Abstract
Ebola virus (Zaire ebolavirus; EBOV) is a highly lethal hemorrhagic disease virus that most recently was responsible for two independent 2014 outbreaks in multiple countries in Western Africa, and the Democratic Republic of the Congo, respectively. Herein, we show that a cytomegalovirus (CMV)-based vaccine provides durable protective immunity from Ebola virus following a single vaccine dose. This study has implications for human vaccination against ebolaviruses, as well as for development of a ‘disseminating’ vaccine to target these viruses in wild African great apes.
The original zoonotic source of the 2014 Ebola virus (Zaire ebolavirus; EBOV) outbreak in Western Africa is currently unclear (1, 2). Following transmission into the human population, the chain of ebolavirus infection is maintained by human-to-human transmission. Contact with wild animals serves as a main conduit for the initial zoonotic transmission of ebolaviruses into the human population (2–7). Fruit bats are believed to be one potential source of human infection, and direct contact or exposure to environments inhabited and frequented by bats has been associated with human outbreaks (2, 4, 7). Great apes (western lowland gorillas and chimpanzees) are a second significant source of transmission due, in large part, to the bushmeat trade which drives humans and wild animals together within an environment conducive to zoonotic transmission (i.e., hunting and butchering) (3–5). Consistent with the importance of this route for zoonotic ebolavirus transmission, a 2014 EBOV outbreak in the Boende Health Zone in the Equateur Province in the Democratic Republic of Congo (DRC), independent from the West Africa epidemic, was a result of handling and preparation of bushmeat (8). Ebolaviruses are also highly lethal in African great apes, and are regarded as a major threat to the survival of chimpanzees and gorillas in the wild (3, 5, 9–12).
Vaccination of great apes has been proposed as one strategy to decrease the transmission of ebolaviruses to humans, whilst at the same time also protecting these wild animal populations from the devastating effects of these viruses (4, 13, 14). We recently proposed the use of a cytomegalovirus (CMV)-based ‘disseminating’ vaccine as one approach to achieve vaccine coverage in the inaccessible and hostile environment of African tropical forest regions, where application of conventional vaccines using baiting/individual darting strategies may prove more difficult, if not impossible (14). CMV is a species-specific β-herpesvirus that is benign except in the immunocompromised host, such as individuals undergoing iatrogenic immunosuppression, AIDS patients (prior to HAART) and the neonate (15). CMV is also highly immunogenic, and has shown promise for development as a vaccine vector platform (16–20). We hypothesize that amongst other ebolavirus vaccine platforms, the established ability of CMV to spread easily through its host population regardless of CMV immune status (14, 21–24) makes this vector platform suited for development as a ‘disseminating’ ebolavirus vaccine that could spread ebolavirus-specific immunity from animal-to-animal without the need for direct vaccination of every individual.
CMVs are extremely host specific (25, 26). In a previous study we showed the ability of a single dose of a murine CMV (MCMV) expressing a CD8 T cell epitope from nucleoprotein (NP) of EBOV (designated MCMV/ZEBOV-NPCTL) to induce durable EBOV-specific CD8+ T cell immunity for at least 33 weeks (> 8 months) post-vaccination (14). In this earlier study, mice vaccinated with MCMV/ZEBOV-NPCTL were protected against disease when challenged with a lethal dose of mouse-adapted EBOV (Mayinga isolate) (ma-EBOV) at 6 weeks post-boost. Previous studies using MCMV recombinants expressing pathogen target epitopes (influenza A and lymphocytic choriomeningitis virus) have shown long-lasting protective immunity (27). In the current study, we wanted to assess whether MCMV/ZEBOV-NPCTL was able to afford durable protective immunity against a lethal EBOV challenge after only a single vaccine dose. We reasoned that the capacity to provide such long-lasting protective immunity would be an attractive if not essential quality for development of CMV as either a ‘disseminating’ vaccine for use in wild African great ape populations, or as a human CMV-based vaccine for conventional use.
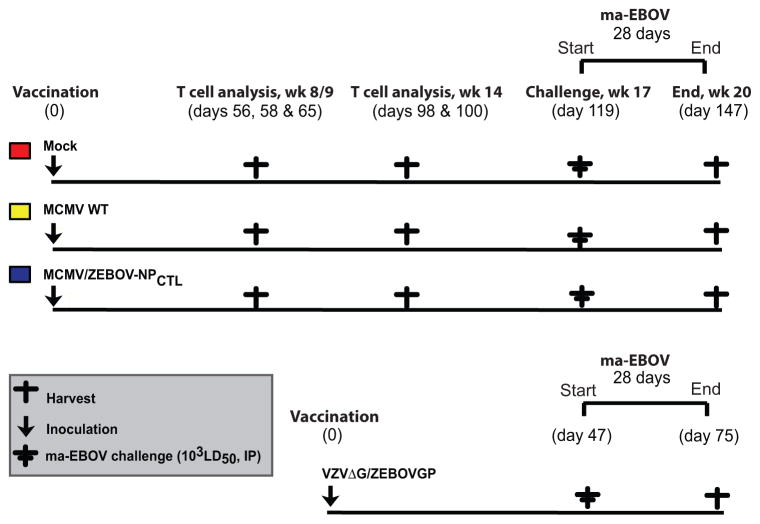
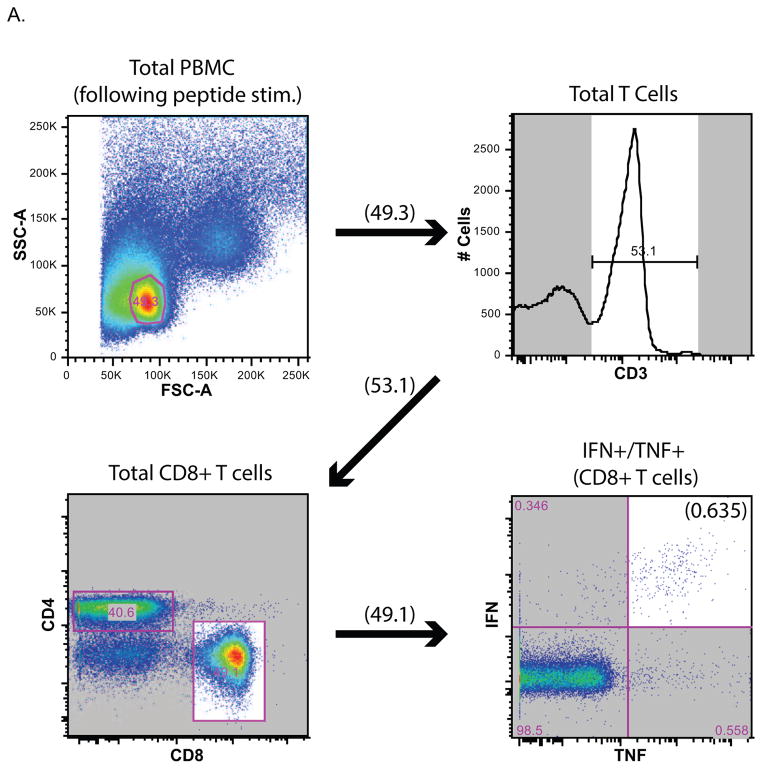
Figure 1 shows a schematic of the mouse-adapted (ma)-EBOV challenge study using MCMV/ZEBOV-NPCTL vaccinated mice. Animal use complied with the Guide for the Use and Care of Laboratory Animals, USDA Animal Welfare Regulations, PHS Policy on Humane Care and Use of Laboratory Animals and other relevant regulations. All procedures received prior approval by IACUC committees at RML, DIR, NIAID, NIH and OHSU. To assess whether vaccine-induced immunity provided durable protection, we challenged mice at 119 days (17 weeks) post-vaccination. This time of challenge was based on the observation that most previous mouse studies (ours included (14)) have only looked at short-term protection, within 6 weeks following the last vaccine dose (28–30). Briefly, female C57BL/6 mice were vaccinated intra-peritoneally (IP) with either MCMV/ZEBOV-NPCTL (Clone 5A1) (5×105 plaque-forming units, pfu), parental MCMV wild-type (MCMV WT), or vaccine diluent (2% FBS in DPBS) (Mock). Excepting a mouse receiving MCMV WT (which died during the vaccine phase) CD8+ T cell responses were assessed in mice (n = 4–5) 8/9 and 14 weeks after vaccination (Figure 2B & C). The gating strategy is shown for a representative MCMV/ZEBOV-NPCTL vaccinated mouse in Figure 2A. Consistent with our earlier study, MCMV/ZEBOV-NPCTL induced EBOV NP-specific CD8+ T cells, which were not observed in either MCMV WT or Mock controls. All MCMV WT and MCMV/ZEBOV-NPCTL, but not Mock groups also had responses against MCMV endogenous proteins M38 and M45 as expected.
Figure 1. Schematic showing mouse groups and sampling regimen in ma-EBOV challenge study of MCMV/ZEBOV-NPCTL.
C57BL/6 (H2b-restricted) mice were immunized using a single IP dose of 5×105 pfu of MCMV/ZEBOV-NPCTL. Control groups received MCMV WT or diluent (Mock). Splenocytes were harvested for analysis of T cell responses in groups of mice at times indicated (week 8/9: days 56, 58, 65 post-vaccination, and prior to challenge: days 96 and 100 post-vaccination). Antigen specific T cells were assayed by using ICS with a 6 hour incubation in the presence of BFA with peptide. After 119 days (approx. 4 months) post-vaccination, mice were challenged with 1×103 LD50 ma-EBOV IP and disease course was followed for 28 days. VZVΔG/ZEBOVGP vaccinated mice served as a vaccine efficacy control group, and received a single IP dose of VZVΔG/ZEBOVGP (5×105 pfu) prior to the ma-EBOV challenge (47 days later).
Figure 2. CD8+ T cell responses following immunization with MCMV/ZEBOVCTL.
Female C57BL/6 H2b-restricted mice were immunized IP using a single inoculation of 5×105 pfu of MCMV/ZEBOV-NPCTL. Control groups received MCMV WT (5×105 pfu) or diluent (Mock). Splenocytes were harvested for analysis of T cell responses. (A) Schematic showing gating strategy for ICS. NP-specific T cells for a representative MCMV/ZEBOV-NPCTL vaccinated mouse is shown. (B) 8/9 weeks (days 56, 58 and 65 post-vaccination), and (C) week 14 (days 98 and 100 post-vaccination). T cells were analyzed by using ICS with a 6 hour incubation in the presence of BFA with indicated peptide as previously described (14). Human prostate-specific antigen (PSA) is an irrelevant control peptide (20), and NP (peptide pool) is an overlapping peptide pool (15-mer, 5 amino acid overlap) representing the full length EBOV NP protein. Levels of responding (IFNγ and TNFα double-positive) CD8+ T cells in individual mice are shown. All mice receiving MCMV had CD8+ T cell responses against MCMV M38 and M45, MCMV endogenous ‘inflationary’ and ‘non-inflationary’ antigens, respectively. Mock-infected mice showed no MCMV-specific T cell responses as expected. All MCMV/ZEBOV-NPCTL immunized mice showed significant CD8-restricted T cell responses against the NP target antigen (2-tailed t-test, p<0.05) consistent with previous results (14). All mice were 29 weeks old at time of vaccination other than the Mock group assessed at Week 14, which were 21 weeks old. ●= not tested.
At week 17 (approx. 4 months) post-vaccination, age-matched mice (n=14) were challenged with 1×103 LD50 ma-EBOV (IP). An additional control group of mice (n=14) received the ‘benchmark’ VSVΔG/ZEBOVGP vaccine (31) to serve as a vaccine efficacy control. Vaccine efficacy was assessed on the basis of morbidity (clinical symptoms and weight loss) and survival (Figure 3). Weight was monitored in mice until day 17 post-challenge, or until all animals had succumbed to EBOV disease. Surviving mice were then followed until 28 days post-challenge, at which time they were humanely euthanized. All MCMV WT and Mock control mice showed signs of severe ma-EBOV disease with clinical symptomology (ruffled hair, reduced mobility and weight loss). 100% of Mock and 90% of MCMV WT mouse groups were euthanized as a result of reaching a pre-defined humane endpoint of EBOV-associated disease by day 7 post-challenge (Figure 3A). In contrast, no EBOV disease was observed in MCMV/ZEBOV-NPCTL vaccinated mice. Although not statistically significant, MCMV/ZEBOV-NPCTL vaccinated mice did show a slight loss in weight suggesting that immunity was not sterilizing in all mice (Figure 3B), which is consistent with results from the earlier study (14). Together, these results indicate that a CMV-based EBOV vaccine can provide long-term protection from EBOV-associated disease and mortality following only a single inoculation at least 119 days (approx. 4 months) post-vaccination.
Figure 3. Efficacy of MCMV/ZEBOV-NPCTL vector against ma-EBOV challenge following a single inoculation at day −119.
Age matched groups of C57BL/6 mice (n=10) were vaccinated with a single IP administration of 5×105 pfu of MCMV/ZEBOV-NPCTL. Additional groups received either diluent (Mock), or VSVΔG/ZEBOVGP (positive control forvaccine efficacy, given 47 days prior to challenge). After 119 days, mice were challenged with 103 LD50 ma-EBOV (IP). Data represent (A) Percent survival. (B) Body weight change over time post-challenge. For body weight, groups were weighed daily until 17 days post-EBOV challenge, or until all animals in a group had succumb to EBOV disease. Vaccinated mice that survived challenge were then monitored until day 28 post-challenge, at which time they were humanely euthanized. Vaccination with MCMV/ZEBOV-NPCTL had a significant impact on survival from ma-EBOV challenge compared to MCMV WT control (p <0.0001) using a Log-rank (Mantel-Cox) Test. MCMV WT and Mock groups showed a significant decrease in bodyweight compared to MCMV/ZEBOV-NPCTL (p-value at least <.05) from day 3 onwards using a one-tailed t-test. No significant differences were seen in body weight between MCMV/ZEBOV-NPCTL and VSVΔG/ZEBOVGP groups at any time post-challenge. All mice were 21 weeks old at time of vaccination.
Although a role for antibodies cannot be formally discounted in this protection, the expression of only a single CD8 T cell EBOV epitope by MCMV/ZEBOV-NPCTL, the absence of detectable EBOV antibodies in vaccinated mice prior to challenge (Table 1) and the presence of EBOV NP-specific CD8+ T cell responses (Figure 2) are consistent with the mode of protection induced by the CMV vector as being primarily T cell mediated. CMV has been shown to induce T cell responses shifted towards ‘effector’ memory (TEM) that are primed for immediate ‘effector’ function at mucosal/epithelial tissue sites (32–34). We previously showed that EBOV NP CD8+ T cell responses had TEM characteristics based on similarity in kinetics of expansion as a MCMV ‘inflationary’ endogenous protein (M38) (14). Using the same study group from this earlier published study (Figure 2 in (14)), splenocytes were harvested at days 442 and 444 (> 14 months) following a single MCMV/ZEBOV-NPCTL IP vaccination (1×105 pfu). We monitored the expression of KLRG-1, a marker consistently upregulated to high levels on TEM but not on TCM antigen-specific CD8+ T cells (36, 37). As shown in Figure 4, EBOV NP-specific CD8+ T cell responses were comparable to the TEM-biased responses directed against M38 rather than to the central memory (TCM) responses against M45.
Table 1. Total anti-EBOV VLP IgG antibody titre in mouse blood samples pre- and post-challenge.
VLPs (GP/NP/VP40) were used as the source of antigen. Pre-challenge Mock samples were used to establish background values. Samples were deemed positive if the signal was greater than the mean of pre-challenge Mock values plus four standard deviations. An ‘in house’ anti-VP40 antibody was used as the positive control. NT = not tested. Samples from 4 mice of each experimental group were analyzed.
| Vaccination | Pre-challenge | # Post-challenge |
|---|---|---|
| Anti-VP40 (positive control) | 25600 | |
| VSVΔG/ZEBOVGP (control) | 6400 | NT |
| VSVΔG/ZEBOVGP (control) | 6400 | NT |
| VSVΔG/ZEBOVGP (control) | 1600 | NT |
| VSV ΔG/ZEBOVGP (control) | 1600 | NT |
| MCMV WT | Neg | NT |
| MCMV WT | Neg | NT |
| MCMV WT | Neg | NT |
| MCMV WT | Neg | NT |
| MCMV/ZEBOV-NP(CTL) | Neg | NT |
| MCMV/ZEBOV-NP(CTL) | Neg | NT |
| MCMV/ZEBOV-NP(CTL) | Neg | NT |
| MCMV/ZEBOV-NP(CTL) | Neg | NT |
Figure 4. MCMV/EBOV-NPCTL induces TEM-biased responses against EBOV NP.
129S1/SvlmJ/Cr H2b-restricted mice were immunized (IP) with a single dose (1×105 pfu) of MCMV/ZEBOV-NPCTL (clone 5D1). These mice are the same groups that were serially followed for T cell responses through week 33 post-vaccination in reference (14). (A) At days 442 and 444 (> 14 months) post-vaccination, splenocytes were harvested and CD8+ T cell responses were determined by ICS using a 6 hour incubation in the presence of BFA with peptides (NP, M38 or M45). (B) EBOV NP-specific CD8+ T cell (IFN+/TNF+) responses were characterized into TEM and TCM on the basis of CD44 and KLRG-1 expression. M38 and M45 responses served as controls for TEM and TCM-biased responses, respectively. All responses were normalized against cells incubated in the absence of peptide. Typical response (B and C) and (D) average responses in total mice tested (n=6) with SD shown. Populations were compared using 1-way ANOVA with Bonferroni’s Post Test.
In summary, we show that a CMV-based ebolavirus vaccine can provide durable immunity for at least 119 days following only a single vaccine dose. These findings have important implications for development of CMV as a disseminating vaccine to prevent ebolavirus in great apes, and possibly a non-disseminating, conventional human CMV (HCMV)-based ebolavirus vaccine for humans. Studies ongoing will determine whether these results translate to protection in the macaque EBOV challenge model, regarded as the ‘gold standard’ for vaccine efficacy assessment in a model representative of ebolavirus infection in great apes, including humans.
Acknowledgments
We thank Dr U. Koszinowski (Max von Pettenkofer-Institute, Ludwig-Maximilians-University, Germany) for providing the pSMfr3 MCMV BAC, and Dr D. Court (NCI-Frederick, MD) for providing the lambda-based recombination system used to construct the original MCMV/ZEBOV-NPCTL construct. We appreciate K. Marshall (VGTI, OR) and J. Bailey (NIAID, MT) for their organization and coordination of animals used in the study. We also thank the members of Rocky Mountain Veterinary Branch (DIR, NIAID, NIH) for assistance with animal care. Finally, we thank Drs H. Ebihara (DIR, NIAID, NIH), A. Marzi (DIR, NIAID, NIH), P. Barry (University of California at Davis, CA), M. Cranfield (Mountain Gorilla Veterinary Project, Baltimore, MD) for insightful discussions. This study was supported by R21 (AI088442) and the Intramural Research Program of the NIAID, NIH; and University of Plymouth, School of Biomedical and Healthcare Sciences internal funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gatherer D. The 2014 Ebola virus disease outbreak in West Africa. J Gen Virol. 2014;95:1619–1624. doi: 10.1099/vir.0.067199-0. [DOI] [PubMed] [Google Scholar]
- 2.Mari Saez A, Weiss S, Nowak K, Lapeyre V, Zimmermann F, Dux A, Kuhl HS, Kaba M, Regnaut S, Merkel K, Sachse A, Thiesen U, Villanyi L, Boesch C, Dabrowski PW, Radonic A, Nitsche A, Leendertz SA, Petterson S, Becker S, Krahling V, Couacy-Hymann E, Akoua-Koffi C, Weber N, Schaade L, Fahr J, Borchert M, Gogarten JF, Calvignac-Spencer S, Leendertz FH. Investigating the zoonotic origin of the West African Ebola epidemic. EMBO Mol Med. 2014;7:17–23. doi: 10.15252/emmm.201404792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizkalla C, Blanco-Silva F, Gruver S. Modeling the impact of Ebola and bushmeat hunting on Western Lowland Gorillas. EcoHealth. 2007;4:151–155. [Google Scholar]
- 4.Groseth A, Feldmann H, Strong JE. The ecology of Ebola virus. Trends Microbiol. 2007;15:408–416. doi: 10.1016/j.tim.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, Froment JM, Bermejo M, Smit S, Karesh W, Swanepoel R, Zaki SR, Rollin PE. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–390. doi: 10.1126/science.1092528. [DOI] [PubMed] [Google Scholar]
- 6.Rouquet P, Froment JM, Bermejo M, Kilbourn A, Karesh W, Reed P, Kumulungui B, Yaba P, Delicat A, Rollin PE, Leroy EM. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg Infect Dis. 2005;11:283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leroy EM, Kumulungui B, Pourrut X, Rouquet P, Hassanin A, Yaba P, Delicat A, Paweska JT, Gonzalez JP, Swanepoel R. Fruit bats as reservoirs of Ebola virus. Nature. 2005;438:575–576. doi: 10.1038/438575a. [DOI] [PubMed] [Google Scholar]
- 8.Prevention CfDCa. Alert - level 2, Practice Enhanced Precautions. 2014. Ebola in Democratic Republic of the Congo. posting date. [Online.] [Google Scholar]
- 9.Walsh PD, Abernethy KA, Bermejo M, Beyers R, De Wachter P, Akou ME, Huijbregts B, Mambounga DI, Toham AK, Kilbourn AM, Lahm SA, Latour S, Maisels F, Mbina C, Mihindou Y, Obiang SN, Effa EN, Starkey MP, Telfer P, Thibault M, Tutin CE, White LJ, Wilkie DS. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- 10.Bermejo M, Rodriguez-Teijeiro JD, Illera G, Barroso A, Vila C, Walsh PD. Ebola outbreak killed 5000 gorillas. Science. 2006;314:1564. doi: 10.1126/science.1133105. [DOI] [PubMed] [Google Scholar]
- 11.Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, Walker F, Le Guenno B. Ebola virus outbreak among wild chimpanzees living in a rain forest of Cote d’Ivoire. J Infect Dis. 1999;179(Suppl 1):S120–126. doi: 10.1086/514296. [DOI] [PubMed] [Google Scholar]
- 12.Ryan SJ, Walsh PD. Consequences of non-intervention for infectious disease in African great apes. PLoS One. 2011;6:e29030. doi: 10.1371/journal.pone.0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warfield KL, Goetzmann JE, Biggins JE, Kasda MB, Unfer RC, Vu H, Aman MJ, Olinger GG, Jr, Walsh PD. Vaccinating captive chimpanzees to save wild chimpanzees. Proc Nat Acad Sci USA. 2014;111:8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis. 2011;5:e1275. doi: 10.1371/journal.pntd.0001275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocarski ESJ, Shenk T, Pass RF. Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology. 5. Vol. 2. Lippincott-Raven Publishers; Philadelphia: 2007. pp. 2701–2772. [Google Scholar]
- 16.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, Legasse AW, Chiuchiolo MJ, Parks CL, Axthelm MK, Nelson JA, Jarvis MA, Piatak M, Jr, Lifson JD, Picker LJ. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–527. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis MA, Hansen SG, Nelson JA, Picker LJ, Frueh K. Vaccine vectors using the unique biology and immunology of cytomegalovirus. In: Reddehase MJ, editor. Cytomegaloviruses: From Molecular Pathogenesis to Intervention. II. Caister Academic Press; 2012. [Google Scholar]
- 18.Redwood AJ, Messerle M, Harvey NL, Hardy CM, Koszinowski UH, Lawson MA, Shellam GR. Use of a murine cytomegalovirus K181-derived bacterial artificial chromosome as a vaccine vector for immunocontraception. J Virol. 2005;79:2998–3008. doi: 10.1128/JVI.79.5.2998-3008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney R, Nakai T, Parkins CJ, Caposio P, Fairweather NF, Sesardic D, Jarvis MA. A single-dose cytomegalovirus-based vaccine encoding tetanus toxin fragment C induces sustained levels of protective tetanus toxin antibodies in mice. Vaccine. 2012;30:3047–3052. doi: 10.1016/j.vaccine.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 20.Klyushnenkova EN, Kouiavskaia DV, Parkins CJ, Caposio P, Botto S, Alexander RB, Jarvis MA. A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer. J Immunother. 2012;35:390–399. doi: 10.1097/CJI.0b013e3182585d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross SA, Arora N, Novak Z, Fowler KB, Britt WJ, Boppana SB. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis. 2010;201:386–389. doi: 10.1086/649903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farroway LN, Gorman S, Lawson MA, Harvey NL, Jones DA, Shellam GR, Singleton GR. Transmission of two Australian strains of murine cytomegalovirus (MCMV) in enclosure populations of house mice (Mus domesticus) Epidemiol Infect. 2005;133:701–710. doi: 10.1017/s0950268805003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 24.Hansen SG, Powers CJ, Richards R, Ventura AB, Ford JC, Siess D, Axthelm MK, Nelson JA, Jarvis MA, Picker LJ, Fruh K. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 2010;328:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy S, Couacy-Hymann E, Metzger S, Nowak K, De Nys H, Boesch C, Wittig R, Jarvis MA, Leendertz FH, Ehlers B. Absence of frequent herpesvirus transmission in a nonhuman primate predator-prey system in the wild. J Virol. 2013;87:10651–10659. doi: 10.1128/JVI.01104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern ER. Pivotal role of animal models in the development of new therapies for cytomegalovirus infections. Antiviral Res. 2006;71:164–171. doi: 10.1016/j.antiviral.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Karrer U, Wagner M, Sierro S, Oxenius A, Hengel H, Dumrese T, Freigang S, Koszinowski UH, Phillips RE, Klenerman P. Expansion of protective CD8+ T-cell responses driven by recombinant cytomegaloviruses. J Virol. 2004;78:2255–2264. doi: 10.1128/JVI.78.5.2255-2264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong G, Richardson JS, Pillet S, Patel A, Qiu X, Alimonti J, Hogan J, Zhang Y, Takada A, Feldmann H, Kobinger GP. Immune parameters correlate with protection against ebola virus infection in rodents and nonhuman primates. Science Transl Med. 2012;4:158ra146. doi: 10.1126/scitranslmed.3004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bukreyev A, Marzi A, Feldmann F, Zhang L, Yang L, Ward JM, Dorward DW, Pickles RJ, Murphy BR, Feldmann H, Collins PL. Chimeric human parainfluenza virus bearing the Ebola virus glycoprotein as the sole surface protein is immunogenic and highly protective against Ebola virus challenge. Virol. 2009;383:348–361. doi: 10.1016/j.virol.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Raja NU, Trubey CM, Juompan LY, Luo M, Woraratanadharm J, Deitz SB, Yu H, Swain BM, Moore KM, Pratt WD, Hart MK, Dong JY. Development of a cAdVax-based bivalent ebola virus vaccine that induces immune responses against both the Sudan and Zaire species of Ebola virus. J Virol. 2006;80:2738–2746. doi: 10.1128/JVI.80.6.2738-2746.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, Daddario KM, Hensley LE, Jahrling PB, Geisbert TW. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 32.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munks MW, Cho KS, Pinto AK, Sierro S, Klenerman P, Hill AB. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J Immunol. 2006;177:450–458. doi: 10.4049/jimmunol.177.1.450. [DOI] [PubMed] [Google Scholar]
- 34.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–299. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baaten BJ, Tinoco R, Chen AT, Bradley LM. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Front Immunol. 2012;3:23. doi: 10.3389/fimmu.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cush SS, Flano E. KLRG1+NKG2A+ CD8 T cells mediate protection and participate in memory responses during gamma-herpesvirus infection. J Immunol. 2011;186:4051–4058. doi: 10.4049/jimmunol.1003122. [DOI] [PMC free article] [PubMed] [Google Scholar]