Abstract
Objectives: Our primary aim was to assess how patients’ characteristics, bleeding pattern, sonographic endometrial thickness (ET) and additional features at unenhanced ultrasound examination (UTVS) and at fluid instillation sonography (FIS) contribute to the diagnosis of intracavitary uterine pathology in women presenting with abnormal uterine bleeding (AUB). We further aimed to report the prevalence of pathology in women presenting with AUB.
Methods: 1220 consecutive women presenting with AUB underwent UTVS, colour Doppler imaging (CDI) and FIS. Most women (n = 1042) had histological diagnosis.
Results: Mean age was 50 years and 37% were postmenopausal. Of 1220 women 54% were normal, polyps were diagnosed in 26%, intracavitary fibroids in 11%, hyperplasia without atypia in 4% and cancer in 3%. All cancers were diagnosed in postmenopausal (7%) or perimenopausal (1%) women. ET had a low predictive value in premenopausal women (LR+ and LR- of 1.34 and 0.74, respectively), while FIS had a LR+ and LR- of 6.20 and 0.24, respectively. After menopause, ET outperformed all patient characteristics for the prediction of endometrial pathology (LR+ and LR- of 3.13 and 0.24). The corresponding LR+ and LR- were 10.85 and 0.71 for CDI and 8.23 and 0.26 for FIS.
Conclusion: About half of the women presenting to a bleeding clinic will have pathology. In premenopausal women, benign lesions are often the cause of AUB. For the prediction of intracavitary pathology ET is of little value in premenopausal women. CDI and FIS substantially improve the diagnostic accuracy.
Keywords: Ultrasonography, endometrial cancer, polyps, leiomyoma, metrorrhagia
Introduction
When a woman presents with abnormal uterine bleeding, her history is taken and further tests are then considered (Dreisler et al., 2013; Bignardi et al., 2009; NICE guideline 44, 2007). Risk factors for uterine intracavitary pathology in general, and endometrial cancer in particular, include postmenopausal status, advanced age and oestrogen exposure e.g. due to anovulation, obesity, late menopause or unopposed oestrogen use (Amant et al., 2005; Bignardi et al., 2009; Van den Bosch et al., 2012).
In the large majority of women, abnormal bleeding is caused by benign lesions such as an endometrial polyp or an intracavitary fibroid (Van den Bosch et al., 1995; Werbrouck et al., 2011, NICE guideline 44, 2007). These lesions are not life-threatening but these women expect their bleeding problem to be solved. In postmenopausal women endometrial thickness measurement at unenhanced transvaginal ultrasound examination (UTVS) is used to triage patients at high risk for endometrial cancer (Smith-Bindman et al., 1998; Dijkhuizen et al., 2000; Gupta et al., 2002; Tabor et al., 2002; Epstein et al., 2004; Clark et al., 2006; Timmermans et al., 2010). A regular and thin endometrium is reassuring, and a thick endometrium warrants further investigation (Van den Bosch et al., 2007; Bignardi et al., 2009).
The primary aim of this study was to evaluate the importance of patients’ demographic characteristics, bleeding pattern, endometrial thickness and additional ultrasound features at UTVS and at fluid instillation sonography (FIS) for the diagnosis of intracavitary uterine pathology in pre- and postmenopausal women presenting with abnormal uterine bleeding.
The secondary aim of the study is to evaluate the prevalence of pathology in women presenting with abnormal uterine bleeding before and after menopause.
Methods
Unenhanced transvaginal sonography (UTVS) with colour Doppler imaging (CDI) followed by fluid instillation sonography (FIS) was performed in 1220 consecutive women presenting with abnormal uterine bleeding at the Leuven Bleeding Clinic from October 2004 till December 2010. A medical history was taken including the following variables: age, menopausal status, weight, height, body mass index (BMI), parity, gravidity, use of hormonal therapy, type of hormonal therapy, bleeding pattern (cyclical, non-cyclical or both cyclical and non-cyclical), amount of bleeding (spotting, ‘normal’ menses, more than ‘normal’ menses), and duration of the bleeding problem. Menopause was defined as the absence of menstruation for at least 12 months in a woman older than 40, after exclusion of pregnancy. If the menopausal status was uncertain, the woman was classified as perimenopausal. All ultrasound examinations were performed by the first author using an Acuson SequoiaTM 512 (Siemens, Erlangen, Germany) equipped with an EV-8C4 endovaginal probe in the first 402 cases and a GE VolusonTM E8 (GE Healthcare, Milwaukee, WI, USA) ultrasound machine equipped with a 6-12MHz 3D endovaginal probe thereafter. At UTVS following variables were recorded: maximal total endometrial thickness in sagittal plane (Leone et al., 2010), the endometrial pattern (presence or absence of a 3-layer pattern), presence of focal intracavitary lesions, type of intracavitary lesion (polyp, fibroid) if present, presence or absence of fibroids (number) elsewhere in the uterus, presence of vascularity within the endometrium. Immediately after UTVS, all patients underwent FIS using saline in the first 402 cases and gel (EndosgelTM, Farco-Pharma GmbH, Köln, Germany) in the remaining cases (Werbrouck et al., 2011). FIS was not performed in 13 cases because of the presence of pre-existing intracavitary fluid (n = 10), vaginal stenosis (n = 2), a high level of suspicion for malignancy (n = 1), and because of technical failure in 34 cases (2.8%). The following variables were recorded at FIS: total endometrial thickness (sum of both single layers measured in sagittal plane), presence of an intracavitary lesion, type of intracavitary lesion (polyp, fibroid, polypoid endometrium). The endometrium is defined as polypoid in case of multiple deep indentations (Leone et al., 2010). Subsequently most women (n = 1042) underwent diagnostic hysteroscopy, endometrial sampling, operative hysteroscopy or hysterectomy. In cases where there were multiple intracavitary lesions a single outcome was allocated to each woman according to the following diagnostic hierarchy: cancer, atypical hyperplasia, polyp, fibroid, hyperplasia without atypia. In reporting the prevalence of pathology for all patients presenting at the bleeding clinic (Table I), the 176 cases without available histology were also included. The diagnosis of the latter was based on FIS (polyp, intracavitary fibroid, atrophy defined as an ET < 5mm, proliferative/secretory endometrium as an ET ≥ 5mm).
Table I. Final diagnosis in the total group, and in the pre-, peri- and post-menopausal group.
| Pre-menopausal | Peri-menopausal | Post-menopausal | Total | |||||
| N | % | N | % | N | % | N | % | |
| Proliferative/Secretory | 344 | 51.3 | 42 | 45.2 | 66 | 14.5 | 452 | 37.1 |
| Hypotrophy/atrophy | 47 | 7.0 | >15 | 16.1 | 140 | 30.8 | 202 | 16.6 |
| Hyperplasia without atypia | 35 | 5.2 | 4 | 4.3 | 9 | 2.0 | 48 | 3.9 |
| Polyp | 123 | 18.4 | 18 | 19.4 | 171 | 37.7 | 313 | 25.7 |
| Fibroid | 95 | 14.2 | 11 | 11.8 | 28 | 6.2 | 134 | 11.0 |
| Atypical hyperplasia | 0 | 0 | 0 | 0 | 1 | 0.2 | 1 | 0.1 |
| Carcinoma | 0 | 0 | 1 | 1.1 | 30 | 6.6 | 31 | 2.5 |
| Other | 26 | 3.9 | 1 | 1.1 | 9 | 2.0 | 36 | 3.0 |
| Total | 670 | 100 | 93 | 100 | 454 | 100 | 1217 | 100 |
In the 176 cases without available histology the diagnosis was based on FIS: atrophy (defined as an ET < 5mm) in 54, proliferative/secretory endometrium (defined as an ET ≥5mm) in 76, polyps in 24, an intracavitary fibroid in 15, miscellaneous in 7.
In order to evaluate the relative importance of the different variables for the prediction of intracavitary pathology, models were designed using stepwise variable selection.
All statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA.) The area under the ROC curve (AUC) and its 95% confidence interval were calculated for each model.
Results
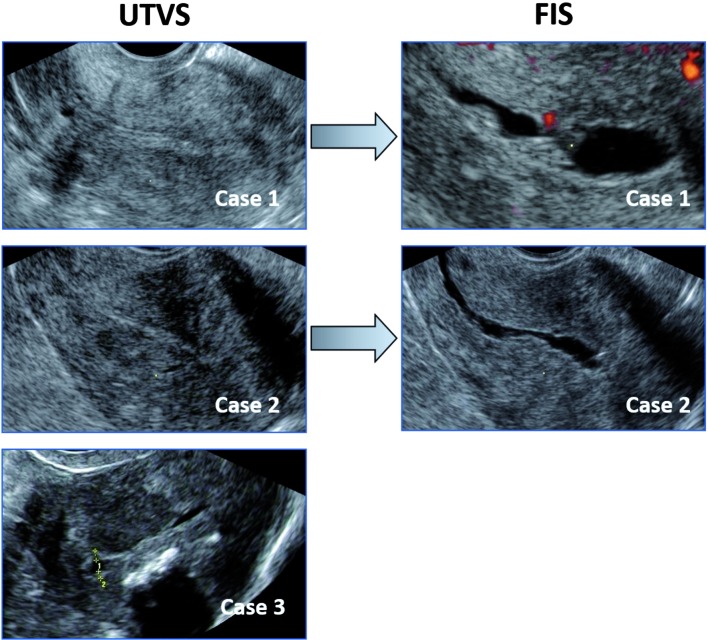
The mean age was 50 years (range 19-86), with an average parity of 1.9 (range 0-12) and a body mass index ranging from 16 to 63 (mean 26). Three patients with incomplete data were excluded from further analysis (1217 cases included). Four hundred and fifty-five patients (37.3%) were postmenopausal, 672 (55.1%) premenopausal and 93 (7.6%) were classified as perimenopausal. The endometrial thickness was not measureable at UTVS in 139 cases (11%). The final diagnosis for all patients presenting at the bleeding clinic is summarized in Table I: of 1217 women 654 (54%) were normal (59% of the premenopausal women and 44% of the postmenopausal group), there were 313 (25.7%) cases of polyps, 134 (11%) intracavitary fibroids, 48 (3.9%) cases of endometrial hyperplasia without atypia, 1 (0.1%) with atypia and 31 (2.5%) endometrial cancers. Cancer was diagnosed in 7% of postmenopausal women and 1% of perimenopausal patients. There were no cancers in premenopausal women. Polyps were diagnosed in 18.4% of premenopausal versus 37.7% of postmenopausal women, and intracavitary fibroids in 14.2% and 6.2%, respectively. Endometrial hyperplasia without atypia was diagnosed in 5.2% of women of reproductive age, in 4.3% of perimenopausal women and in 2% after menopause. The median ET at UTVS in cancer cases was 16.8 mm (range 3.3 mm-78.9 mm) and in 6 cases the endometrium was not measurable at UTVS. In 3 cases of cancer the reported ET at UTVS was ≤ 5 mm (4.8 mm, 4.8 mm and 3.3 mm, respectively). However, in all three cases possible intracavitary pathology had been suggested based on the irregularity of the endometrium at FIS in two cases and because of incomplete visualization due to fibroids in the third patient (Fig. 1). According to the International Endometrial Tumor Analysis (IETA)-recommendations (Leone et al., 2010) the endometrium in the latter case should have been reported as “not measurable”.
Fig. 1. Images at unenhanced ultrasound (UTVS) and at fluid instillation sonography (FIS) (for case 1 and 2) of endometrial cancer with an endometrial thickness < 5 mm: FIS demonstrates an irregular lesion in case 1 and 2 (in case 1 it is associated with visible vascularity within the irregularity).
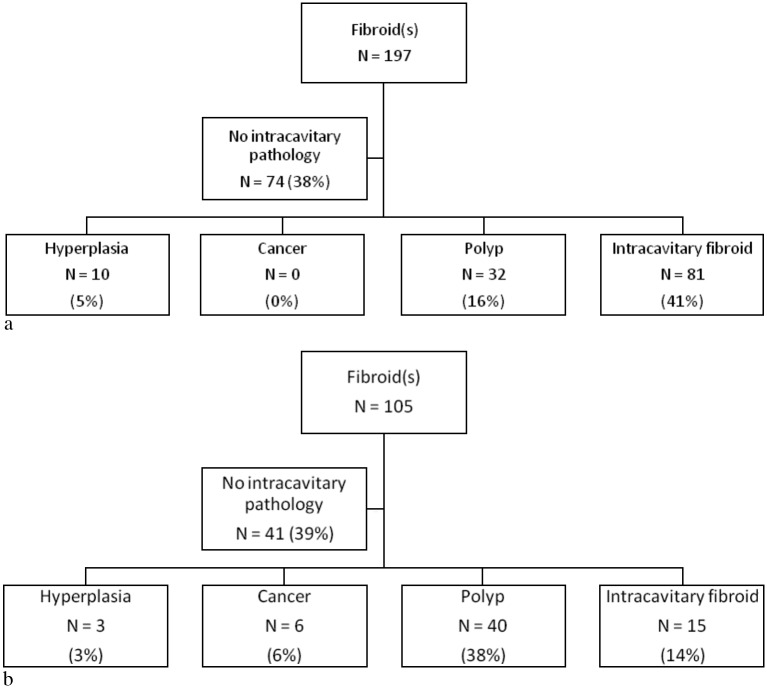
Uterine fibroids outside the endometrial cavity were reported in 27% of patients at UTVS: in 29% of premenopausal women and 23% of women after menopause (Fig. 2). In the majority of women in whom fibroids had been reported on UTVS, intracavitary pathology was also present: endometrial hyperplasia in 4%, endometrial cancer in 2%, polyps in 23% and intracavitary fibroids in 32%. In the premenopausal group with fibroids at UTVS, hyperplasia, polyps and intracavitary fibroids were detected in 5%, 16% and 41%, respectively (Fig. 2a). In postmenopausal women with fibroids reported at UTVS, hyperplasia, endometrial cancer, polyps and intracavitary fibroids were diagnosed in 3%, 6%, 38% and 14%, respectively (Fig. 2b). Intramural or subserous fibroids were the only structural pathology in 10% of cases (124/1220) of the total group: 11% and 9% in pre- and postmenopausal women, respectively.
Fig. 2. Incidence of intracavitary pathology in patients with uterine fibroids recorded at UTVS: (a) premenopausal group; (b) postmenopausal group.
For the prediction of focal intracavitary lesions in premenopausal women, patient’s age was selected as the single most relevant patient characteristic, although the predictive value was low (AUC 0.61; 95% CI 0.56-0.66). Measurements of ET at UTVS did not significantly improve prediction (AUC 0.64; 95% CI 0.59-0.69) in the premenopausal group.
In postmenopausal women, age and BMI were selected as the most important patient’s characteristics for predicting cancer (AUC 0.72; 95% CI 0.61-0.82) and focal benign lesions (AUC 0.60; 95% CI 0.54-0.66). If ET was included in the analysis together with patient’s characteristics, ET was selected as the only relevant predictive factor for both endometrial cancer (AUC 0.80; 95% CI 0.72-0.89) and benign focal intracavitary lesions (AUC 0.80; 95% CI 0.72-0.89) after the menopause.
The diagnostic accuracy for intracavitary pathology using both UTVS and FIS is summarized in Table II for premenopausal and Table III for postmenopausal women.
Table II. Unenhanced transvaginal ultrasound (UTVS), color Doppler imaging (CDI) and fluid instillation sonography (FIS) for the diagnosis of intracavitary pathology* in premenopausal women.
| POSTmenopausal | Sens | Spec | PPV | NPV | LR+ | LR- |
|---|---|---|---|---|---|---|
| UTVS | % | % | % | % | ||
| Endometrial thickness | ||||||
| > 3 mm | 95 | 5 | 38 | 65 | 1.01 | 0.87 |
| > 4 mm | 93 | 12 | 39 | 73 | 1.05 | 0.62 |
| > 5 mm | 86 | 20 | 40 | 71 | 1.09 | 0.29 |
| Optimal (> 5 mm) | 57 | 58 | 45 | 69 | 1.34 | 0.74 |
| Focal lesion | 44 | 94 | 81 | 72 | 6.81 | 0.60 |
| CDI | ||||||
| Vessel(s) within the endometrium | 29 | 96 | 81 | 72 | 6.81 | 0.60 |
| FIS | ||||||
| Lesion | 79 | 87 | 80 | 87 | 6.20 | 0.24 |
Sens = sensitivity; spec = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR- = negative likelihood ratio.
* Endometrial polyps and intracavitary fibroids.
Table III. Unenhanced ultrasound (UTVS), color Doppler imaging (CD) and fluid instillation sonography (FIS) for the diagnosis of intracavitary pathology* in postmenopausal women.
| POSTmenopausal | Sens | Spec | PPV | NPV | LR+ | LR- |
|---|---|---|---|---|---|---|
| UTVS | % | % | % | % | ||
| Endometrial thickness | ||||||
| > 3 mm | 93 | 45 | 74 | 79 | 1.68 | 0.16 |
| > 4 mm | 90 | 64 | 81 | 79 | 2.48 | 0.16 |
| > 5 mm | 83 | 74 | 84 | 72 | 3.13 | 0.24 |
| Optimal (> 5 mm) | 83 | 74 | 84 | 72 | 3.13 | 0.24 |
| Focal lesion | 39 | 95 | 93 | 49 | 7.86 | 0.64 |
| CDI | ||||||
| Vessel(s) within the endometrium | 31 | 97 | 95 | 46 | 10.85 | 0.71 |
| FIS | ||||||
| Lesion | 76 | 91 | 93 | 70 | 8.23 | 0.26 |
Sens = sensitivity; spec = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR- =negative likelihood ratio.
* Endometrial polyps, intracavitary fibroids and endometrial cancer.
In premenopausal women, the LR+ and LR- for intracavitary uterine pathology was 1.34 and 0.74 respectively for ET at UTVS using a cut-off of 9 mm, 6.63 and 0.74 for the presence of one or more dominant vessels at CDI and 6.20 and 0.24 for FIS. In postmenopausal women the corresponding LR+ and LR- were 3.13 and 0.24 for ET using a cut-off of 5 mm, 10.85 and 0.71 for CDI and 8.23 and 0.26 for FIS. In premenopausal women, the retained variables predicting intracavitary pathology at multivariate analysis were age, fibroids at UTVS, a polypoid endometrial pattern at FIS (inverse association) and the presence of a lesion seen at FIS: model with an AUC of 0.88 (0.85-0.92). The diagnostic accuracy of UTVS, CDI and FIS for endometrial cancer is summarized in Table IV, and shows high negative predictive values, ranging from 94 to 100% but very low positive predictive values ranging from 9 to 19%.
Table IV. Unenhanced ultrasound (UTVS), color Doppler imaging (CD) and fluid instillation sonography (FIS) for the diagnosis of endometrial cancer in postmenopausal women.
| POSTmenopausal | Sens | Spec | PPV | NPV | LR+ | LR- |
|---|---|---|---|---|---|---|
| UTVS | % | % | % | % | ||
| Endometrial thickness | ||||||
| > 3mm | 100 | 2 | 9 | 100 | 1.29 | 0 |
| > 4mm | 96 | 32 | 10 | 99 | 1.41 | 0.13 |
| > 5mm | 88 | 40 | 11 | 98 | 1.47 | 0.31 |
| Lesion | 50 | 76 | 15 | 94 | 2.07 | 0.67 |
| CDI | ||||||
| Vessel(s) within the endometrium | 47 | 82 | 19 | 95 | 2.59 | 0.65 |
| FIS | ||||||
| Lesion | 70 | 51 | 11 | 95 | 1.42 | 0.59 |
Sens = sensitivity; spec = specificity; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR- =negative likelihood ratio.
In postmenopausal women the best model predicting endometrial cancer included the presence of one or more vessels within the endometrium using colour Doppler imaging, endometrial thickness and a polypoid endometrial pattern at FIS: AUC 0.84 (0.76-0.92). For the prediction of benign focal lesions (endometrial polyps and intracavitary fibroids), age, endometrial thickness, the presence of a lesion at FIS and a polypoid endometrial pattern at FIS were the selected variables: AUC 0.90 (0.86-0.94).
Discussion
We have shown that, other than menopausal status, the knowledge of a patient’s history is of little value for predicting the presence or absence of intracavitary pathology in women with abnormal bleeding. We have also shown that benign focal intracavitary lesions are present in one third of premenopausal women and in almost half of postmenopausal women with abnormal uterine bleeding. After the menopause, our data support the use of measurements of ET at UTVS as the cornerstone for diagnosing of intracavitary pathology, whereas ET measurements are of no value in premenopausal women. Finally, our study confirms the importance of CDI and FIS for the diagnosis of intracavitary pathology in both pre- and postmenopausal women (Dijkhuizen et al., 2000; de Kroon et al., 2003; Timmerman et al., 2003; Opolskiene et al., 2011).
The strengths of this study are the prospective design, the large number of consecutive women included and the fact that FIS, hysteroscopy and endometrial sampling were performed in the vast majority of patients. The fact that the study was carried out in a single centre by one operator may be seen as a strength in terms of the protocol being applied reproducibly within the trial, but may also be a weakness as the results may be less generalizable. We acknowledge that the prevalence of pathology may vary between centres, some reporting higher malignancy rates (Amant et al., 2005; Opolskiene et al., 2011). Our lower prevalence of cancer may be explained by some being diagnosed as emergencies on biopsies carried out in the general outpatient clinic, in which case the patient would not have attended the bleeding clinic at our hospital. The fact that 10% of cancer cases were below the 5mm ET threshold is unexpectedly high and may be due to a selection bias: easier to diagnose cancer were more likely to have been picked up prior to referral to the bleeding clinic, resulting in a higher prevalence of difficult cases with endometria that are hard to visualize at ultrasound examination. It is also important to acknowledge that our results should not be extrapolated to an asymptomatic population (Menzies et al., 2011; Worley et al., 2011; Breijer et al., 2012). The fact that part of the fluid instillations was performed with saline and the other with gel could have influenced the results. However this issue has been addressed in a publication analysing two consecutive cohorts of 402 women undergoing SIS or GIS and showing a comparable diagnostic accuracy in both groups (Werbrouck et al., 2011).
After menopausal status, age was the most relevant patient’s characteristic. In premenopausal women, focal intracavitary lesions (fibroids and polyps) become more prevalent with advancing age. After the menopause, endometrial cancer risk also increases with advancing age, a finding which is concordant with previous reports (Van den Bosch et al., 1996; Prudie & Green, 2001; Amant et al., 2005).
An endometrial thickness measurement should be reported at every ultrasound examination (Leone et al., 2010). However, in premenopausal women, because of cyclical endometrial changes, ET is less reliable for the detection of pathology. This is illustrated by the fact that ET measurement at UTVS did not improve the predictive value for intracavitary lesions in premenopausal women. In postmenopausal women, in general a thin endometrium rules out intracavitary uterine pathology, and is particularly effective at excluding the presence of cancer.
Cut-off values for total endometrial thickness between 3 and 5 mm have been proposed to define the presence or absence of an increased risk of endometrial malignancy, but these are only valid if the endometrium is clearly visible throughout the cavity. The fact that about one in five cancer cases did not show a clearly visible endometrium on UTVS in our study confirms that if the endometrium is not measurable, it should be considered abnormal until proven otherwise (Van den Bosch et al., 2007; Leone et al., 2010). We believe that FIS is indicated in all women with postmenopausal bleeding if the entire endometrium cannot be visualized clearly.
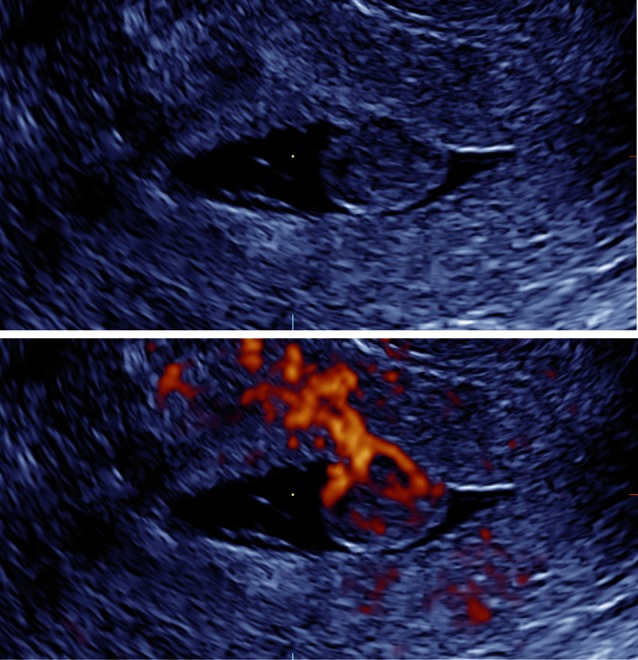
Colour Doppler imaging enables the visualization of vessels within the endometrium. In endometrial polyps the ‘pedicle artery sign’ (Timmerman et al., 2003) is often visible using CDI (Fig. 3), a fibroid often shows circumferential vascularization (Fig. 4), and the visualization of one or more dominant vessels within the endometrium with CDI is commonly associated with endometrial cancer (Opolskiene et al., 2011). In our study increased vascularity was reported in half of the cancer cases and in one third of the patients with focal intracavitary lesions. Small vessels may not be detected with CDI and transient myometrial contractions may result in an intermittent absence of flow signals (Van den Bosch et al., 2002), leading to false negative results as reflected by the suboptimal LR- both in pre- and postmenopausal women (Table II and III).
Fig. 3. Endometrial polyp (gel instillation sonography at grey scale and power Doppler imaging): note the pedicle artery.
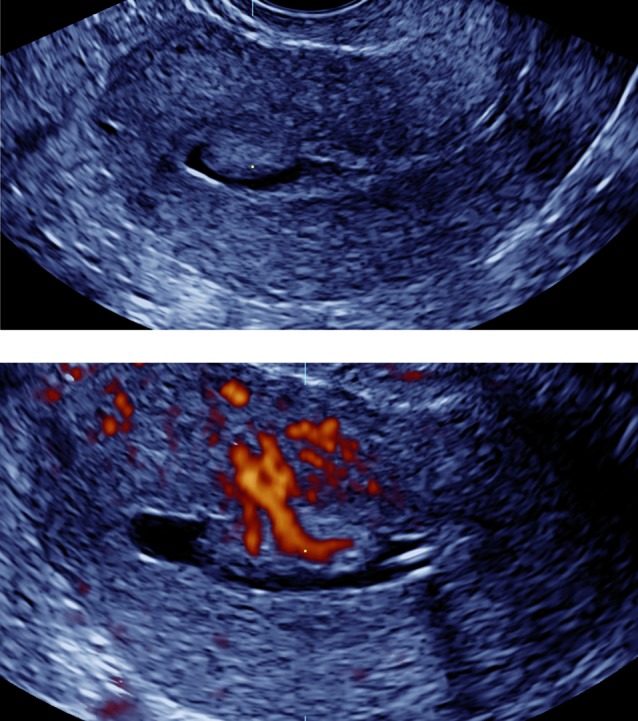
Fig. 4. Intracavitary fibroid with circumferential vascularization (gel instillation sonography at grey scale and power Doppler imaging).
When carrying out FIS, the fluid instilled into the uterine cavity offers a negative contrast facilitating the visualization of the endometrial lining at ultrasound examination. FIS has demonstrated a diagnostic reliability for endometrial polyps and intracavitary fibroids that is comparable to hysteroscopy (de Kroon et al., 2003; Dijkhuizen et al., 2000).
In our series intramural or subserosal fibroids without associated intracavitary pathology were reported in 10% of cases. Other pathology such as endometrial atrophy or ovulatory dysfunction could be responsible for the abnormal bleeding in some of these women, suggesting the limited clinical relevance of diagnosing extra-cavitary fibroids. However, finding a fibroid does not lower the likelihood of endometrial cancer. In our series about 1 in 5 endometrial cancer cases also had uterine fibroids. Especially in postmenopausal women, conservative treatment including myomectomy, myoma morcellation, uterine artery embolization or endometrial ablation (NICE guideline 44, 2007) should be considered only after malignancy has been excluded.
In summary, our study demonstrates that, other than menopausal status, other patient characteristics have a limited value in the diagnosis of intracavitary pathology. Moreover, we showed the lack of relevance of endometrial thickness measurements in premenopausal women. Benign focal intracavitary lesions are the most likely structural cause of abnormal uterine bleeding in all age groups. Some of those lesions may remain unnoticed on UTVS and fluid instillation sonography should therefore be considered in case of abnormal uterine bleeding.
Acknowledgments
Dirk Timmerman is a senior clinical investigator of Scientific Research Fund (FWO) Flanders.
Tom Bourne is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Amant F, Moerman P, Neven P, et al. Endometrial cancer. Lancet. 2005;366:491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- Bignardi T, Van den Bosch T, Condous G. Abnormal uterine and post-menopausal bleeding in the acute gynaecology unit. Best Pract Res Clin Obstet Gynaecol. 2009;23:595–607. doi: 10.1016/j.bpobgyn.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Breijer MC, Peeters JA, Opmeer BC, et al. Capacity of endometrial thickness measurement to diagnose endometrial carcinoma in asymptomatic postmenopausal women: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2012;40:621–629. doi: 10.1002/uog.12306. [DOI] [PubMed] [Google Scholar]
- Clark TJ, Barton PM, Coomarasamy A, et al. Investigating postmenopausal bleeding for endometrial cancer: cost-effectiveness of initial diagnostic strategies. BJOG. 2006;113:502–510. doi: 10.1111/j.1471-0528.2006.00914.x. [DOI] [PubMed] [Google Scholar]
- de Kroon C, De Bock GH, Dieben SWM, et al. Saline contrast hydrosonography in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2003;110:938–947. doi: 10.1111/j.1471-0528.2003.02472.x. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen FP, De Vries LD, Mol BW, et al. Comparison of transvaginal ultrasonography and saline infusion sonography for the detection of intracavitary abnormalities in premenopausal women. Ultrasound Obstet Gynecol. 2000;15:372–376. doi: 10.1046/j.1469-0705.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Dreisler E, Poulsen LG, Antonsen SL, et al. EMAS clinical guide: Assessment of the endometrium in peri and postmenopausal women. Maturitas. 2013;75:181–190. doi: 10.1016/j.maturitas.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Epstein E, Valentin L. Managing women with post-menopausal bleeding. Best Pract Res Obstet Gynaecol. 2004;18:125–143. doi: 10.1016/j.bpobgyn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Gupta JK, Chien PFW, Voit D. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand. 2002;81:799–816. doi: 10.1034/j.1600-0412.2001.810902.x. [DOI] [PubMed] [Google Scholar]
- Leone F, Timmerman D, Bourne T, et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol. 2010;35:103–112. doi: 10.1002/uog.7487. [DOI] [PubMed] [Google Scholar]
- Menzies R, Wallace S, Ennis M, et al. Significance of abnormal sonographic findings in postmenopausal women with and without bleeding. J Obstet Gynaecol Can. 2011;33:944–951. doi: 10.1016/s1701-2163(16)35020-4. [DOI] [PubMed] [Google Scholar]
- NICE. clinical guideline 44 Heavy menstrual bleeding. guidance.nice.org.uk/cg44 NICE’s ‘The guidelines manual’ 2007. 2009 [Google Scholar]
- Opolskiene G, Sladkevicius P, Valentin L. Prediction of endometrial malignancy in women with postmenopausal bleeding and sonographic endometrial thickness ≥ 4.5 mm. Ultrasound Obstet Gynecol. 2011;37:232–240. doi: 10.1002/uog.8871. [DOI] [PubMed] [Google Scholar]
- Prudie DM, Green AC. Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol. 2001;15:341–354. doi: 10.1053/beog.2000.0180. [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R, Kerlikowske K, Feldstein VA, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280:1510–1517. doi: 10.1001/jama.280.17.1510. [DOI] [PubMed] [Google Scholar]
- Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol. 2002;99:663–670. doi: 10.1016/s0029-7844(01)01771-9. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Verguts J, Konstantinovic ML, et al. The pedicle artery sign based on sonography with color Doppler imaging can replace second-stage tests in women with abnormal vaginal bleeding. Ultrasound Obstet Gynecol. 2003;22:166–171. doi: 10.1002/uog.203. [DOI] [PubMed] [Google Scholar]
- Timmermans A, Opmeer B, Khan K, et al. Endometrial thickness measurement for detecting endometrial cancer in women with postmenopausal bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:160–167. doi: 10.1097/AOG.0b013e3181e3e7e8. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Coosemans A, Morina M, et al. Screening for uterine tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26:257–266. doi: 10.1016/j.bpobgyn.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Van Schoubroeck D, Vergote I. A thin and regular endometrium on ultrasound is very unlikely in patients with endometrial malignancy. Ultrasound Obstet Gynecol. 2007;29:674–679. doi: 10.1002/uog.4031. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Van Schoubroeck D, Lu C, et al. Color Doppler and gray-scale ultrasound evaluation of the postpartum uterus. Ultrasound Obstet Gynecol. 2002;20:586–591. doi: 10.1046/j.1469-0705.2002.00851.x. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Vandendael A, Van Schoubroeck D, et al. Age, weight, body mass index and endometrial thickness in postmenopausal women. Acta Obstet Gynecol Scand. 1996;75:181–182. doi: 10.3109/00016349609033315. [DOI] [PubMed] [Google Scholar]
- Van den Bosch T, Vandendael A, Van Schoubroeck D, et al. Combining vaginal ultrasonography and office endometrial sampling in the diagnosis of endometrial disease in postmenopausal women. Obstet Gynecol. 1995;85:349–352. doi: 10.1016/0029-7844(94)00421-9. [DOI] [PubMed] [Google Scholar]
- Werbrouck E, Veldman J, Luts J, et al. Detection of endometrial pathology using saline infusion sonography versus gel instillation sonography: a prospective cohort study. Fertil Steril. 2011;95:285–288. doi: 10.1016/j.fertnstert.2010.04.074. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Jr, Dean KL, Lin SN, et al. The significance of a thickened endometrial echo in asymptomatic postmenopausal patients. Maturitas. 2011;68:179–181. doi: 10.1016/j.maturitas.2010.10.007. [DOI] [PubMed] [Google Scholar]