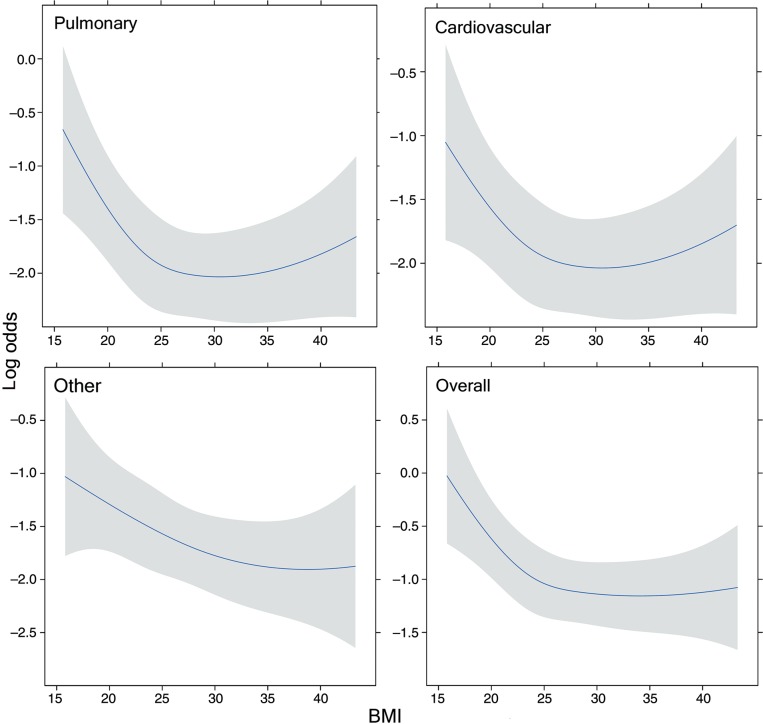
The final model for complications contained the following predictors: BMI, sex, age at operation, CAD, PS and year of operation. The log odds of complications as a function of BMI (adjusted for the other variables in the model) are shown for four types of complications in Fig. 2. The number of deaths was sufficiently small so that generating a trend line was not appropriate. The incidence of complications decreased with increasing BMI up to about 25 and stabilized above that value. There was a suggestive uptick for very obese levels for pulmonary complications, but this did not reach statistical significance. Interaction between BMI and sex was explored, but the differences fell well within confidence bands.
Figure 2:
Complications by category as a function of BMI (adjusted for sex, age, coronary artery disease, PS and smoking status). The grey bands represent 95% CIs.
DISCUSSION
Obesity is associated with an increase in cancer risk and cardiovascular disorders, and is accompanied by decreased life expectancy and impaired quality-of-life in the general population [13]. Excess body weight/mass has been explored as a predictor of postoperative outcomes after a variety of surgical procedures. A recent review of the link between excess BMI and outcomes after nonbariatric general surgery identified mixed results, with increased morbidity and mortality associated with extreme obesity, whereas being overweight or moderately obese did not confer an increase in perioperative risk [14]. Similar mixed findings have been reported for outcomes from cardiovascular surgery. In contrast, being underweight is consistently associated with increased risk of operative morbidity and mortality, both in cardiac [15] and noncardiac [16] surgery.
Little information on the effects of BMI on outcomes after pulmonary surgery has been published. Obesity is associated with increased operating time [1], but whether obesity is associated with increased risk is uncertain based on recent clinical reports [2–5]. In contrast, little information has been published on the association of low BMI and outcomes after major lung resection. The growing obesity epidemic mandates that surgeons understand the implications of the extremes of BMI for perioperative and long-term outcomes after major lung resection.
We explored the interactions of BMI and acute outcomes after major lung resection using our institutional database. Our patient population changed over the more than three decades encompassed by our study, as evidenced by an increasing incidence of obesity and decreasing frequencies of complications. Taking these factors into account, we found no important increase in perioperative risk in any category associated with overweight or obese status. In fact, overweight or obese status was associated with a decreased risk of cardiovascular complications compared with patients who were not obese/overweight. These findings are similar to those reported by Smith et al. [4], Dhakal et al. [5] and Thomas et al. [17]. The first two of these studies dichotomized BMI (obese vs other and overweight vs other, respectively) which limits comparisons of outcomes at the extremes. Our findings are similar to those of Thomas et al., who demonstrated no increased risk in overweight and obese patients, and suggested a possible protective effect of obesity on some perioperative outcomes. Overall, we identified no strong evidence that the ‘obesity paradox’ applies to major lung resection, although the vertices of the outcomes curves centred on the intersection of overweight and obese, indicating that the risks were lowest at that point. We did note that there was an uptick in complication rates at the extremes of obesity; this range of the obesity curve was not explored by Thomas et al., and a more definitive answer awaits further study. The small numbers of patients in the very obese category may have precluded the identification of a statistically significant risk for this subgroup. Overall, the overweight/obese category is not uniform, and adverse outcomes risks are determined by a complex set of characteristics.
Being underweight in our study carried important increased risks of postoperative complications, particularly pulmonary complications and mortality. Low BMI was recently identified by Thomas et al. [17] as an independent risk factor for increased complications after major lung resection, including pulmonary complications and death. It also has been identified as a risk factor for increased complications in a wide range of categories for nonbariatric general surgery [18]. Low body weight is often associated with low serum albumin, and the latter has been shown to adversely influence postoperative morbidity and mortality following thoracic surgery in general and pneumonectomy in particular [19, 20].
There are a number of potential explanations for these findings. Adipose tissue, particularly nonvisceral adipocytes, secretes cytokines that regulate inflammation, endovascular homeostasis and insulin sensitivity. Adipose tissue is capable of scavenging inflammatory toxins, and lipoproteins that are often increased in the obese can bind to and neutralize endotoxins [14, 21]. In contrast, low BMI may not be a specific cause of increased risk, but instead may be a result of other acute or chronic processes that themselves increase risk such as smoking and chronic obstructive pulmonary disease (COPD). Extreme loss of muscle mass (sarcopenia) may be related to decreased effectiveness of muscles of respiration and relative inactivity, both of which may contribute to increased perioperative risks, especially for pulmonary complications. Sarcopenia is common in patients with COPD and in those with lung cancer [22, 23].
It is likely that the use of BMI as a risk predictor will be supplanted by more sophisticated techniques in the near future. Sarcopenia is not confined to low BMI, but is common in patients in all BMI categories [23, 24]. The challenge of identifying patients with sarcopenic obesity requires adoption of new standards of risk classification. Differentiating abdominal (visceral) adiposity from general adiposity requires measurement of abdominal girth waist-to-hip ratio, or waist-to-height ratio. Computed tomographic measurement of fat content and fat–lean muscle ratio may permit a more definitive determination of both adiposity and sarcopenia [23]. Static measurements may not be sufficient, however. It is unclear whether being chronically underweight or experiencing weight loss leading to being underweight carries the most risk, so assessment of weight change may be important in the future. Weight change trajectories, including normal weight trending downwards and very obese trending upwards, are among the highest risk settings for increased mortality risk [25].
Potential shortcomings of this study include the long period of time over which data were collected, which influences demographics, patient selection, operative techniques and perioperative management. Weight loss was not assessed, and in patients who received induction therapy it was not always possible to determine whether baseline weight or posttreatment weight was recorded. Albumin and other nutritional measures were not routinely recorded, making it impossible to correlate BMI with these variables. No other measures of adiposity or muscle mass were performed to assess their value relative to BMI in relation to perioperative outcomes.
In summary, patients who are overweight or mildly obese are at somewhat lower risk for complications after major lung resection compared with patients in other BMI categories. However, we identified no consistent statistically significant protective effect of obesity on outcomes. There was a suggestion that extreme obesity put patients at increased risk. In contrast, being underweight was associated with a substantial increased risk of perioperative complications, particularly pulmonary complications and death. Knowledge of the relationship of BMI to perioperative risk for major lung resection is essential in proper risk stratification. Routine use of more sophisticated methods of assessing lean body mass, the amount and distribution of adiposity and weight change trajectory should be considered.
Funding
This work was supported by the Donald J. Ferguson, MD, Research Fund.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr A. Sihoe (Hong Kong, China): I note with interest that the patients who are underweight actually are at higher risk, so I'm just wondering, although you've alluded to the fact that you didn't look at the nutritional status, do you think this could have some relationship to the albumin levels? For example, would that be linked to the underweight and, in turn, to the complications in this cohort of patients?
Dr Ferguson: My guess, and it's just a guess, is that the majority of patients who were underweight were underweight as a result of more recent weight loss and this probably also would be reflected in their nutritional status, yes.
Dr Sihoe: That leads on to my second question, actually. You mentioned the rate of weight loss. I mean, would it be feasible or meaningful to look at the patient's premorbid BMI, is that data collectable?
Dr Ferguson: In our database it's not collectable, but I think there are ways of collecting reasonably useful information by taking more careful histories. The information about weight loss trajectory is relatively new. The highest risk patients are those who were underweight who had had recent weight loss and those who were obese who had had important recent weight gain. So even those extremes and the trajectories going in opposite directions predispose those patients to substantially increased risk. This is after general surgical procedures, but I think the lesson could apply to thoracic operations as well.
Dr G. Rocco (Naples, Italy): Mark, that was a very interesting talk. Back home, we are looking at the relation between cardiopulmonary exercise, be it either low tech or high tech, and BMI, to try to see whether there is a relation between the two. Have you looked at that kind of particular issue?
Dr Ferguson: Unlike you and some of our colleagues, we haven't routinely done exercise testing as part of our preoperative workup. So our data are quite limited and I don't have information about the relationship between the two at this point.
Dr Rocco: Can you speculate whether you think there could be an actual relation? Because, maybe your patients perform better from a cardiopulmonary test point of view and that could result in a better predictor.
Dr Ferguson: I think the handicap that we have in discussing this now is that BMI is a crude surrogate for other important elements such as sarcopenia, and obese and sarcopenic patients would probably perform poorly on either type of exercise test. Underweight and sarcopenic patients would also perform poorly, whereas you may find that underweight or obese patients who are not sarcopenic have paradoxically good exercise capacity. So I think we need to hone down a little bit better on the distribution and amount of adiposity and the ratio between adiposity and lean muscle mass to better define the performance characteristics of the patients. And that could theoretically be done almost in an automated manner using the CT scans that we already have available. We just haven't availed ourselves of the opportunity to do that.
Dr Sihoe: Could I ask one more question. Based on the results of this quite big study, would you consider a low BMI now to be a trigger for additional nutritional supplementation or management preoperatively or perioperatively?
Dr Ferguson: Yes, the problem is the results of attempts at acute nutritional repletion are quite mixed. I think that there is some evidence in more recent years that there may be some benefit, so I think that's an area worthy of investigation.
REFERENCES
- 1.St Julien JB, Aldrich MC, Sheng S, Deppen SA, Burfeind WR, Jr, Putnam JB, et al. Obesity increases operating room time for lobectomy in the society of thoracic surgeons database. Ann Thorac Surg. 2012;94:1841–7. doi: 10.1016/j.athoracsur.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petrella F, Radice D, Borri A, Galetta D, Gasparri R, Solli P, et al. The impact of preoperative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. Results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. 2011;39:738–44. doi: 10.1016/j.ejcts.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Launer H, Nguyen DV, Cooke DT. National perioperative outcomes of pulmonary lobectomy for cancer in the obese patient: a propensity score matched analysis. J Thorac Cardiovasc Surg. 2013;145:1312–8. doi: 10.1016/j.jtcvs.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Smith PW, Wang H, Gazoni LM, Shen KR, Daniel TM, Jones DR. Obesity does not increase complications after anatomic resection for non-small cell lung cancer. Ann Thorac Surg. 2007;84:1098–106. doi: 10.1016/j.athoracsur.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 5.Dhakal B, Eastwood D, Sukumaran S, Hassler G, Tisol W, Gasparri M, et al. Morbidities of lung cancer surgery in obese patients. J Thorac Cardiovasc Surg. 2013;146:379–84. doi: 10.1016/j.jtcvs.2013.02.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247–54. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 7.AJCC Cancer Staging Manual. American Joint Committee on Cancer. 7th edn. New York, NY: Springer; 2010. [Google Scholar]
- 8.Royston P, White IR. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Software. 2011;45:1–20. [Google Scholar]
- 9.Barnard J, Rubin DB. Small sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–55. [Google Scholar]
- 10.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons; 1987. [Google Scholar]
- 11.R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; ISBN 3-900051-07-0 http://www.R-project.org/ (22 January 2014, date last accessed) [Google Scholar]
- 12.Harrell FER., Jr . rms: Regression Modeling Strategies. R package version 3.6-3. 2013; http://CRAN.R-project.org/package=rms. (22 January 2014, date last accessed) [Google Scholar]
- 13.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 14.Valentijn TM, Galal W, Tjeertes EK, Hoeks SE, Verhagen HJ, Stolker RJ. The obesity paradox in the surgical population. Surgeon. 2013;11:169–76. doi: 10.1016/j.surge.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Effects of obesity and small body size on operative and long-term outcomes of coronary artery bypass surgery: a propensity-matched analysis. Ann Thorac Surg. 2005;79:1976–86. doi: 10.1016/j.athoracsur.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg. 2013;37:2561–8. doi: 10.1007/s00268-013-2162-y. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PA, Berbis J, Falcoz PE, Le Pimpec-Barthes F, Bernard A, Jougon J, et al. on behalf of the EPITHOR Group. National perioperative outcomes of pulmonary lobectomy for cancer: the influence of nutritional status. Eur J Cardiothorac Surg. 2014;45:652–9. doi: 10.1093/ejcts/ezt452. [DOI] [PubMed] [Google Scholar]
- 18.Mullen JT, Moorman DW, Davenport DL. The obesity paradox: body mass index and outcomes in patients undergoing nonbariatric general surgery. Ann Surg. 2009;250:166–72. doi: 10.1097/SLA.0b013e3181ad8935. [DOI] [PubMed] [Google Scholar]
- 19.Bagan P, Berna P, De Dominicis F, Das Neves Pereira JC, Mordant P, De La Tour B, et al. Nutritional status and postoperative outcome after pneumonectomy for lung cancer. Ann Thorac Surg. 2013;95:392–6. doi: 10.1016/j.athoracsur.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity: results from the National VA Surgical Risk Study. Arch Surg. 1999;134:36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 21.Amundson DE, Djurkovic S, Matwiyoff GN. The obesity paradox. Crit Care Clin. 2010;26:583–96. doi: 10.1016/j.ccc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Corsonello A, Antonelli Incalzi R, Pistelli R, Pedone C, Bustacchini S, Lattanzio F. Comorbidities of chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2011;17(Suppl 1):S21–28. doi: 10.1097/01.mcp.0000410744.75216.d0. [DOI] [PubMed] [Google Scholar]
- 23.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am J Clin Nutr. 2010;91:1133S–7S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]
- 24.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Tumin D, Qian Z. Obesity and mortality risk: new findings from body mass index trajectories. Am J Epidemiol. 2013;178:1591–9. doi: 10.1093/aje/kwt179. [DOI] [PMC free article] [PubMed] [Google Scholar]