Dear Editor in Chief
Breast cancer is one of the most frequent cancers around the world and in Iran. Both genetic and environmental factors have an important role in the developing of this disease. Transforming growth factor beta (TGF- β) protein is one of the genetic factors for breast cancer (1). It is a multi-functional cytokine found in almost all human tissue cells and regulates cellular processes such as cell division, differentiation, death, adhesion, and motility (2). In normal cells, TGF- β might act mainly as a tumor suppressor and inhibits cellular proliferation or promote cellular differentiation and apoptosis. TGF-ß1 inhibits the proliferation of carcinomas in early stages of breast cancer (1). As cancer cells progress, regulatory mechanism of this protein becomes more complex because of changes in TGF-ß1 expression and loss of negative cell signaling. Consequently, the more tumors advance, the more TGF-ß1 express, which can be associated with phenotype and impaired clinical feature of this disease (3). Therefore, defining TGF-ß molecular mechanisms and its involvement in the process of carcinogenesis is a major challenge.
The TGF- ß 1 gene is located on chromosome 19q13 and linkage studies and genetic models show that TGF-ß Polymorphisms have relation with risk of developing breast cancer. Common variants of TGF-ß1 gene may affect the production, secretion, or activity of this cytokine. Five different TGF-ß1gene polymorphisms have been studied regarding breast cancer susceptibility (4). Polymorphism at codon 10(T29C) that result to Leu10Pro substitution in the signal peptide is most extensively studied polymorphism in the TGF-ß1gene (5). C allele of T29C is associated with increased TGF- ß1 serum levels, the result of association between this polymorphism and this disease is inconsistent in different populations. In Caucasian women, T allele of the T29C polymorphism was associated with increased risk of breast cancer (6). In contrast, in Korean and European population woman carrying C allele are more susceptible to breast cancer (7). Some studies also show no association between this polymorphism and breast cancer. According to these results, we investigated whether TGFβ1 gene polymorphism in codon 10 is associated with Breast cancer in Iranian population.
This study as a case-control was performed between 60 women patients who had suffering from breast cancer attending Different hospitals of Yazd (central part of Iran) during the period from February 2012 - October 2014 and 60 healthy controls. Patient mean age was 51.2 years. The control did not have any history of cancer and other disease with known genetic predisposition. The study was confirmed by the local Ethics Committee of our center and all cases participated in this study voluntarily. Genomic DNA of the samples was extracted according to Saremi et al. protocol and the isolated DNA was quantified by the nanodrop and agarose gel (8). Amplification Refractory Mutation System-Polymerase Chain Reaction (ARMS-PCR) was applied for detection of the polymorphisms of TGF-b1 gene at codon 10 (T29C) (rs1982073). We used target DNA, allele specific ARMS primer, and the common primer in two separate complimentary reactions for each allele. By this means, homozygote or heterozygote individuals for this mutation can be detected (9). Primers for detection of this polymorphism were Sense (common) primer 5'-gttgtgggtttccaccattag-3', Antisense (ARMS) C-primer (pro 10 allele) 5'-ctccgggctgcggctgctgcC-3', Antisense (ARMS) T-primer (Leu 10 allele) 5'- ctccgggctgcggctgctgcT-3'. These primers amplify 346 bp fragment that includes our polymorphism. We used internal primers (F5′-TGCCCTGTGCAGCTGTGGGTTGATT- 3′; R5′-GCCCCA-GCTGCTCACCATCGCTATC- 3′) amplifying a limited region of p53 gene located at chromosome 17p13.1, present in all samples as a positive control (10). Negative control was also applied for checking DNA contamination. Each Polymerase chain reaction contain 100 ng DNA, 10 mM Tris-HCl, pH 8.4, 50 mM KCl, 2 mM MgCl2, 0.001% gelatin, 0.2 mM dNTP, and 1 μl from specific primers. (TGF-B1 sense, TGF-B1 antisense C or TGF-B1 antisense T, P53 Forward and P53 Reverse), 2 U Taq DNA polymerase and H2O up to 55 μL and reactions were performed under following condition: first at 95 °C for 5min; and 35 cycles at (94 °C for 1 min, 60 °C for 1 min and 72 °C for 2 min) and final extension at 72 °C for 10 min and hold at 4 °C. PCR products were identified by 2% agarose gel electrophoresis in 1X TBE buffer and the presence of each allele was analyzed by checking specific bands under UV light and documented by gel documentation system. Each experiment was repeated three times to confirm our results.
The statistical software SPSS (version20) was used to analyze the data. Allele and genotype frequencies were compared with a 2×2contingency table using Fischer’s exact test and chi-squared. P <0.05 was considered as significant.
In total, 60 Iranian patients with breast cancer and 60 healthy controls recruited in this study. The specific band of single nucleotide polymorphism of the TGF-β gene at codon 10 was analyzed and counted (Fig. 1). The allele and genotype frequency of codon 10 TGFβ1 gene polymorphism in our patients were compared with those in control subjects to analyze the difference between the two groups. The result of ARMS-PCR showed there was no significant difference between the patient and the control group (P>0.05).
Fig. 1:
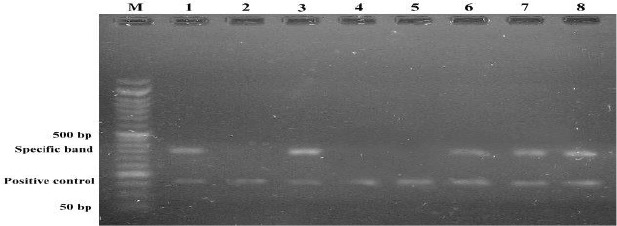
Result of ARMS-PCR. Lane M molecular weight marker. Each two successive lanes are corresponding to one sample amplified by different allele specific primer. Lanes 1&2, 3&4 (Homozygote TT), Lanes 5&6(Homozygote CC), and lanes 7&8 are heterozygote TC. Specific bands are 346 bp
Table1 shows frequencies of genotypes and alleles in the breast cancer cases and the control group. This result indicates more frequent genotype and allele in both cases and controls was TC and T (Leu), respectively.
Table 1:
Genotype and allelic frequencies of TGFbeta codon 10 polymorphisms in Iranian breast cancer patients and healthy controls (n= 60 each)
| Codon10 | Patients n(%) | Controls n(%) | P-value | Odds Ratio(95%CI) |
|---|---|---|---|---|
| Genotype frequency | ||||
| TT | 18(30) | 16(26.7) | 0.84 | 1.17(0.53–2.61) |
| TC | 29(48.3) | 32(53.3) | 0.71 | 0.819(0.40–1.67) |
| CC | 13(21.7) | 12(20) | 1.00 | 1.10(0.458–2.67) |
| Allele frequency | ||||
| T(Leu) | 65(54.2) | 64(53.3) | 1.00 | 1.03(0.62–1.71) |
| C(Pro) | 55(45.8) | 56(46.7) |
In conclusion, we found no association between T29C polymorphism of TGF β1 and breast cancer in Iranian women. Our result accompanies some other studies regarding this polymorphism and the disease. Nevertheless, further functional studies with more subjects can provide stronger results. These findings may have significant role in identifying involved genes and their variation in the disease as well as screening breast cancer patient for further treatment.
Acknowledgements
Our research group is thankful to patients and controls. We also appreciate Yazd Research and Clinical Center for Infertility staff for their kind cooperation. We declare that there is no conflict of interest.
References
- 1. Benson JR. (2004). Role of transforming growth factor beta in breast carcinogenesis. Lancet Oncol, 5( 4): 229–239. [DOI] [PubMed] [Google Scholar]
- 2. Kretzschmar M. (2000). Transforming growth factor-beta and breast cancer: Transforming growth factor-beta/SMAD signaling defects and cancer. Breast Cancer Res, 2( 2): 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorsch SM, Memoli VA, Stukel TA, Gold LI, Arrick BA. (1992). Immunohistochemical staining for transforming growth factor beta 1 associates with disease progression in human breast cancer. Cancer Res, 52( 24): 6949–6952. [PubMed] [Google Scholar]
- 4. Kaklamani VG, Baddi L, Liu J, et al. (2005). Combined genetic assessment of transforming growth factor-beta signaling pathway variants may predict breast cancer risk. Cancer Res, 65( 8): 3454–3461. [DOI] [PubMed] [Google Scholar]
- 5. Ziv E, Cauley J, Morin PA, Saiz R, Browner WS. (2001). Association between the T29-->C polymorphism in the transforming growth factor beta1 gene and breast cancer among elderly white women: The Study of Osteoporotic Fractures. JAMA, 285( 22): 2859–2863. [DOI] [PubMed] [Google Scholar]
- 6. Hishida A, Iwata H, Hamajima N, Matsuo K, Mizutani M, Iwase T, Miura S, Emi N, Hirose K, Tajima K. (2003). Transforming growth factor B1 T29C polymorphism and breast cancer risk in Japanese women. Breast Cancer, 10( 1): 63–69. [DOI] [PubMed] [Google Scholar]
- 7. Lee KM, Park SK, Hamajima N, Tajima K, Yoo KY, Shin A, Noh DY, Ahn SH, Hirvonen A, Kang D. (2005). Genetic polymorphisms of TGF-beta1 & TNF-beta and breast cancer risk. Breast Cancer Res Treat, 90( 2): 149–155. [DOI] [PubMed] [Google Scholar]
- 8. Saremi MA, Saremi M, Tavallaei M. (2008). Rapid genomic DNA extraction (RGDE). Forensic Science International : Genetics, Suppl. Ser. 1: 63– 65. [Google Scholar]
- 9. Li X, Yue ZC, Zhang YY, Bai J, Meng XN, Geng JS, Fu SB. (2008). Elevated serum level and gene polymorphisms of TGF-beta1 in gastric cancer. J Clin Lab Anal, 22( 3): 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perrey C, Turner SJ, Pravica V, Howell WM, Hutchinson IV. (1999). ARMS-PCR methodologies to determine IL-10, TNF-alpha, TNF-beta and TGF-beta 1 gene polymorphisms. Transpl Immunol, 7( 2): 127–128. [DOI] [PubMed] [Google Scholar]


