Abstract
All gynecologists are faced with ovarian tumors on a regular basis, and the accurate preoperative diagnosis of these masses is important because appropriate management depends on the type of tumor. Recently, the International Ovarian Tumor Analysis (IOTA) consortium published the Assessment of Different NEoplasias in the adneXa (ADNEX) model, the first risk model that differentiates between benign and four types of malignant ovarian tumors: borderline, stage I cancer, stage II-IV cancer, and secondary metastatic cancer. This approach is novel compared to existing tools that only differentiate between benign and malignant tumors, and therefore questions may arise on how ADNEX can be used in clinical practice. In the present paper, we first provide an in-depth discussion about the predictors used in ADNEX and the ability for risk prediction with different tumor histologies. Furthermore, we formulate suggestions about the selection and interpretation of risk cut-offs for patient stratification and choice of appropriate clinical management. This is illustrated with a few example patients. We cannot propose a generally applicable algorithm with fixed cut-offs, because (as with any risk model) this depends on the specific clinical setting in which the model will be used. Nevertheless, this paper provides a guidance on how the ADNEX model may be adopted into clinical practice.
Keywords: Ovarian neoplasms, ultrasonography, CA-125, decision support techniques, practical guidance
Introduction
Although often overlooked, the preoperative characterization of an adnexal mass is of crucial importance for selecting the optimal management strategy. Firstly, accurate differentiation between benign and malignant tumors can lead to referral of patients with malignant tumors to gynecological oncology centers for further diagnosis or staging, followed by debulking surgery and/or administration of systemic therapy. This is an important factor that positively influences prognosis (Earle et al., 2006; Engelen et al., 2006; Woo et al., 2012; Bristow et al., 2013). Benign ovarian masses can be managed expectantly or by conservative surgical management with reduced morbidity and fertility preservation (Carley et al., 2002). Secondly, optimal treatment of adnexal malignancies depends on the type of tumor. Borderline tumors can be treated with less aggressive techniques than invasive tumors, which is of interest when fertility preservation is desired (Tinelli et al., 2006; Daraï et al., 2013). Furthermore, stage I ovarian cancer may be managed more conservatively than stage II-IV disease (Trimbos et al., 2003). Finally, for cancers of other primary origin metastasized to the ovary, treatment depends on the type of tumor. Recently, the International Ovarian Tumor Analysis (IOTA) group proposed the Assessment of Different NEoplasias in the adneXa (ADNEX) model. This is the first risk model to differentiate between benign, borderline tumors, stage I invasive, stage II-IV invasive ovarian cancer and secondary metastatic cancer (Van Calster et al., 2014). Such a ‘multiclass’ or polytomous model is uncommon and poses new practical challenges. The aim of this paper is to discuss the ADNEX model in more detail and provide some guidance for clinical management.
Explanation of ADNEX
Intended population
The ADNEX model was developed and validated using data from the IOTA phase 1-3 datasets, consisting of prospectively collected patients who were referred for an ultrasound examination to one of the participating centers for a known or a suspected adnexal mass (Timmerman et al., 2005; Van Holsbeke et al., 2009; Van Calster et al., 2012; Kaijser et al., 2014). Patients were eligible for inclusion if they presented with at least one adnexal mass that was judged not to be a physiological cyst, and if they were selected for surgical intervention by the managing clinician following local protocols. Exclusion criteria were refusal for transvaginal ultrasonography, pregnancy at the time of presentation and surgical intervention more than 120 days after the ultrasound examination. If a patient presented with multiple masses, the mass with the most complex morphology on ultrasound examination was selected for analysis. If more than one mass with similar morphology was present, the largest or the most easily accessible mass was used. The model was developed on 3506 patients recruited between 1999 and 2007, temporally validated on 2403 patients recruited between 2009 and 2012, and then updated on all 5909 patients in the final analysis. Twenty-four centers in 10 countries were involved (Van Calster et al., 2014).
Selection of predictors
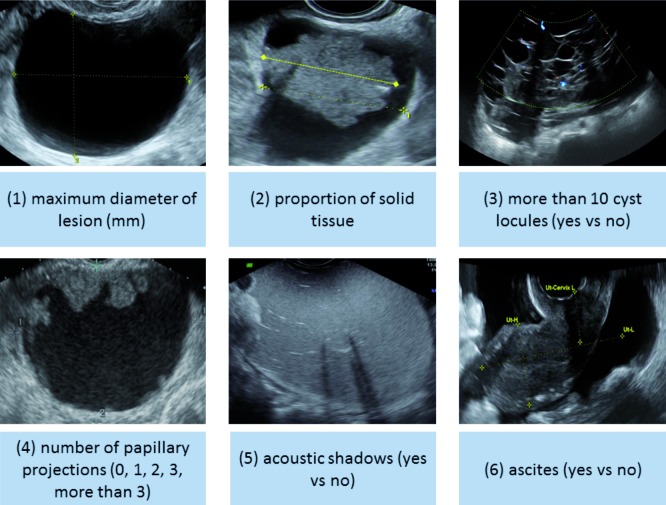
The ADNEX model consists of three clinical predictors and six ultrasound predictors. The clinical predictors are age (years), serum CA-125 (U/mL) and type of center to which the patient has been referred for ultrasound examination. Type of center has been divided into oncology centers versus other hospitals, and is further discussed in the next section. The ultrasound predictors are the maximal diameter of the lesion (mm), proportion of solid tissue (%), number of papillary projections (0, 1, 2, 3, > 3), presence of more than 10 cyst locules (yes/no), acoustic shadows (yes/no), and presence of ascites (yes/no) (Fig. 1). The proportion of solid tissue is defined as the ratio of the maximal diameter of the largest solid component and the maximal diameter of the lesion.
Fig. 1. Ultrasound characteristics selected as predictors in the ADNEX model.
The ADNEX predictors were selected as follows. Clinicians with experience in the characterization of adnexal tumors a priori selected a limited set of variables that were considered as potentially useful predictors. This was based on the variables’ perceived predictive value to distinguish between the four types of malignant ovarian pathologies of interest, subjectivity, dependency on the experience of the ultrasound examiner, and impact on the patient. This approach is in accordance with current methodological recommendations (Steyerberg, 2009). In addition, variable selection was also based on an analysis of consistency of the variables across the IOTA study centers (Wynants et al., 2013). As a result, it was deliberately decided not to include color Doppler variables or the presence of cyst wall irregularities. Ten potential predictor variables were selected, on which further statistical variable selection was performed (Van Calster et al., 2014). This resulted in the omission of one variable (family history of ovarian cancer). As a result ADNEX contains nine predictors that have strong diagnostic value.
Six predictors in the ADNEX model are ultrasound variables. A concern might be the experience required to accurately measure these variables. However, there is now evidence available that suggests that ultrasound-based prediction models and rules retain their performance in the hands of relatively inexperienced doctors and sonographers (Nunes et al., 2012; Alcazar et al., 2013; Sayasneh et al., 2013a; Sayasneh et al., 2013b) on the condition that the examiners are familiar with the IOTA terms and definitions and properly use the IOTA examination and measurement techniques (Timmerman et al., 2000).
Type of center
One of the ADNEX predictors is the type of center, divided into oncology centers and other hospitals. An ‘oncology center’ was defined as a tertiary referral center with a specific gynecological oncology unit. The prevalence of different types of malignant tumors is higher in oncology centers compared to other centers (Table I). Using the data on all 5909 patients, we observed malignancy rates between 22 and 66% in oncology centers, and between 0 and 30% in other centers (Van Calster et al., 2014). This is largely explained by the observation that patients with masses that look suspicious are more often referred to specialized centers for assessment and treatment. Nevertheless, although the ADNEX model contains eight patient or tumor-specific predictors, type of center was still predictive (Van Calster et al., 2014).
Table I. Baseline risks for the different final diagnoses using the combined IOTA phase 1-3 dataset (n = 5909).
| Overall | Oncology center | Other center | |
| Benign | 68.2% | 48.8% | 83.7% |
| Malignant | 31.8% | 51.2% | 16.3% |
| Borderline | 6.3% | 9.3% | 3.8% |
| Stage I invasive | 7.4% | 10.3% | 4.6% |
| Stage II-IV invasive | 14.1% | 24.3% | 6.4% |
| Secondary metastatic | 4.0% | 7.3% | 1.6% |
If the same patient would be evaluated at a local community hospital and then also by a clinician in an oncology center, the risk of malignancy for the same tumor would be higher in the latter setting than in the former. Intuitively this might sound confusing, but as Candido-dos-Reis (2014) stated, type of center may still be a surrogate for clinical signs of malignancy that are not in the ADNEX model. Potential examples can be symptoms for ovarian cancer, such as pain and bloating or mode of detection. This is an issue for further research.
Note that type of center is the weakest of all nine ADNEX predictors (Van Calster et al., 2014). This implies that the higher baseline risks of malignant tumor types in oncology centers are largely (but not completely) explained by patient- and tumor-specific predictors. Nonetheless, the inclusion of type of center is important to obtain more reliable risk predictions.
Reference standard
The reference standard for the ADNEX model was based on histopathological examination of excised tissue, and for malignant tumors also on surgical staging using the classification of the International Federation of Gynecology and Obstetrics (FIGO) (Prat, 2014). In the IOTA database, 21 histological groups were used (11 benign and 10 malignant groups) which were reduced to five for the ADNEX model: benign, borderline tumors, stage I invasive, stage II-IV invasive ovarian cancer and secondary metastatic cancer.
Risk prediction
When we consider the discrimination between benign and malignant adnexal masses, the area under the receiver operating characteristic curves (AUC) of the ADNEX model was 0.954 (95% confidence interval 0.947 to 0.961) on the development data and 0.943 (0.934 to 0.952) on the validation data (Van Calster et al., 2014). Using a previously proposed cut-off of 10% (Timmerman et al., 2005), the sensitivity for malignancy was 96.5% and specificity 71.3% on the validation data. The model discriminated well between benign tumors and each of four types of malignancy, with AUCs between 0.85 (benign versus borderline) and 0.99 (benign versus stage II-IV cancer) (Van Calster et al., 2014).
In the most recent meta-analysis evaluating the performance of prediction models and rules to characterize adnexal pathology, the IOTA logistic regression model LR2 (Timmerman et al., 2005; Van Holsbeke, 2012; Van Calster et al., 2012) and the IOTA ‘simple rules’(Timmerman et al., 2008; Timmerman et al., 2010) performed best for the overall discrimination between benign and all malignant masses (Kaijser et al., 2014). The ADNEX model seems to perform similar to, or even slightly better than, both LR2 (AUC 0.92) and simple rules (Testa et al., 2014).
Validation AUCs for discrimination between malignant subtypes varied between 0.71 and 0.95. The model discriminated well between stage I cancer and borderline tumors (validation AUC 0.75) and between stage I cancer and secondary metastatic cancer (validation AUC 0.71) (Van Calster et al., 2014). It performed very well in distinguishing stage II-IV cancer from other malignancies (validation AUCs for stage II-IV cancer versus borderline tumors was 0.95, versus stage I cancer was 0.87, and versus secondary metastatic cancer was 0.82) (Van Calster et al., 2014). Finally, the validation AUC for borderline versus secondary metastatic cancer was 0.87 (Van Calster et al., 2014).
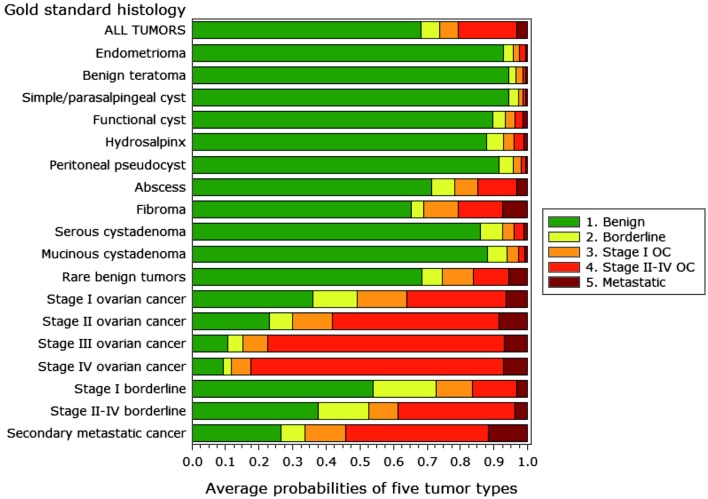
It is interesting to look at the final histological outcome in relation to the average predicted risks for the five ADNEX groups because this illustrates the relative difficulty of classifying different types of adnexal masses (Fig. 2). As might be expected, benign teratomas and simple cysts were given the highest likelihood of being benign tumors. On average, the predicted probability of being benign was around 90% or higher for eight of eleven benign histologies. The ADNEX model had more difficulties with abscesses, rare benign tumors, and fibromas, with average predicted probabilities of being benign of around 70%. Regarding malignant masses, the average risk of being classified as an advanced stage cancer by the ADNEX model increased with FIGO stage. Conversely, the predicted risk of borderline or stage I ovarian cancer decreased with increasing FIGO stage. Finally, the average predicted risk of secondary metastatic cancer is highest for histologically proven metastatic cancers, but notice that also fibromas, rare benign tumors and invasive ovarian cancers were assigned rather high risks of metastatic cancer.
Fig. 2. Average predicted risks for different histologies.
The final ADNEX model is available online and in mobile applications (www.iotagroup.org/adnexmodel/).
Clinical implications of the ADNEX model
As with any decision support tool, there does not exist a single fixed approach to use the model in clinical practice. Risk models aim to provide accurate risk estimates for individual patients. How these estimates are used to inform patients or make decisions depends on a multitude of factors, such as personal preferences, patient characteristics (e.g. age and co-morbidity), the patient’s values, local protocols and regional guidelines. In this section, we will discuss some general suggestions for using the ADNEX model in clinical practice.
Cut-offs
Using risk predictions to guide patient stratification and treatment selection implies the introduction of cut-offs. The choice of cut-offs is not straightforward and may not be in accordance with modern evidence based medicine (EBM). Indeed, Greenhalgh and colleagues (2014), in their recent discussion of the EBM crisis stated that “inflexible rules and technology driven prompts may produce care that is management driven rather than patient centered” and that “real EBM demands individualized evidence, is characterized by expert judgment rather than mechanical rule following“ (Greenhalgh et al., 2014). The rigid use of a cut-off may result in suboptimal and even unethical judgment.
Nevertheless, instead of inflexibly using cut-offs both as a surrogate for clinical patient-centered decision making and as a generally applicable algorithm, a critical determination of cut-offs to provide risk stratification could be the “golden standard” in optimizing patient-centered health care. Selecting cut-offs may be dependent on clinician, center, local protocols or guidelines.
For example, consider defining a risk cut-off for the selection of patients with benign masses for expectant management. Some centers will prefer the risk to be below 1%, while in other centers a cut-off of 5% may be chosen. On the other hand, referral to a tertiary oncology center for treatment of the most suspicious tumors by skilled gynecological oncologists may be more or less restricted. For one center it may be most important to have a high sensitivity, by choosing a low cut-off for malignancy (e.g. 5-10%). This leads to the appropriate referral of most malignancies to a gynecological oncologist. For another center, it may be more important to have a high specificity, by choosing a much higher cut-off value for malignancy (e.g. 30%). This limits the number of false positive results, i.e. patients with benign pathology that are referred to the gynecological oncologist. A wide spectrum of strategies could be encountered in different countries, with different health systems and referral protocols.
Furthermore, once these cut-offs have been chosen, they should be interpreted for each individual patient depending on her characteristics (e.g. age, history, comorbidity, operability, symptoms, fertility wish) and values. We will try to illustrate this in the next sections.
Benign versus malignant
When applying the ADNEX model for an individual patient, probabilities for a benign tumor and for four types of malignant tumors are obtained. When summing the latter four, the overall predicted risk of malignancy is obtained. As described above, the ADNEX model seems to discriminate at least as well between benign and malignant adnexal lesions as the simple rules or LR2. Note that future studies comparing ADNEX with other prediction models still have to confirm this finding. A center-specific cut-off value for relative risk stratification could be chosen for clinical management. When the patient’s risk is situated below the cut-off value, the mass can be considered as benign. Taking into account the patient’s values and symptomatology, conservative follow-up or laparoscopic cystectomy or adnexectomy may be appropriate. Above the cut-off, the adnexal lesion may be considered suspicious enough for referral to a gynecological oncologist for appropriate treatment. In this second step, differential diagnosis of the mass by the ADNEX model may help to optimize management (See 3.3).
Note that the model is based on patients selected for surgery, with histopathological examination as standard reference. These patients in fact all had an indication for surgical intervention. This implies that the population on which the model was developed and validated is at higher risk than a population that includes patients who are selected for expectant management. This means that below the cut-off, the true risk of being malignant is expected to be even lower.
In the future, once the model can be validated on populations including patients that are conservatively followed (as in IOTA phase 5), better discrimination between benign and malignant tumors can probably be reached.
Differential diagnosis of malignancy: absolute risk versus relative change in risk
The ADNEX model calculates absolute risk estimates for four types of malignant tumors. These predicted risks for a specific patient can be an important result on which to base clinical management. Of the four groups of malignant tumors, especially the secondary metastases to the ovaries and the borderline malignant ovarian tumors are of particular interest to identify preoperatively. However, due to the low prevalence of most malignant outcome categories, predicted risks for these categories will not always be very high. As an example, secondary metastatic cancer has a baseline risk of only 4% (Table I). Hence it is more difficult to observe very high-predicted risks for this category compared to stage II-IV ovarian cancer (baseline risk 14.1%). We observed a maximum predicted risk of 68.4% for being borderline, 48.9% for being stage I cancer, 99.6% for being stage II-IV cancer, and 44.6% for being secondary metastatic cancer. Therefore, it is not realistic to expect that the predicted risk for being borderline, stage I cancer or secondary metastatic cancer could be as high as 80%. This does not imply that the model does not function well: the AUCs for pairs of outcome categories were in fact quite high for a model that aims to differentiate between five categories. Rather, this finding may imply that the ADNEX model is a very interesting tool to assess whether there is an increased possibility of one of the more rare categories. In that sense, it is useful to check how the predicted risks for an individual patient compare with the baseline risks. For this purpose, the relative risk, i.e. absolute predicted risk divided by baseline risk, and their corresponding positive predictive value (PPV) can be computed (Table II). For example, patients with a relative risk of having a borderline tumor above 2 have an absolute predicted risk above 12.6%. In this subgroup of patients, 23.9% were eventually diagnosed with a borderline tumor in our dataset.
Table II. Relative risk of each tumor subgroup and corresponding positive predictive value (PPV).
| Relative risk | ||||||
|---|---|---|---|---|---|---|
| ≤ 1 | > 1 | > 2 | > 3 | > 4 | ||
| Borderline | Absolute predicted risk | ≤ 6.3 | > 6.3 | > 12.6 | > 18.9 | > 25.2 |
| Observed PPV (%) | 2.0 | 18.4 | 23.9 | 26.0 | 29.7 | |
| Stage I cancer | Absolute predicted risk | ≤ 7.4 | > 7.4 | > 14.8 | > 22.2 | > 29.6 |
| Observed PPV (%) | 2.2 | 16.5 | 21.2 | 26.6 | 30.7 | |
| Stage II-IV cancer | Absolute predicted risk | ≤ 14.1 | > 14.1 | > 28.2 | > 42.3 | > 56.4 |
| Observed PPV (%) | 1.4 | 56.5 | 66.2 | 71.3 | 75.8 | |
| Secondary | Absolute predicted risk | ≤ 4.0 | > 4.0 | > 8.0 | > 12.0 | > 16.0 |
| metastatic cancer | Observed PPV (%) | 1.0 | 13.5 | 18.4 | 26.4 | 31.6 |
Relative risk: rate of change of the absolute predicted risk versus the baseline risk.
Observed PPV: the observed positive predictive value, i.e. the percentage of patients with a given outcome among those with a given relative risk for that outcome as observed in the sample of 5909 patients on which the final ADNEX coefficients were obtained. Note that this is an observed percentage that is unadjusted for clustering by center.
The ADNEX model is the first known model able to discriminate between different types of malignant tumors, hence it is not straightforward how to use it clinically. In this respect knowledge of these relative risks as additional information to the predicted risks might help to adjust management to the individual patient. We believe that the aim should not be to classify tumors into a single subgroup of malignancy and to manage the patients accordingly. Rather, it is more sensible to assess per type of malignancy whether the predicted risk and/or relative risk is high or low.
Secondary metastases to the ovaries (Krukenberg tumors) originate in 76% of cases in the stomach, 11% in the intestines (usually colon or rectum), 4% in the breast, 3% in the biliary system, 3% in the appendix, and the remaining 3% in miscellaneous sites, such as pancreas, uterine cervix, urinary bladder (including urachus), and renal pelvis (Irving et al., 2006). When an ovarian malignancy is suspected on vaginal ultrasound, preoperative staging is planned by using chest x-ray, computed tomography (CT) of the abdomen, or magnetic resonance imaging (MRI) to evaluate the extension of the disease. When the ADNEX model would give an increased risk of secondary metastatic cancer (e.g. a relative risk > 3 or > 4, corresponding PPV 26.4 and 31.6%), this could assist the clinician to plan appropriate diagnostics to exclude another primary origin of malignancy. Depending on the clinical situation these additional investigations could include a PET-CT, MRI whole body diffusion, gastroscopy, colonoscopy, and x-ray mammography.
Borderline malignant epithelial tumors are primarily non-invasive but can (rarely) lead to invasive metastases. Extent of surgery depends on (surgical) staging and wish of fertility preservation (Cadron et al., 2007). If the latter is not desired by the patient, surgery consists of hysterectomy with bilateral adnexectomy, omentectomy and collection of peritoneal biopsies and cytology. If the patient wants to preserve her fertility, fertility sparing surgery (unilateral adnexectomy or cystectomy or unilateral adnexectomy with contralateral cystectomy if bilateral disease, with omentectomy and peritoneal biopsies) is possible. This procedure is usually done by laparotomy, but might also be done by laparoscopy. These fertility-sparing procedures could be considered when the ADNEX model points to an increased risk for a borderline malignancy (e.g. a relative risk > 3 or > 4, corresponding PPV 26.0 and 29.7%).
The surgical treatment of invasive ovarian cancer stage I depends on the histology and degree of differentiation of the tumor (Vergote et al., 2001). Sex cord-stromal tumors and germ cell tumors can mostly be operated by fertility-sparing procedures. In the case of epithelial ovarian cancer, this depends on degree of differentiation or the histological type (e.g. expansile versus infiltrative invasive mucinous carcinoma) and is only possible in stage Ia (Muyldermans et al., 2013). In these patients, usually a laparotomy is performed with preoperative histopathological examination on frozen section.
In the case of suspected advanced stage ovarian cancer, a diagnostic laparoscopy may be performed to assess feasibility of primary surgical debulking. The option of neo-adjuvant chemotherapy followed by debulking surgery might be preferred in certain circumstances (Vergote et al., 2010).
The ADNEX model can also help the clinician to plan the appropriate strategy when invasive ovarian cancer is anticipated, but will probably contribute less here, as other imaging, surgical staging and patients’ characteristics will more strongly determine the suitable strategy.
Use of ADNEX with or without the CA-125 marker
Although the ADNEX model includes the serum CA-125 level, the online and mobile applications allow for risk calculations without this information. If doing so, a warning is given “Calculate results without serum CA-125 level? The field is indeed optional but this will decrease the discrimination between Stage II-IV invasive tumors and the other malignancy subtypes”. A possible way to use ADNEX, especially when CA-125 is not always measured at your hospital, is by first applying the ADNEX model without serum CA-125 level and using these risk predictions to differentiate benign and malignant tumors. Results indicate that the omission of CA-125 has limited impact on discrimination between benign and all malignant tumors: the validation AUC was 0.943 with CA-125 level included as a predictor, and 0.932 without using CA-125 level as a predictor (Van Calster et al., 2014). Second, in case of high (enough) risk of malignancy, the CA-125 level could be included to update risk predictions. This results in a superior differentiation between borderline, stage I invasive, stage II-IV invasive and secondary metastatic cancer (Van Calster et al., 2011, 2014). In this way, the measurement of the serum CA-125 level may be restricted to patients with an increased risk of having ovarian cancer.
Practical examples
Case 1
We assess a 59-year-old woman in a gynecological oncology center. The CA125 level is 153 U/ml. On transvaginal ultrasound, we describe a solid ovarian mass with a maximal lesion diameter of 59 mm, a maximal diameter of the largest solid component of 59 mm as well, and without acoustic shadowing. There is presence of fluid outside the pouch of Douglas (ascites). If we introduce these parameters in the ADNEX model (in this case, application for smartphone), we obtain the results and column charts as illustrated in Figure 3. First we add the risk predictions for the four malignant subgroups to obtain the total risk of malignancy, which is 95.3% for this patient. Thus the tumor is likely to be malignant. Then, we look at the differentiation between four malignant subgroups and observe a predicted risk for secondary metastatic cancer of 21.2% (compared to a baseline risk of 4.0%) and a risk for stage II-IV ovarian cancer of 67.9% (compared to a baseline risk of 14.1%). The predicted risks for the other subgroups are lower (4.6% for stage I cancer and 1.6% for borderline) and are also smaller than the baseline risks.
Fig. 3. Illustration of the ADNEX model for case 1.
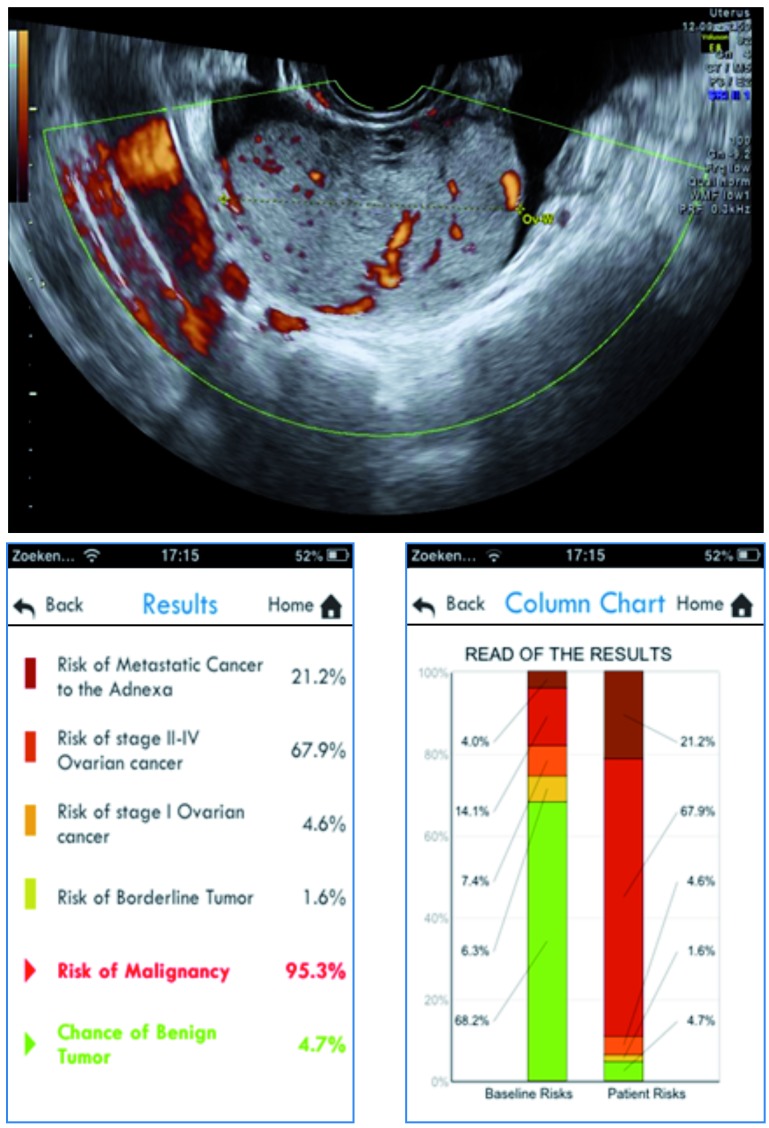
The corresponding relative risks (i.e. ratio of predicted risk and baseline risk) for secondary metastatic cancer and stage II-IV ovarian cancer are 5.3 and 4.8, respectively. The relative risks for borderline or stage I ovarian cancer are below 1. With the knowledge of the increased risk for advanced stage ovarian cancer and more importantly secondary metastatic cancer, clinicians may adjust their preoperative diagnostics by excluding a tumor of other primary origin, such as gastric cancer or breast cancer. In case of a solid tumor these investigations may include PET-CT, MRI whole body diffusion, gastroscopy, colonoscopy, or mammography. This is important as appropriate therapy depends on the origin of the primary tumor. This patient was eventually diagnosed with metastatic gastric cancer (Krukenberg tumor).
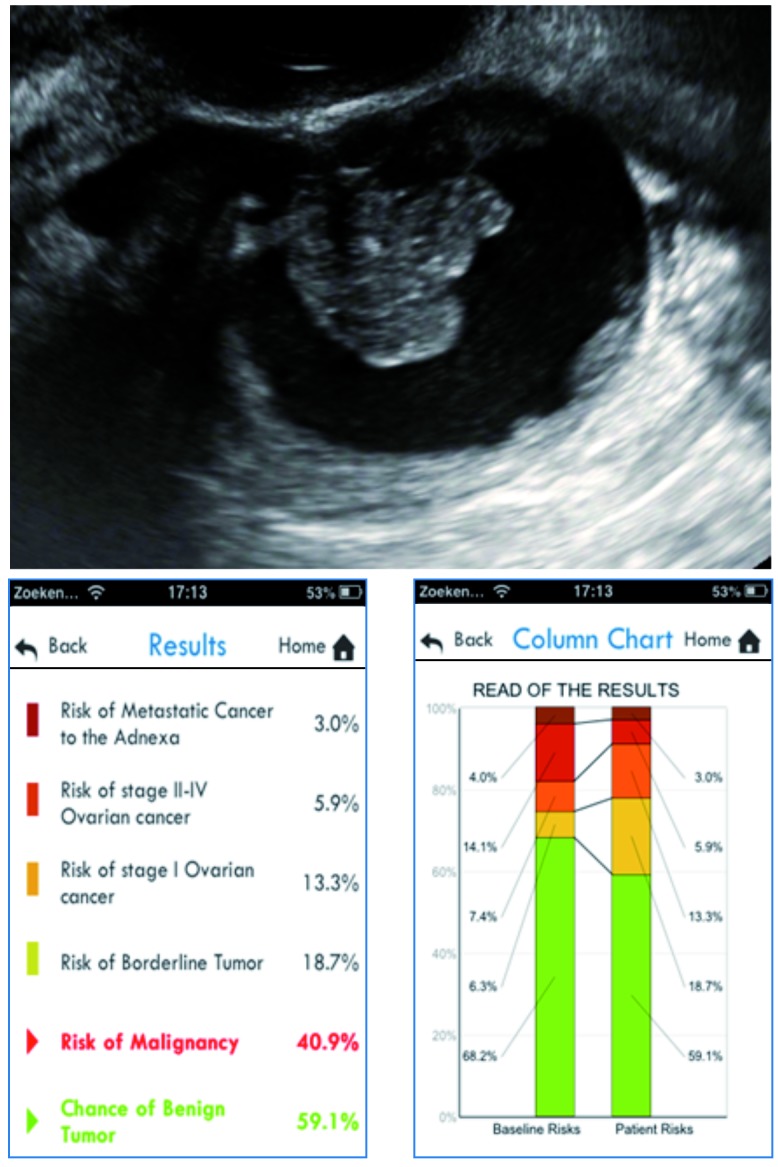
Case 2
We evaluate a 48-year-old woman in a gynecological oncology center. She is diagnosed with a unilocular-solid adnexal mass with a maximal diameter of 66 mm. The maximal diameter of the largest solid component is 27 mm (one papillary projection), and there is no acoustic shadowing or ascites. CA125 is 22 U/ml. If we introduce these parameters in the ADNEX model using the smartphone application, we obtain the results and column charts as illustrated in Figure 4. The total risk of malignancy is 40.9%. The predicted risk for stage I ovarian cancer is 13.3% (compared to a baseline risk of 7.4%), the predicted risk for a borderline tumor is 18.7% (compared to a baseline risk of 6.3%). The predicted risks for the other subgroups are lower (5.9% for stage II-IV cancer and 3.0% for secondary metastatic cancer) and are also smaller than the baseline risks. The relative risk for stage I ovarian cancer and for a borderline tumor is 1.8 and 3, respectively. The relative risks for advanced stage ovarian cancer and secondary metastatic cancer are below 1. This patient was eventually diagnosed with a borderline ovarian tumor.
Fig. 4. Illustration of the ADNEX model for case 2.
Discussion
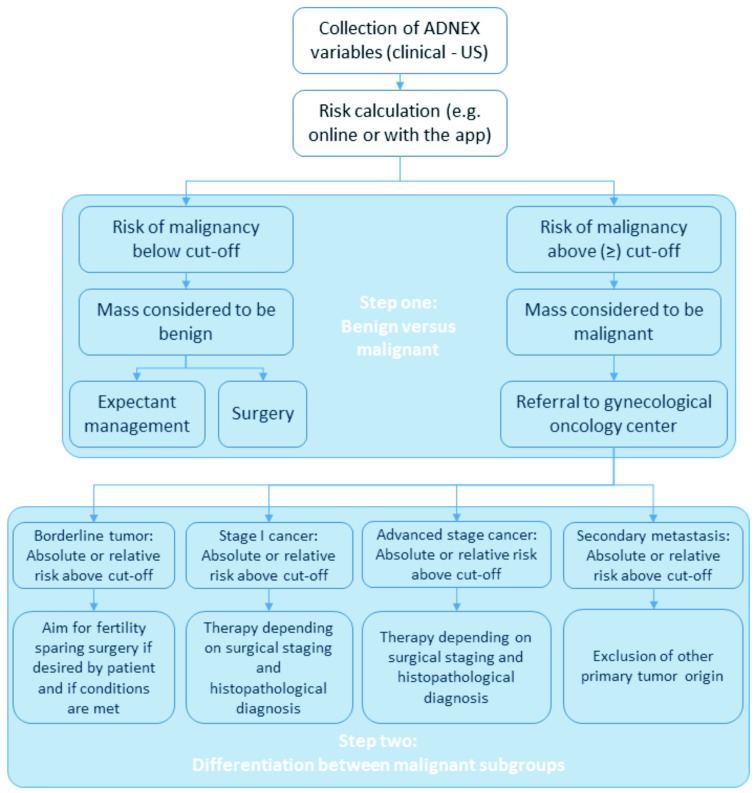
The ADNEX model is the first risk model that differentiates between benign and four subgroups of malignant adnexal tumors. The model consists of three clinical predictors and six ultrasound predictors, which can be evaluated by examiners familiar with the IOTA terms and definitions.Although the ADNEX model includes the serum CA-125 level, the online and mobile application allow for risk calculations without this measurement. A two-step approach could be adopted to make clinical use of the predicted risks from ADNEX (see Figure 5 for an example). First the risk calculation can be used to discriminate between benign and malignant masses based on the specific risk cut-off value used by individual centers to define malignancy, where the adopted cut-off may depend on the local healthcare policy. The discrimination between benign and malignant masses can be done without CA-125 should this be desired, because results indicate no loss of performance in terms of AUC. Second, we can differentiate between the four subgroups of malignant tumors using the predicted risks for these subgroups. In this step, absolute predicted risks as well as the relative change of these risks versus the baseline risks provide clinically useful information to select an appropriate patient-specific management strategy. We cannot propose a generally applicable algorithm with fixed cut-offs, because this depends on the specific clinical setting where the model will be used. Nevertheless, this paper provides guidance on how the ADNEX risk model may be adopted into clinical practice.
Fig. 5. Example of a two-step approach towards the clinical use of ADNEX predicted risks.
Acknowledgments
This study is supported by the Flemish Government: FWO project G049312N, Flanders’ Agency for Innovation by Science and Technology (IWT) project IWT-TBM 070706-IOTA3, and iMinds 2013. Kirsten Van Hoorde and Laure Wynants are doctoral fellows of the Institute of Science and Technology (IWT). Dirk Timmerman is Senior Clinical Investigator of the Research Foundation - Flanders (Belgium) (FWO). Tom Bourne is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- Alcázar JL, Pascual MA, Olartecoechea B, et al. IOTA simple rules for discriminating between benign and malignant adnexal masses: prospective external validation. Ultrasound Obstet Gynecol. 2013;42:467–471. doi: 10.1002/uog.12485. [DOI] [PubMed] [Google Scholar]
- Bristow RE, Chang J, Ziogas A. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121:1226–1234. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- Cadron I, Leunen K, Van Gorp T. Management of borderline ovarian neoplasms. J Clin Oncol. 2007;25:2928–2937. doi: 10.1200/JCO.2007.10.8076. [DOI] [PubMed] [Google Scholar]
- Candido-dos-Reis FJ. Ovarian cancer risk model needs a more meaningful clinical parameter. BMJ. 2014;349:g6689. doi: 10.1136/bmj.g6689. [DOI] [PubMed] [Google Scholar]
- Carley ME, Klingele CJ, Gebhart JB, et al. Laparoscopy versus laparotomy in the management of benign unilateral adnexal masses. J Am Assoc Gynecol Laparosc. 2002;9:321–326. doi: 10.1016/s1074-3804(05)60411-2. [DOI] [PubMed] [Google Scholar]
- Daraï E, Fauvet R, Uzan C. Fertility and borderline ovarian tumor: a systematic review of conservative management, risk of recurrence and alternative options. Hum Reprod Update. 2013;19:151–166. doi: 10.1093/humupd/dms047. [DOI] [PubMed] [Google Scholar]
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98:172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]
- Engelen MJA, Kos HE, Willemse PHB, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106:589–598. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, Howick J, Maskrey N, et al. Evidence based medicine: a movement in crisis? BMJ. 2014;348:g3725. doi: 10.1136/bmj.g3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving JA, Vasques DR, McGuinness TB. Krukenberg tumor of renal pelvic origin: report of a case with selected comments on ovarian tumors metastatic from the urinary tract. Int J Gynecol Pathol. 2006;25:147–150. doi: 10.1097/01.pgp.0000185405.08556.a0. [DOI] [PubMed] [Google Scholar]
- Kaijser J, Sayasneh A, Van Hoorde K, et al. Presurgical diagnosis of adnexal tumours using mathematical models and scoring systems: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:449. doi: 10.1093/humupd/dmt059. [DOI] [PubMed] [Google Scholar]
- Muyldermans K, Moerman P, Amant F, et al. Primary invasive mucinous ovarian carcinoma of the intestinal type: importance of the expansile vs infiltrative type in predicting recurrence and lymph node metastases. Eur J Cancer. 2013;49:1600–1608. doi: 10.1016/j.ejca.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Nunes N, Yazbek J, Ambler G, et al. A prospective evaluation of the IOTA Logistic Regression Model (LR2) for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2012;40:355–359. doi: 10.1002/uog.11088. [DOI] [PubMed] [Google Scholar]
- Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124:1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Sayasneh A, Kaijser J, Preisler J, et al. A multicenter prospective external validation of the diagnostic performance of IOTA simple descriptors and rules to characterize ovarian masses. Gynecol Oncol. 2013;130:140–146. doi: 10.1016/j.ygyno.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Sayasneh A, Wynants L, Preisler J. Multicenter external validation of IOTA prediction models and RMI by operators with varied training. Br J Cancer. 2013;108:2448–2454. doi: 10.1038/bjc.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW. Clinical prediction models: a practical approach to development, validation,and updating. New York: Springer; 2009. [Google Scholar]
- Testa A, Kaijser J, Wynants L, et al. Strategies to diagnose ovarian cancer: new evidence from phase 3 of the multicenter international IOTA study. Br J Cancer. 2014;111:680–688. doi: 10.1038/bjc.2014.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by the IOTA group. BMJ. 2010;341:c6839. doi: 10.1136/bmj.c6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman D, Testa AC, Bourne T, et al. Logistic regression model to distinguish between the benign and malignant adnexal mass before surgery: a multicenter study by the International Ovarian Tumor Analysis group. 2005;23:8794–8801. doi: 10.1200/JCO.2005.01.7632. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31:681–690. doi: 10.1002/uog.5365. [DOI] [PubMed] [Google Scholar]
- Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol. 2000;16:500–505. doi: 10.1046/j.1469-0705.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- Tinelli R, Tinelli A, Tinelli FG, et al. Conservative surgery for borderline ovarian tumors: a review. Gynecol Oncol. 2006;100:185–191. doi: 10.1016/j.ygyno.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Trimbos JB, Vergote I, Bolis G, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J Natl Cancer Inst. 2003;95:113–125. [PubMed] [Google Scholar]
- Van Calster B, Valentin L, Van Holsbeke C, et al. A novel approach to predict the likelihood of specific ovarian tumor pathology based on serum CA-125: a multicenter observational study. Cancer Epidemiol Biomarkers Prev. 2011;20:2420–2428. doi: 10.1158/1055-9965.EPI-11-0422. [DOI] [PubMed] [Google Scholar]
- Van Calster B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014;349:g5920. doi: 10.1136/bmj.g5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holsbeke C, Van Calster B, Bourne T, et al. External validation of diagnostic models to estimate the risk of malignancy in adnexal masses. Clin Cancer Res. 2012;18:815–825. doi: 10.1158/1078-0432.CCR-11-0879. [DOI] [PubMed] [Google Scholar]
- Van Holsbeke C, Van Calster B, Testa AC, et al. Prospective internal validation of mathematical models to predict malignancy in adnexal masses: results from the International Ovarian Tumor Analysis study. Clin Cancer Res. 2009;15:684–691. doi: 10.1158/1078-0432.CCR-08-0113. [DOI] [PubMed] [Google Scholar]
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176–182. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIc or IV ovarian cancer. New Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- Woo YL, Kyrgiou M, Bryant A, et al. Centralisation of services for gynaecological cancers – a Cochrane systematic review. Gynecol Oncol. 2012;126:286–290. doi: 10.1016/j.ygyno.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Wynants L, Timmerman D, Bourne T, et al. Screening for data clustering in multicenter studies: the residual intraclass correlation. BMC Med Res Methodol. 2013;13:128. doi: 10.1186/1471-2288-13-128. [DOI] [PMC free article] [PubMed] [Google Scholar]