Abstract
Recent development of analytical methods for lipid hydroperoxides and preparation of highly pure lipid hydroperoxides have revealed the important new pathophysiological roles of oxidized phospholipids. Generation of reactive oxygen species and subsequent oxidative stress leads to random oxidation of membrane phospholipids. However, recent studies have reported that anionic phospholipid molecules such as phosphatidylserine (PS) and cardiolipin are preferentially oxidized during apoptosis, resulting in efficient apoptosis execution and apoptotic cell clearance by phagocytes. This review is exclusively focused on selective production of oxidized PS (oxPS) during apoptosis as well as the novel roles of oxPS under pathophysiological conditions.
Keywords: anti-inflammatory, apoptosis, arteriosclerosis, lipid peroxidation, phosphatidylserine
From the 80s to the 90s, a number of studies on roles of lipid peroxidation in a variety of diseases and/or animal disease models were carried out.1– 3 However, whether generation of lipid hydroperoxides resulted in or from diseases was usually argued. It is assumed to be due to the absence of precise methods for analyzing lipid hydroperoxides at that time. Recent development of analytical methods using mass spectrometry4 as well as preparation of highly pure lipid hydroperoxides5 have revealed the following novel pathophysiological roles of oxidized phospholipids: specific epitopes of innate immunity receptors, modification of intracellular signal transduction, pro- and anti-inflammatory activities, enhancement of reactive oxygen species (ROS) generation, angiogenesis, calcification of atherosclerotic plaques, inhibition of acquired immunity and enhancement of blood coagulation.6, 7 These roles of oxidized phospholipids manifested in the above studies were predominantly related to those of oxidatively modified products of phosphstidylcholine (PC), the most abundant phospholipid in the membrane. Phosphatidylserine (PS) is also known to be a preferential target of in vivo oxidation.8 The aim of this review is to exclusively focus on the production of oxidized phosphatidylserine (oxPS) and its biological activities.
PRODUCTION OF oxPS
It is well known that non-enzymatic oxidation of phospholipids including PS in membranes can be initiated by free radicals or non-radical ROS under many pathological conditions.6 Here, selective, but not random oxidation of negatively charged phospholipids such as PS and cardiolipin (CL) is described in detail.
Asymmetric distribution of phospholipids in plasma membranes
Bretscher9 has first postulated the concept of “phospholipid asymmetry” suggesting that phospholipids in plasma membrane are distributed asymmetrically between two leaflets. Indeed, in normal cells the choline-containing phospholipids, PC and sphingomyelin, reside mainly in the outer leaflet of plasma membrane, whereas the aminophospholipids, PS and, to a lesser extent, phosphatidylethanolamine (PE), are confined to the inner leaflet.10 It seems that at least three lipid translocators are related to maintain this asymmetry. ATP-dependent flippases, members of type 4 P-type ATPases, also referred to as aminophospholipid translocases, are specific for aminophospholipids, with a preference for PS over PE, and catalyze the inward translocation of aminophospholipids.11 Another ATP-dependent translocator floppases, members of ATP-binding cassette transporters, catalyze the efflux of phospholipids from the inner to the outer leaflet with little selectivity for polar head group of the phospholipids.12 Energy-independent scramblases are non-selective and catalyze bidirectional transbilayer movement of phospholipids Ca2+-dependently but ATP-independently.13, 14
PS externalization to the cell surface commonly found in apoptotic cells is likely to be associated with the inhibition of flippases as well as the activation of scramblases in the plasma membrane.15
oxPS generation in apoptotic cells
There is accumulating evidence showing that apoptosis induced by various stimuli in a number of different cell types is accompanied by preferential oxidation of PS.8, 16–21 Furthermore, it has been reported that oxidation and externalization of PS almost simultaneously occur during apoptosis.22, 23
As the source of oxidizing equivalents required for redox catalysis of PS oxidation was not identified, we determined which kind of ROS oxidized PS in the cells undergoing apoptosis. 24 We used H2O2-resistant HP100 cells derived from HL-60 cells, which expressed catalase (CAT) 2.5 times more than HL-60 cells but contained the same levels of primary antioxidant enzymes (glutathione peroxidase and superoxide dismutase) and apoptosis-related proteins (Bcl-2 and Bax) as HL-60 cells. HP100 cells but not HL-60 cells exerted a higher resistance to apoptosis induced by anti-cancer reagent melphalan (Mel). This resistance to Mel-induced apoptosis in HP100 cells was abolished by pretreatment with a CAT inhibitor 3-amino-1,2,4-triazole (3-AT), suggesting that overexpression of CAT in HP100 cells was mainly responsible for their resistance to Mel-induced apoptosis. Treatment of HL-60 cells with Mel induced ROS production in the cells. However, no increase in ROS generation in HL-60 cells co-treated with exogenous CAT following Mel treatment. On the other hands, there was no ROS generation in naïve HP100 cells both before and after Mel treatment although Mel caused ROS production in HP100 cells pretreated with 3-AT as well. Thus, it was suggested that ROS generated in the cells treated with Mel is predominantly H2O2. Mel induced also PS oxidation and externalization as well as cytochrome c (cyt c) from mitochondria into cytosol in HL-60 cells but not HP100 cells. In addition, CAT inhibition by 3-AT restored the sensitivity of HP100 cells to PS oxidation and externalization after Mel exposure. This suggested that Mel-induced H2O2 indeed plays a pivotal role in implementation of apoptosis (PS oxidation, PS externalization and cyt c release) as a required messenger.
The presence of oxPS on the apoptotic cell surface
We demonstrated for the first time that oxPS exists both within and on the surface of apoptotic cells.25
To determine PS on the cell surface, Jurkat cells following anti-Fas antibody treatment were labeled with membrane-impermeable fluorescamine, a probe for visualizing lipids that contain primary amino groups. Their total lipids were extracted and subjected to two-dimensional high-performance thin-layer chromatography (HPTLC). Thereafter the HPTLC plate was first sprayed with N,N,N', N'-tetramethyl-p-phenylenediamine dihydrochloride (TMPD) to detect the oxidation of PS and then exposed to UV lights to determine externalized PS. Since fluorescamine and TMPD interact with a primary amine in the polar head group and hydroperoxide in acyl residue of oxPS, respectively, we can detect both externalization and oxidation of PS on the same HPTLC plate, i.e., prove the presence of oxPS on the surface of the cells. Additionally, to determine PS unbound to fluorescamine, i.e., PS within the cells, the same HPTLC plate was sprayed with ninhydrin, another probe that reacts with primary amino groups. The above methods enabled direct detection of oxPS both within and on the surface of cells on the same HPTLC plate. The experiments using the above methods suggested that treatment of Jurkat cells with anti-Fas antibody increased oxPS within the cells and caused oxPS to appear on the cell surface.
The mechanisms underlying oxidation of PS during apoptosis
It is considered that a potential mechanism underlying selective oxidation of CL and PS by H2O2 during apoptosis relies on the specific interactions between positively charged cyt c (net charge is +8e at neutral pH) and negatively charged phospholipids such as PS in the cytosolic leaflet of the plasma membrane and CL in the mitochondria.23, 26 Cyt c is a globular protein containing heme and its heme iron has 6 coordination bonds. In cyt c, heme iron has two axial bonds: one with His17 on the proximal side of heme and one with Met80 on the distal side. 23 When cyt c binds to membrane PS or CL with electrostatic forces, hydrophobic interactions and hydrogen bonding, the protein globule is partially unfolded, likely leading to disruption of Fe-Met80 coordination bond followed by enhancement of reactivity of cyt c to ROS such as H2O2. 23, 26 Cyt c seems to get peroxidase activity by such conformational changes. Presumably, highly oxidized heme (compound I or II) and protein-based tyrosyl radical formed in the presence of H2O2 in PS- or CL-activated peroxidase forms of cyt c can subtract an electron from unsaturated acyl chains of PS or CL followed by the addition of oxygen and formation of oxPS (PSOOH) or oxCL (CLOOH), similar to peroxidase reaction to cyclooxygenase (COX).23 Newly formed oxPS (PSOOH) or oxCL (CLOOH) may function as substrates for cyt c peroxidase activity and further propagate lipid peroxidation, even in the absence of H2O2. In cell-free model experiments, 27 cyt c-derived tyrosyl radical was measured by low-temperature electron paramagnetic resonance spectroscopy in the presence of cyt c, phospholipid-containing liposomes and H2O2. The production of tyrosyl radical depended strongly on the presence of PS-containing liposomes, supporting the above hypothesis indirectly.
It has been suggested that peroxidase activity of cyt c is closely related to PS oxidation and externalization as mentioned above. Therefore, we determined whether departure of cyt c from mitochondria is required for production of oxPS as well as its externalization during Fas-mediated apoptosis in Jurkat cells using two selective inhibitors of caspase-8 (Casp-8) and caspase-3 (Casp-3) functioning upstream and downstream from mitochondria, respectively.28 As shown in Fig. 1, inhibition of Casp-8 reduced mitochondrial cyt c release into the cytosol (Fig. 1A), the amount of oxPS not only within but also on the surface of Jurkat cells (Fig. 1B), Casp-3 activation, and apoptotic cell number after treatment with anti-Fas antibody. In contrast, selective inhibition of Casp-3 was unable to suppress cyt c release (Fig. 1A), and the amount of oxPS both within and on the surface of the cells after anti-Fas antibody (Fig. 1B) as expected, although it inhibited activation of Casp-3 and apoptosis. These results strongly suggested that mitochondrial event, especially cyt c departure from mitochondria plays a critical role in production of oxPS within the cells and subsequent its appearance on the cell surface during apoptosis.
Fig. 1.
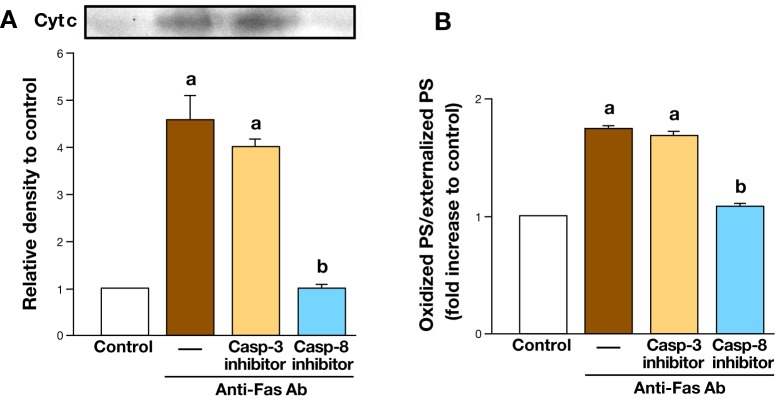
Effect of caspase inhibitors on cyt c release into the cytosol (A) as well as oxidation of PS exposed to the cell surface (B) in Jurkat cells after treatment with anti-Fas antibody.28 aP < 0.01 versus control, bP < 0.01 versus anti-Fas antibody alone.
Production of oxPS under pathological conditions (in vivo)
Pulmonary phospholipid peroxidation after inhalation exposure of mice to single-walled carbon nanotubes was identified in three relatively minor classes of anionic phospholipids, PS, CL and phosphatidylinositol (PI).29 This non-random peroxidation of phospholipids coincided with the accumulation of apoptotic cells in the lung. In another animal models of lung injury (hyperoxic acute lung injury30 and gamma-radiation-induced lung injury31), the preferential formation of oxPS and oxCL in the lung was also detected. These lung injuries were all accompanied by apoptotic cell accumulation in the lung.
In mouse model of Alzheimer disease (AD), which showed aberrant expression of human myeloperoxidase in astrocytes, there was selective accumulation of two anionic phospholipid hydroperoxides, oxPS (PSOOH) and oxPI (PIOOH) in the brains.32 Furthermore, in postmortem brain samples from AD patients, accumulation of the same individual molecular species of oxPS and oxPI was observed.32 In addition to the above brain diseases, it has been demonstrated that selective oxidation of CL and PS in rat cortical neurons is triggered during staurosporine-induced apoptosis.33
Total body irradiation to mice induced intestinal injury accompanied by apoptosis as well as selective accumulation of oxCL and oxPS in small intestines.34
It has been reported that anti-phospholipid antibodies in sera from patients with alcoholic liver disease (ALD) target apoptotic cells by specifically recognizing oxPS, suggesting the production of oxPS in ALD.35
BIOACTIVITIES OF oxPS
Role in apoptotic cell clearance
It has been established that PS serves as a recognizable “eat-me” signal for phagocytes through its translocation from the inner to the outer leaflet of the plasma membrane during apoptosis.36 However, oxPS also appears on the surface of apoptotic cells during apoptosis.25 Therefore, it is important to determine whether oxPS on the surface of apoptotic cells enhances the recognition and engulfment of apoptotic cells by phagocytes. Several receptors for apoptotic cell uptake have been identified on the surface of the phagocytes. They include the lectins that bind altered sugars on apoptotic cells, CD36 (in conjunction with integrins alphaVbeta3 and alphaVbeta5) that binds thrombospondin, LRP1/CD91 (in conjunction with calreticulin) that binds complement C1q, CD14 that binds intercellular adhesion molecule 3 (ICAM3), and the scavenger receptors that bind oxidized LDL.37 Furthermore, PS is recognized either directly via receptors such as brain angiogenesis inhibitor 1 (BAI1), T-cell immunoglobulin- and mucin-domain-containing 4 (TIM-4), TIM-1, and Stabilin-2 or indirectly via bridging molecules such as milk fat globule-EGF factor 8 (MFG-E8), growth-arrest-specific 6 (Gas6), or protein S.37 MFG-E8 is expressed and secreted by professional phagocytes, associates with integrins alphaVbeta3/alphaVbeta5 on phagocytes, and binds PS on apoptotic cells.38
As for oxPS, it has been reported that the oxPS, but not non-oxidized PS, serves as a preferred ligand for class B scavenger receptor CD36-mediated phagocytosis by macrophages. 39 Moreover, it has been shown that MFG-E8 preferentially interacts with oxPS, and to a lesser extent, with non-oxidized PS.40 In another study,8 liposomes containing oxPS inhibited phagocytosis of apoptotic cells more potently than non-oxidized PS. Furthermore, non-apoptotic cells treated with liposomes containing both oxPS and non-oxidized PS were more efficiently phagocytosed than cells treated with non-oxidized PS alone.8
Taken together, these findings indicate that oxPS may act in combination with naïve PS as an important signal on the cell surface to facilitate the recognition of apoptotic cells. In other words, oxPS is likely to enhance the clearance of apoptotic cells by phagocytes. In addition, it has been reported that oxPS acts as a “non-enzymatic scramblase” to facilitate translocation of both PS and oxPS molecules into the cell surface.41
Anti-inflammatory activities
Protection against endothelial barrier dysfunction and acute lung injury
The tight intercellular barrier of endothelial cell (EC) maintaining low permeability is adequately regulated by a counterbalance of barrier-protective and barrier-disruptive bioactive molecules in the circulation.42 Birukova et al. showed that oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphoserine (oxPAPS) potently protected pulmonary EC barrier function and induced the remodeling of pulmonary EC actin cytoskeleton.42 In that study, oxPAPS inhibited the increase in permeability in human pulmonary arterial EC induced by lipopolysaccharide (LPS), a major component of the outer membrane of Gram-negative bacteria. oxPAPS also attenuated neutrophil accumulation and myeloperoxidase activation in bronchoalveolar lavage fluid in mice treated with LPS (in vivo model of acute lung injury), and suppressed lung barrier dysfunction in the mice. Furthermore, oxPAPS diminished pulmonary EC barrier dysfunction induced by interleukin (IL)-6, an inflammatory mediator or thrombin, an edemagenic mediator. The protection by oxPAPS against thrombin-induced pulmonary EC barrier dysfunction was attributed to both attenuation of Rho-dependent pathway of endothelial contraction leading to hyperpermeability and stimulation of Rac-mediated EC barrier recovery. These results suggest that oxPAPS potently suppresses endothelial barrier dysfunction induced by inflammatory and edemagenic agents in vitro and in vivo potentially due to attenuation of Rho signaling as well as stimulation of Rac signaling. Since the protective effect of oxPAPS is much more than that of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (oxPAPC), it seems that negatively charged polar head group but not oxidized sn-2 residue of oxPAPS may play some important role in its protective ability although PAPS, non-oxidized form, shows no protective effects.
Inhibition of LPS recognition by TLR4
LPS is a potent activator of macrophages and a causal agent of endotoxin shock. 43 LPS is well known to induce production of pro-inflammatory mediators such as tumor necrosis factor-alpha (TNF-alpha), IL-1beta, IL-6, ROS and nitric oxide (NO), leading to death from endotoxin shock in animal models.44, 45 Moreover, it has been shown that LPS enhances cytokine and/or NO production in macrophages, 46 microglial cells 47 and ECs.48 The process of these cellular responses involves recognition of LPS by Toll-like receptor (TLR) 4 on the surface of the host cells, followed by activation of nuclear factor (NF)-kappa B.49 TLR is a receptor family protein related to innate immunity and to date, 12 functional TLRs have been identified in mice and 10 in humans.50 TLR4 has now been established as the receptor for LPS. The activation of innate immunity response by LPS starts from the interaction of LPS with LPS-binding protein (LBP). LBP in the serum removes LPS from the outer membrane of the bacteria and delivers LPS to soluble CD14 (sCD14) and/or membrane-bound CD14 and then LPS bound to CD14 is transferred to TLR4-myeloid differentiation 2 (MD-2) complex. The formation of dimeric LPS/MD-2/TLR4 complex initiates the intracellular signaling.51
According to a recent study,52 oxPAPS inhibited LPS-induced elevation of E-selectin mRNA in human umbilical vein endothelial cells (HUVECs), indicating that it acted as an antagonist on induction of TLR4 downstream genes. oxPAPS prevented binding of LPS to LBP in an in vitro competitive assay. Moreover, oxPAPS formed complexes with sCD14 and prevented interaction of LPS with sCD14 in vitro.
Taken together, it was suggested that oxPAPS binds to LBP and sCD14, thus preventing recognition of LPS via MD-2/TLR4 complex, leading to inhibition of LPS-induced inflammatory reaction.
Inhibition of respiratory burst
Neutrophils are recruited to inflamed sites upon infections and exert microbicidal activities. The assembly and activity of NADPH oxidase (NOX) are essential for neutrophil microbicidal activities. Neutrophils engulf invading microbes and kill them by ROS such as hydroxyl radicals and hypochlorous acids derived from superoxide anions generated by NOX together with microbicidal peptides and proteases in phagolysosomes.53, 54 Neutrophil NOX (NOX2), also referred to as respiratory burst oxidase is a multicomponent enzyme system composed of membrane proteins (p22phox and gp91phox, which form cytochrome b558) and cytosolic proteins (p47phox, p67phox, p40phox and Rac2), which assemble at membrane sites upon cell activation. The importance of this enzyme in host defenses is highlighted by the fact that loss of function mutations of NOX subunits cause chronic granulomatous disease in which the phagocyte enzyme is dysfunctional, leading to life-threatening bacterial and fungal infections. In contrast, excessive ROS generation can damage surrounding tissues. Thus, NOX activation and ROS production have to be tightly regulated.
It has been shown that ROS produced by activated neutrophils promotes oxPS production. 18 Blüml et al. reported that oxPAPS inhibited ROS production in phorbol myristate acetate (PMA)- or formyl-methionyl-leucyl-phenylalanine (FMLP)-stimulated neutrophils in a dose dependent manner.55 In contrast, unoxidized PAPS dose-dependently increased the production of ROS in neutrophils, suggesting that there is an oxidation state-dependent regulatory role of oxPS on neutrophil respiratory burst formation. oxPAPS did not inhibit up-regulation of CD11b which is involved in adhesion of activated neutrophil to the endothelium, or phagocytosis. It has been known that MAPK (ERK1/2 and p38) are activated during respiratory burst and there is involvement of these kinases in the production of ROS.56 However, oxPAPS did not inhibit activation of ERK1/2 and p38 in neutrophils stimulated with either PMA or FMLP.
Taken together, it was shown that oxPAPS inhibited respiratory burst in neutrophils induced by various stimuli without inhibiting MAPK activation.
Atherogenic activities
Induction of VEGF via UPR
Endoplasmic reticulum (ER) is the site for proper folding of newly synthesized proteins and formation of three-dimensional conformation of proteins, and only proteins assured of quality in ER are released into the secretary pathway. ER stress induced by hypoxia, nutrient deprivation, acidosis and certain chemicals disturbs the protein quality control leading to accumulation of the incorrect folded proteins in ER, and this triggers the activation of the following three ER transmembrane proteins, which generate an adaptive response called the unfolded protein response (UPR): inositol-requiring enzyme 1 (IRE1), double-stranded RNA-activated protein kinase (PKR)-like ER kinase (PERK) and activating transcription factor-6 (ATF6).57 The activation of these transducer proteins triggers signaling cascades, which induce downstream an adaptive UPR via protein kinases and transcription factors. The PERK/eIF2alpha/ATF4 and IRE1/ASK1/JNK cascades can trigger the induction of vascular endothelial growth factor (VEGF).58
It has been shown that oxPAPS stimulates gene expression of well-characterized angiogenesis inducers (VEGF, IL-8 and COX-2) in HUVECs.59 oxPAPS induced VEGF via activation of ATF4 branch of UPR in HUVECs.60 According to a recent report, VEGF induction by oxPAPS in ECs depended on microRNA-663 (miR-663).61 Given that miR-663 is required for oxPAPC-induced elevation of ATF4 and its target gene TRIB (tribbles pseudokinase),61 it is suggested that oxPAPS may also induce ATF4 and its downstream gene VEGF in a miR-663-dependent manner, playing a role in angiogenesis especially in atherosclerotic plaques.
Procoagulation
Protein C is a vitamin-K dependent anti-coagulant serine protease serving as a negative feedback regulator against coagulation.62 When thrombin generated by blood coagulation binds to thrombomodulin (TM) on the EC membrane, protein C is activated by thrombin-TM complex to be an activated protein C (APC). This conversion is augmented by a specific receptor for protein C, endothelial cell protein C receptor. APC converts activated coagulation factors V (Va) and VIII (VIIIa) to the inactive forms by proteolysis with an APC cofactor protein S, resulting in inhibition of blood coagulation. In addition to its role in coagulation, APC decreases inflammatory responses by inhibition of nuclear translocation of NF-kappa B.62
Protein C inhibitor (PCI) is a serine protease inhibitor belonging to the class of serpins.63 PCI, originally an inhibitor of APC, inactivates lots of other serine proteases including blood coagulation factors, fibrinolytic enzymes, tissue kallikrein, and the sperm protease acrosin.64
It has been shown that oxPAPS, PAPS and oxPAPE directly bind PCI and stimulate its inhibitory activity toward APC.64 Positively charged amino acids in the H-helix of PCI were involved not only in heparin binding, but also in the binding of oxPAPS, PAPS and oxPAPE.
Given that PCI and annexin V, a protein binding to the head group of PS, were found to be endogenously colocalized in atherosclerotic plaques, it was suggested that oxPAPS in vivo could promote blood coagulation and thrombus formation as well as inflammation at sites of tissue injury by stimulating the inhibition of APC by PCI.
Immunosuppressive activities
oxPAPS inhibited the proliferation of peripheral blood T cells induced by anti-CD3/CD28 monoclonal antibody, demonstrating a novel immunosuppressive molecule for adaptive immunity. 65 In that study, oxPAPC prevented the development of Th1-type responses, T cell proliferation and the induction as well as the effector phase of CD8+ effector cytotoxic T lymphocytes induced through stimulation via TCR/CD3 complex, although there were no data for oxPAPS. Furthermore, T cells activated in the presence of oxPAPC failed to proliferate in response to restimulation, a phenomenon called anergy. If oxPS functions similarly to oxPC, oxPS may also induce T cell anergy, resulting in avoiding overwhelming Th1-driven immune responses at the inflammation sites. Further examinations for oxPS will be required to resolve the above issues.
CONCLUDING REMARKS
The oxidative stress associated with apoptosis results in the selective oxidation of PS in the cytoplasmic layer of the plasma membranes, followed by egress of oxPS to the surface of the apoptotic cells and effective recognition of oxPS by phagocytes. This is no longer than a mere non-specific oxidation of phospholipids, but seems to be a finely tuned system via non-random oxidation of PS for clearance of apoptotic cells by phagocytes to prevent the inflammation (Fig. 2). oxPS has some biological activities such as anti-inflammatory activities, atherogenic activities, and immunosuppressive activities other than the role in apoptotic cell clearance as mentioned above.
Fig. 2.
Selective oxidation of PS and externalization of oxPS in apoptotic cells as well as recognition of oxPS on the cell surface by macrophages. Its proposed mechanisms are indicated by bold arrows.
In contrast, oxPC has more novel functions and is recognized as a lipid mediator. In the future, we have to examine using highly pure oxPS whether oxPS has the same functions as oxPC and furthermore detect undiscovered biological activities of oxPS. In addition, it is also important to detect the specific receptor for oxPS in the phagocytes.
The author declares no conflict of interest.
REFERENCES
- 1.Reckenagel RO . A new direction in the study of carbon tetrachloride hepatotoxicity. Life Sci. 1983; 33: 401-8. . [DOI] [PubMed] [Google Scholar]
- 2.Ytrehus K , Hegstad AC . Lipid peroxidation and membrane damage of the heart. Acta Physiol Scand. 1991; S599: 81-91. . [PubMed] [Google Scholar]
- 3.Demopoulos HB , Flamm ES , Pietronigro DD , Seligman ML . The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980; 492: 91-119. . [PubMed] [Google Scholar]
- 4.Tyurin VA , Yanamala N , Tyuria YY , Klein-Seetharaman J , Macphee CH , Kagan VE . Specificity of lipoprotein-associated phospholipase A2 toward oxidized phosphatidylserine: liquid chromatography-electrospray ionization mass spectrometry characterization of products and computer modeling of interactions. Biochemistry. 2012; 51: 9736-50. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibusuki D , Nakagawa K , Asai A , Oikawa S , Masuda Y , Suzuki T . Preparation of pure lipid hydroperoxides. J Lipid Res. 2008; 49: 2668-77. . [DOI] [PubMed] [Google Scholar]
- 6.Bochkov VN , Oskolkova OV , Birukov KG , Levonen AL , Binder CJ , Stöckl J . Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010; 12: 1009-59. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devies SS , Guo L . Lipid peroxidation generates biologically active phospholipids including oxidatively N-modified phospholipids. Chem Phys Lipids. 2014; 181: 1-33. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kagan VE , Gleiss B , Tyurina YY , Tyrin VA , Elestrom-Magnusson C , Liu SX .A role for oxidative stress in apoptosis: oxidation and externalization of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J Immunol. 2002; 169: 487-99. . [DOI] [PubMed] [Google Scholar]
- 9.Bretscher MS . Asymmetrical lipid bilayer structure for biological membranes. Nat New Biol. 1972; 236: 11-2. . [DOI] [PubMed] [Google Scholar]
- 10.Verkleij AJ , Post JA . Membrane phospholipids asymmetry and signal transduction. J Membrane Biol. 2000; 178: 1-10. . [DOI] [PubMed] [Google Scholar]
- 11.Folmer DE , Oude Elferink RP , Paulusma CC . P4 ATPases – lipid flippases and their role in disease. Biochim Biophys Acta. 2009; 1791: 628-35. . [DOI] [PubMed] [Google Scholar]
- 12.Contreras FX , Sanchez-Magraner L , Alonso A , Goni FM . Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010; 584: 1779-86. . [DOI] [PubMed] [Google Scholar]
- 13.Bevers EM , Williamson PL . Phospholipid scramblase: an update. FEBS Lett. 2010; 584: 2724-30. . [DOI] [PubMed] [Google Scholar]
- 14.Suzuki J , Fujii T , Imao T , Ishihara K , Kuba H , Nagata S . Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013; 288: 13305-16. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fadeel B , Xue D , Kagan V . Programmed cell clearance: molecular regulation of the elimination of apoptotic cell corpses and its role in the resolution of inflammation. Biochem Biophys Res Commun. 2010; 396: 7-10. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabisiak JP , Tyurina YY , Tyurin VA , Lazo JS , Kagan VE . Random versus selective membrane phospholipid oxidation in apoptosis: role of phosphatidylserine. Biochemistry. 1998; 37: 13781-90. . [DOI] [PubMed] [Google Scholar]
- 17.Fabisiak JP , Kagan VE , Ritov VB , Johnson DE , Lazo JS . Bcl-2 inhibits selective oxidation and externalization of phosphatidylserine during paraquat-induced apoptosis. Am J Physiol. 1997; 272: C675-84. . [DOI] [PubMed] [Google Scholar]
- 18.Arroyo A , Modriansky M , Serinkan FB , Bello RI , Matsura T , Tyurin VA .NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in DMSO-differentiated HL-60 cells: role in phagocytic clearance. J Biol Chem. 2002; 277: 49965-75. . [DOI] [PubMed] [Google Scholar]
- 19.Shvedova AA , Tyurina YY , Kawai K , Tyurin VA , Kommineni C , Castranova V .Selective peroxidation and externalization of phosphatidylserine in normal human epidermal keratinocytes during oxidative stress induced by cumene hydroperoxide. J Invest Dermatol. 2002; 118: 1008-18. . [DOI] [PubMed] [Google Scholar]
- 20.Koty PP , Tyurina YY , Tyurin VA , Liu SX , Kagan VE . Depletion of Bcl-2 by an antisense oligonucleotide induces apoptosis accompanied by oxidation and externalization of phosphatidylserine in NCI-H226 lung carcinoma cells. Mol Cell Biochem. 2002; 234-5: 125-33. . [PubMed] [Google Scholar]
- 21.Matsura T , Serinkan BF , Jiang J , Kagan VE . Phosphatidylserine peroxidation/externalization during staurosporine-induced apoptosis in HL-60 cells. FEBS Lett. 2002; 524: 25-30. . [DOI] [PubMed] [Google Scholar]
- 22.Matsura T , Kai M , Yamada K , Shvedova AA , Kagan VE . Fine-tuning phagocytic clearance of apoptotic cells by phosphatidylserine oxidation. J Clin Biochem Nutr. 2003; 34: 11-24. . [Google Scholar]
- 23.Kagan VE , Borisenko GG , Tyurina YY , Tyurin VA , Jiang J , Potapovich AI .Oxidative lipidomics of apoptosis: redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free Radic Biol Med. 2004; 37: 1963-85. . [DOI] [PubMed] [Google Scholar]
- 24.Matsura T , Kai M , Jiang J , Babu H , Kini V , Kusumoto C .Endogenously generated hydrogen peroxide is required for execution of melphalan-induced apoptosis as well as oxidation and externalization of phosphatidylserine. Chem Res Toxicol. 2004; 17: 685-96. . [DOI] [PubMed] [Google Scholar]
- 25.Matsura T , Togawa A , Kai M , Nishida T , Nakada J , Ishibe Y .The presence of oxidized phosphatidylserine on Fas-mediated apoptotic cell surface. Biochim Biophys Acta. 2005; 1736: 181-8. . [DOI] [PubMed] [Google Scholar]
- 26.Kagan VE , Bayir HA , Belikova NA , Kapralov O , Tyurina YY , Tyurin VA .Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009; 46: 1439-53. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang J , Serinkan BF , Tyurina YY , Borisenko GG , Mi Z , Robbins PD .Peroxidation and externalization of phosphatidylserine associated with release of cytochtome c from mitochondria. Free Radic Biol Med. 2003; 35: 814-25. . [DOI] [PubMed] [Google Scholar]
- 28.Yamashita A , Morikawa H , Tajima N , Teraoka M , Kusumoto C , Nakaso K .Mechanisms underlying production and externalization of oxidized phosphatidylserine in apoptosis: involvement of mitochondria. Yonago Acta Med. 2012; 55: 11-20. . [PMC free article] [PubMed] [Google Scholar]
- 29.Tyurina YY , Kisin ER , Murray A , Tyurin VA , Kapralova VI , Sparvero LJ .Global phospholipidomics analysis reveals selective pulmonary peroxidation profiles upon inhalation of single-walled carbon nanotubes. ACS Nano. 2011; 5: 7342-53. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tyurina YY , Tyurin VA , Kaynar AM , Kapralova VI , Wasserloos K , Li J .Oxidative lipidomics of hyperoxic acute lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am J Physiol Lung Cell Mol Physiol. 2010; 299: L73-85. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyurina YY , Tyurin VA , Kapralova VI , Wasserloos K , Mosher M , Epperly MW .Oxidative lipidomics of γ-radiation-induced lung injury: mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Radiat Res. 2011; 175: 610-21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maki RA , Tyurin VA , Lyon RC , Hamilton RL , DeKosky ST , Kagan VE .Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J Biol Chem. 2009; 284: 3158-69. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyurin VA , Tyurina YY , Feng W , Mnuskin A , Jiang J , Tang M .Mass-spectrometric characterization of pohspholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J Neurochem. 2008; 107: 1614-33. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyurina YY , Tyurin VA , Epperly MW , Greenberger JS , Kagan VE . Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008; 44: 299-314. . [DOI] [PubMed] [Google Scholar]
- 35.Vay D , Rigamonti C , Vidali M , Mottaran E , Alchera E , Occhino G .Anti-phospholipid antibodies associated with alcoholic liver disease target oxidized phosphatidylserine on apoptotic cell plasma membranes. J Hepatol. 2006; 44: 183-9. . [DOI] [PubMed] [Google Scholar]
- 36.Verhoven B , Schlegel RA , Williamson P . Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J Exp Med. 1995; 182: 1597-601. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hochreiter-Hufford A , Ravichandran KS . Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013; 5: a008748. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanayama R , Tanaka M , Miwa K , Shinohara A , Iwamatsu A , Nagata S . Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002; 417: 182-7. . [DOI] [PubMed] [Google Scholar]
- 39.Greenberg ME , Sun M , Zhang R , Febbraio M , Silverstein R , Hazen SL . Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006; 203: 2613-25. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borisenko GG , Iverson SL , Ahlberg S , Kagan VE , Fadeel B . Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: implications of macrophage clearance of apoptotic cell. Cell Death Differ. 2004; 11: 943-5. . [DOI] [PubMed] [Google Scholar]
- 41.Tyurina YY , Tyurin VA , Zhao Q , Djukic M , Quinn PJ , Pitt BR .Oxidation of phosphatidylserine: a mechanism for plasma membrane phospholipid scrambling during apoptosis?. Biochem Biophys Res Commun. 2004; 324: 1059-64. . [DOI] [PubMed] [Google Scholar]
- 42.Birukova AA , Fu P , Chatchavalvanich S , Burdette D , Oskolkova O , Bochkov VN .Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol. 2007; 292: L924-35. . [DOI] [PubMed] [Google Scholar]
- 43.Lu YC , Yeh WC , Ohashi PS . LPS/TLR4 transduction pathway. Cytokine. 2008; 42: 145-51. . [DOI] [PubMed] [Google Scholar]
- 44.Klosterhalfen B , Hauptmann S , Offner FA , Amo-Takyi B , Tons C , Winkeltau G .Induction of heat shock protein 70 by zinc-bis-(DL-hydrogenaspartate) reduces cytokine liberation, apoptosis, and mortality rate in a rat model of LD100 endotoxiemia. Shock. 1997; 7: 254-62. . [DOI] [PubMed] [Google Scholar]
- 45.Carrillo-Vico A , Lardone PJ , Naji L , Fernández-Santos JM , Martín-Lacave I , Guerrero JM .Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res. 2005; 39: 400-8. . [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama T , Fujita M , Koide N , Mori I , Yoshida T , Mori H .2-Aminopurine inhibits lipopolysaccharide-induced nitric oxide production by preventing IFN-beta production. Microbiol Immunol. 2004; 48: 957-63. . [DOI] [PubMed] [Google Scholar]
- 47.Jantaratnotai N , Utaisincharoen P , Piyachaturawat P , Chongthammakun S , Sanvarinda Y . Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006; 78: 571-7. . [DOI] [PubMed] [Google Scholar]
- 48.Huang KT , Kuo L , Liao JC . Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase. Biochem Biophys Res Commun. 1998; 245: 33-7. . [DOI] [PubMed] [Google Scholar]
- 494.Palsson-McDermott EM , O’Neill LAJ . Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004; 113: 153-62. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawai T , Akira S . Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011; 34: 637-50. . [DOI] [PubMed] [Google Scholar]
- 51.Calabrese V , Cighetti R , Peri F . Molecular simplification of lipid A structure: TLR4-modulating cationic and anionic amphiphiles. Mol Immunol. 2014June14. 10.1016/j.molimm.2014.05.011. [Epub ahead of print]. . [DOI] [PubMed] [Google Scholar]
- 52.Schlieffen EV , Oskolkova OV , Schabbauer G , Gruber F , Blüml S , Genest M .Multi-hit inhibition of circulating and cell-associated components of the Toll-like receptor 4 pathway by oxidized phospholipids. Arterioscler Thromb Vasc Biol. 2009; 29: 356-62. . [DOI] [PubMed] [Google Scholar]
- 53.El-Benna J , Dang PMC , Gougerot-Pocidalo MA . Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol. 2008; 30: 279-89. . [DOI] [PubMed] [Google Scholar]
- 54.Nauseef WM , Borregaard N . Neutrophils at work. Nat Immunol. 2014; 15: 602-11. . [DOI] [PubMed] [Google Scholar]
- 55.Blüml S , Rosc B , Lorincz A , Seyerl M , Kirchberger S , Oskolkova O .The oxidation state of phospholipids controls the oxidative burst in neutrophil granulocytes. J Immunol. 2008; 181: 4347-53. . [DOI] [PubMed] [Google Scholar]
- 56.Dang PM , Morel F , Gougerot-Pocidalo MA , Benna JE . Phosphorylation of the NADPH oxidase component p67(PHOX) by ERK2 and p38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003; 42: 4520-26. . [DOI] [PubMed] [Google Scholar]
- 57.Nagelkerke A , Bussink J , Sweep FCGJ , Span PN . The unfolded protein response as a target for cancer therapy. Biochim Biophys Acta. 2014; 1846: 277-84. . [DOI] [PubMed] [Google Scholar]
- 58.Salminen A , Kauppinen A , Hyttinen JMT , Toropainen E , Kaarniranta K . Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med. 2010; 16: 535-42. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bochkov VN , Philippova M , Oskolkova O , Kadl A , Furnkranz A , Karabeg E .Oxidized phospholipids stimulate angiogenesis via autocrine mechanisms, implicating a novel role for lipid oxidation in the evolution of atherosclerotic lesions. Circ Res. 2006; 99: 900-8. . [DOI] [PubMed] [Google Scholar]
- 60.Oskolkova OV , Afonyushkin T , Leitner A , Schlieffen EV , Gargalovic PS , Lusis AJ .ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood. 2008; 112: 330-9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Afonyushkin T , Oskolkova OV , Bochkov VN . Permissive role of miR-663 in induction of VEGF and activation of the ATF4 branch of unfolded protein response in endothelial cells by oxidized phospholipids. Atherosclerosis. 2012; 225: 50-5. . [DOI] [PubMed] [Google Scholar]
- 62.McKelvey K , Jackson CJ , Xue M . Activated protein C: a regulator of human skin epidermal keratinocyte function. World J Biol Chem. 2014; 5: 169-79. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silverman GA , Bird PI , Carrell RW , Church FC , Coughlin PB , Gettins PG .The serpins are an expanding superfamily of structurally similar but functionally diverse proteins: evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001; 276: 33293-6. . [DOI] [PubMed] [Google Scholar]
- 64.Malleier JM , Oskolkova O , Bochkov V , Jerabek I , Sokolikova B , Perkmann T .Regulation of protein C inhibitor (PCI) activity by specific oxidized and negatively charged phospholipids. Blood. 2007; 109: 4769-76. . [DOI] [PubMed] [Google Scholar]
- 65.Seyerl M , Blüml S , Kirchberger S , Bochkov VN , Oskolkova O , Majdic O .Oxidized phospholipids induce anergy in human peripheral blood T cells. Eur J Immunol. 2008; 38: 778-87. . [DOI] [PubMed] [Google Scholar]



