Abstract
Ovarian cancer is the second most important pelvic gynaecologic malignancy and nowadays still kills 80% of patients. New treatment options are mandatory. Although it has been shown that ovarian cancer is an immunogenic tumor, the possibility of developing immunotherapy has been neglected for a long time. This article focuses on the importance of the immune system in the development and progression of cancer and the possibilities and problems of dendritic cell-based immunotherapy to influence the immune system.
Keywords: Ovarian cancer, immunotherapy, DC, dendritic cell, targeted therapy, cancer
Introduction
Ovarian cancer is the second most important pelvic gynaecologic tumor. According to the National Cancer Institute, the number of new cases is 12.3 per 100000 women per year. There are multiple histological subtypes of ovarian tumors, but 85% is epithelial in origin of which high-grade serous ovarian carcinomas are the most frequent ones. The major issue with ovarian cancer is that it can spread inside the abdomen before causing symptoms. Patients start showing symptoms once the tumor is metastasized over the peritoneum and causes the rise of ascites. As a silent killer, ovarian cancer is consequently most often diagnosed at an advanced stage with poor prognosis: 36-53 months at FIGO stage III and 20 months at stage IV. This is already a substantial increase compared to the situation 15 years ago. The reason is the improved surgical insights and combination with chemotherapy. Surgery is the cornerstone and should be radical to resect all macroscopic tumor burdens. Chemotherapy in the form of carboplatin-paclitaxel completes the primary treatment. In 2010, Vergote et al. (2010) could demonstrate that the overall survival was equally beneficial if chemotherapy was given first and then followed by surgery or vice versa. This important finding caused an enormous reduction in comorbidity due to radical surgery in the ovarian cancer patients with widespread disease. However, in case of relapse, therapeutic options are limited, especially if the relapse occurs within 6 months after completion of primary treatment. Consequently, nowadays, 80% of patients will still die of their disease.
It is clear that new treatments are necessary for ovarian cancer. The discovery of chemotherapy has certainly increased the survival of many patients in the past. However, adverse effects are inevitable and most tumors will reach a point of complete chemo-resistance. New treatments therefore have to be oriented differently. More and more attention is being paid to the targeted therapies. In these treatments, there is a focus present that is specific for the tumor or even for the tumor in a specific patient (personalized treatment). This focus can be variable: molecular, genetic, immunological ... For ovarian cancer, some international interest has already been shown, with for example an EU (European Commission)-approval for the use of Bevacizumab (Avastin®), an antibody against VEGF (vascular endothelial growth factor), in selected cases of ovarian cancer (Heitz et al., 2012). But there are many more possibilities in ovarian cancer to explore. This article will focus on the development of dendritic cell (DC) immunotherapy as a targeted treatment.
Cancer immunoediting: the natural process
In 2011, Schreiber et al. (2011) suggested a mechanism that likely takes place in the human body once malignant cells arise in an organ, based on the immune system. They called it the immunoediting concept. It exists of three major time points: elimination, equilibrium and escape. In the elimination phase the immune system will destroy early developing tumors. If the body succeeds, the case is closed. However, it might be that the eradication is not complete and a single cell remains in a dormant state, controlled by the immune system. This is the second time point, the equilibrium. However, at some point, the tumor cells can escape this and start multiplying. The tumor grows rapidly and will start to cause symptoms. This time point is called the escape and is facilitated by 2 possible pathways: 1. mechanisms that eventually cause antigen loss and 2. the infiltration of immunosuppressive cells, that will block an effective immune response.
One of the cells controlling tumor development in this proposed scheme is the DC. DC were discovered in 1976 by Ralph Steinman. They are professional antigen presenting cells, circulating in our body, and comprise less than 1% of all circulating white blood cells. In an immature phase (DCi) they can capture targets. In the tumor milieu, as described by Schreiber RD et al, these targets are Tumor Associated Antigens (TAA) presented by the tumor. By ingestion of the TAA, DC start to mature (DCm) and migrate to the lymph nodes, where they make contact with T cells that will than traffic to the target organ (the tumor) to selectively destroy the malignant cells.
Dendritic cell immunotherapy as an answer to escape
Cancer immunotherapy can be defined as the treatment of cancer by inducing, enhancing or suppressing the immune response. Traditionally, this was categorized in 2 groups, active or passive immunotherapy. In the active group, an immune response was to be expected from the body upon administration of the active immunotherapy, whereas in passive immunotherapy, a preformed antibody was given and no real reaction of the body was expected to exert full function. However, recently, this vision was critised. Galluzzi et al. (2014) published an overview on, according to the authors, ten existing immunotherapeutic strategies and how they do not fit in the historical picture of active and passive immunotherapy. The reality of all existing types of immunotherapy and their interactions with the human system and the tumor are far too complex to divide into two categories. Dendritic cell (DC) immunotherapy is one of these immunotherapeutic strategies, formerly classified in the group of active immunotherapy.
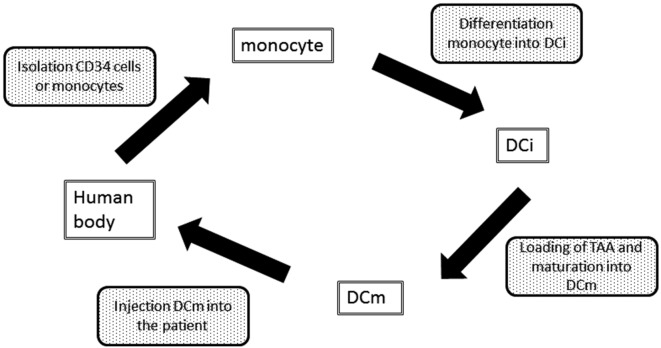
DC immunotherapy is an attempt to increase the number of efficient DCm (and consequently tumor-specific T cells) in order to shift the balance from immunosuppression towards immune surveillance or to reprogram the immune system away from the ‘escape’ phase towards the equilibrium or elimination phase (Fig. 1) (Gilboa, 2007). Although some reports are now being published on augmenting the already existing DC in the body, ex vivo DC culturing is nowadays still the state-of-the-art. Ex vivo DC immunotherapy can schematically be presented as shown in Figure 2. It is a laboratory process, starting from the patient’s own white blood cells. However, several variations are possible at each step of the process. Table I gives an overview of possible variations. Until now, none of them has clearly shown to be superior to the others, therefore, there is no international consensus in how to culture and inject DCm. Also, there is no agreement on the number of DCm that have to be injected and at what frequency.
Fig. 1. Schematic picture of the immune balance during cancer development and the effect that is hoped for by immunotherapy.
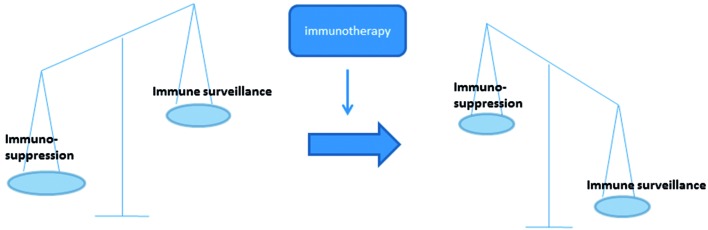
Fig. 2. Schematic picture of ex vivo dendritic cell immunotherapy.
DCi: Immature DC; DCm: mature DC; TAA: tumor-associated antigen
Table I. Non-limiting overview on possibilities in the ex vivo culturing process of dendritic cells.
| Step in the culturing process | Possibilities | |
| Initiation of the culture | From bone marrow CD34 precursor cells | |
| From monocytes | Plastic adherence | |
| CD14 bead selection | ||
| Counterflow centrifugal elutration | ||
| Culturing material | Plastic bags | |
| Adherent surface | Flasks or cell factories | |
| Non-adherent surface | ||
| Differentiation into DCi | IL-4 and GM-CSF | |
| IL-15 and GM-CSF | ||
| IFNα and GM-CSF | ||
| + addition of AA to the culturing medium | ||
| TAA | One specific TAA | Peptide or RNA |
| A few specific TAA | Peptide or RNA | |
| Total tumoral mRNA | ||
| Whole tumor cell lysate | obtained by irradiation, freeze-thaw cycles, ICD inducing agents | |
| Fusion cells composed of autologous DCs and tumor cells | ||
| Synthetic long peptides | ||
| Maturation DCi | TriMix electroporation | |
| One or more pro-inflammatory cytokines | TNFα, IL-1b, IL-6, IRX-2, CD137, IFNg | |
| + PGE2 | ||
| + TLR | ||
| + LPS | ||
| + PolyI:C | ||
| + addition of Rapamycin to the culturing medium | ||
| Culturing time | Ranging from 3-10 days | |
| Injection of DC | Intradermal | |
| Intravenous | ||
| Intranodal | ||
| Subcutaneous | ||
| Intratumoural | ||
AA: arachidonic acid; CD: cluster of differentiation; DC: dendritic cell; DCi: immature dendritic cell; GM-CSF: granulocyte macrophage colony-stimulating factor; ICD: immunogenic cell death; IL: interleukin; IFN: interferon; IRX-2: mix of cytokines; LPS: lipopolysaccharide; mRNA: messenger ribonucleic acid; PGE2: prostaglandin E2; TAA: tumor-associated antigen; TLR: toll-like receptor; TNF: tumor necrosis factor; TriMix: mRNA encoding CD70, CD40L and a constitutively active TLR4.
Immunosuppressive cells as an important cause of escape
Immunosuppressive cells are attracted to the tumor microenvironment due to the secretion of chemokines, cytokines and other mediators, produced by the tumor and immune cells present in and round the tumor. They will on their turn also start producing several mediators that will strengthen their effects and will cause more negative influence on other cells, leading to a downward spiral of effects all contributing to the escape of tumor cells from the immune surveillance. There are several players within this group of immunosuppressive cells of which the most relevant in pelvic gynaecological tumors currently are the regulatory T cells (Treg), the Myeloid-Derived Suppressor Cells (MDSC) and the Tumor-Associated Macrophages (TAM) (Baert et al., 2015).
As a consequence, DC immunotherapy should not only evoke a positive immune response, but also overcome these immunosuppressive effects and this appears to be a problem. The sole use of DC immunotherapy does not seem to be sufficient to create this answer to the escape. Large clinical effects are consequently still lacking when using DC immunotherapy as a targeted treatment. The last years, more and more attention is being paid to the concept of chemo-immunotherapy.
It might seem contradictory that chemotherapy will beneficially influence the immune system. For a long period of time, it was believed that chemotherapy only destroyed the white blood cells and therefore could certainly not contribute to the immune system in a positive way, until literature reports appeared showing a beneficial effect of chemotherapy (Antonia et al., 2006; Chu et al., 2012; Coosemans et al., 2013). The explanation is most probably multifactorial. The direct cytotoxic effect of chemotherapy destroys the tumor cells, which will lead to an increase in TAA. Moreover, some cytotoxic agents will also cause an Immunogenic Cell Death (ICD) of tumor cells, like etoposide and doxorubicin for example, leading to an increased secretion of DAMPs (Damage-Associated Molecular Patterns) (Vacchelli et al., 2014). Due to their immunostimulatory effects and their ability to lead to a better antigen presentation to DC, they increase the visibility of the massively released TAA to the immune system. On top of that, chemotherapy can render tumor cells more sensitive to granzyme, which is released by cytotoxic T cells and is able to start the process of programmed cell death in cancer cells. Chemotherapy can also increase the expression of Fas on tumor cells which makes them also visible for cytotoxic T cell mediated killing (Kadam and Abhang, 2015). But what about the decrease in white blood cells that is inextricably linked with chemotherapy? The majority of decreasing white blood cells are neutrophils that probably are not that important to establish an anti-tumor immune response. On the other hand, experiments have shown that the T cells that will experience a certain decrease and have to recover, will preferentially be the T cells that recognized the release TAA. Moreover, it has been shown that the decrease of white blood cells will also include a decrease in immunosuppressive cells. Every chemotherapy has its own specifications in this respect. For example, cyclophosphamide would be able to reduce Treg and gemcitabine is most probably able to reduce MDSC. Recently, a nice overview has been published (Galluzzi et al., 2012). Finally, the use of chemotherapy can also lead to an increased tumor infiltration of T cells (Mattarollo et al., 2011).
It is clear that the ideas and prejudices on chemotherapy have to be abandoned. However, it is not yet clear how, at what dose and at what time point chemotherapy should be integrated in the new immunotherapeutic options to create a powerful answer. This is the subject of further research.
Clinical studies on DC immunotherapy in ovarian cancer
Though still limited, there is some interest in the development of DC immunotherapy in ovarian cancer. Table IITable II gives an overview on existing studies. A total of 148 patients have been included in 13 studies over the past 14 years. As mentioned in table I, there is an enormous variety in the culturing process of the DC, which is confirmed when looking to these clinical data. Results vary tremendously. So far, only two groups have undertaken additional steps to influence the immunosuppressive cells (Chu et al., 2012; Kandalaft et al., 2013).
Table II. Overview on DC immunotherapy in ovarian cancer.
| Author | Tumorantigen | Type of immunotherapy | Number of patients | Clinical outcome |
| Brossart 2000 | MUC-1 or HER-2 | DC + peptide | 3 | 1 : SD > 8m; 1 SD during 8 w |
| Hernando 2002 | Lysate + KLH | DC + lysate and KLH | 6 | 3 SD |
| Loveland 2006 | mannan-MUC1 fusionprotein | DC + peptide | 1 | SD |
| Homma 2006 | Tumor cells | DC/tumor-fusie vaccin + rhIL-12 | 4 | Only available in 1 patient: PD with transient decease of CA125 |
| Hernando 2007 | α-FR | DC + mRNA-α-FR | 1 | PR |
| Peethambaram 2009 | HER-2 | Mix of PBMC and DC + a recombinant fusion antigen of HER2 | 4 | 2 SD |
| Chu 2012 | HER2-neu + hTERT + PADRE | DC + peptide +/- cyclophosphamide 2 days prior to vaccination + pneumococcal vaccination | 11 | 2 PD during vaccination, 3 PD between 6-26 m follow up, 6 CR |
| Rahma 2012 | P53 | Peptide + IL2 SC vs DC + peptide + IL2 IV | 21 | 4 NED after 2y, 16 PD |
| Kandalaft 2013 | Lysate | A/ In 6 patients: IV bevacizumab + metronomic cyclophosphamide PO, followed by bevacizumab + lysate loaded DC B/ In 3/6 patients this was followed by 1/ lymphodepletion + 2/ transfer of autologous T cells in combination with the vaccin |
6 | A/ 2 PR, 2 SD, 2 PD B/ 1 PR, 1 SD, 1 PD |
| Coosemans 2013 | WT1 | DC + mRNA-WT1 | 2 | PD with prolonged OS if chemotherapy was administered after immunotherapy stop |
| Mitchell 2014 | MUC-1 | DC + peptide | 26 | Decrease in CA125 in 5 patients, of which 2 PD, 2 PR, 1 CR |
| Kobayashi 2014 | WT1, MUC-1, CA125 | DC + peptide | 56 | After 3m: 32 PD, 14 SD, 2 PR, 8 not evaluable |
| Bapsy 2014 | Lysate | DC + lysate IV | 7 | 4 PD, 2 SD, 1 PR |
CR, complete remission; CA125, cancer antigen 125; DC, dendritic cell; IL, interleukin; KHL, keyhole limpet hemocyanin; MUC-1, mucin 1; mRNA, messenger ribonucleic acid; NED, no evidence of disease; PBMC, peripheral blood mononuclear cell; OS, overall survival; PD, progressive disease; PR, partial remission; rh, recombinant human; SC: subcutaneous; SD, stable disease; TAA, tumor-associated antigen; TERT, telomerase reverse transcriptase; WT1, Wilms’ tumor gene 1; y, year; m, month; w, weeks;SC, subcutaneous; IV, intravenous; PO, orally; HER-2, human epidermal growth factor 2; PADRE, DR-restricted Th helper epitope.
Conclusion
Nowadays, 80% of ovarian cancer patients still die of their disease. There is an urgent need for new therapies. Immunotherapy has been neglected for a long time as a substantial candidate in ovarian cancer. Today, 13 clinical studies on DC immunotherapy are available. However, at the same time it also becomes clear that DC immunotherapy alone will not be able to induce an adequate immune response and shift the immune balance again towards immune surveillance. Immunosuppressive cells hampering this immune response appear to be very important players. The association of chemotherapy with DC immunotherapy could offer a possibility in overcoming this immunosuppression. Further studies will be needed to explore what chemotherapy at what dose and time point in the conventional treatment of the patient will be most beneficial.
References
- Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive-stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- Baert T, Timmerman D, Vergote I, et al. Immunological parameters as a new lead in the diagnosis of ovarian cancer. Facts Views Vis Obgyn. 2015;1:67–72. [PMC free article] [PubMed] [Google Scholar]
- Bapsy PP, Sharan B, Kumar C, et al. Open-label, multi-center, non-randomized, single-arm study to evaluate the safety and efficacy of dendritic cell immunotherapy in patients with refractory solid malignancies, on supportive care. Cytotherapy. 2014;16:234–244. doi: 10.1016/j.jcyt.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Brossart P, Wirths S, Stuhler G, et al. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- Chu CS, Boyer J, Schullery DS, et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunol Immunother. 2012;61:629–641. doi: 10.1007/s00262-011-1081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coosemans A, Vanderstraeten A, Tuyaerts S, et al. Immunological response after WT1 mRNA-loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Res. 2013;33:3855–3859. [PubMed] [Google Scholar]
- Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Vacchelli E, Bravo San Pedro J, et al. Classification of current anticancer immunotherapies. Oncotarget. 2014;5:12472–12508. doi: 10.18632/oncotarget.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando JJ, Park TW, Kübler K, et al. Vaccination with autologous tumour antigen-pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunol Immunother. 2002;51:45–52. doi: 10.1007/s00262-001-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando JJ, Park TW, Fischer HP, et al. Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-alpha for relapsed metastatic ovarian cancer. Lancet Oncol. 2007;8:451–454. doi: 10.1016/S1470-2045(07)70142-0. [DOI] [PubMed] [Google Scholar]
- Heitz F, Harter P, Barinoff B, et al. Bevacizumab in the treatment of ovarian cancer. Adv Ther. 2012;29:723–735. doi: 10.1007/s12325-012-0041-9. [DOI] [PubMed] [Google Scholar]
- Homma S, Sagawa Y, Ito M, et al. Cancer immunotherapy using dendritic/tumour-fusion vaccine induces elevation of serum anti-nuclear antibody with better clinical responses. Clin Exp Immunol. 2006;144:41–47. doi: 10.1111/j.1365-2249.2006.03029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam CY, Abhang SA. Serum levels of soluble Fas ligand, granzyme B and cytochrome c during adjuvant chemotherapy of breast cancer. Clin Chim Acta. 2015;438:98–102. doi: 10.1016/j.cca.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Kandalaft LE, Powell DR, Jr, Chiang CL, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Chiba A, Izawa H, et al. The feasibility and clinical effects of dendritic cell-based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. J Ovarian Res. 2014;7:48.: doi: 10.1186/1757-2215-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland BE, Zhao A, White S, et al. Mannun-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- Mattarollo SR, Loi S, Duret H, et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 2011;71:4809–4820. doi: 10.1158/0008-5472.CAN-11-0753. [DOI] [PubMed] [Google Scholar]
- Mitchell PL, Quinn MA, Grant PT, et al. A phase 2, single-arm study of an autologous dendritic cell treatment against mucin 1 in patients with advanced epithelial ovarian cancer. J Immunother Cancer. 2014;2:16. doi: 10.1186/2051-1426-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peethambaram PP, Melisko ME, Rinn KJ, et al. A phase I trial of immunotherapy with lapuleucel-T (APC8024) in patients with refractory metastatic tumors that express HER-2/neu. Clin Cancer Res. 2009;15:5937–5944. doi: 10.1158/1078-0432.CCR-08-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahma OE, Ashtar E, Czystowska M, et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother. 2012;61:373–384. doi: 10.1007/s00262-011-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2014;3 doi: 10.4161/onci.27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIc or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]