Abstract
Background:
Circulating tumour cells (CTC) in the blood have been accepted as a prognostic marker in patients with metastatic colorectal cancer (CRC). Only limited data exist on the prognostic impact of CTC in patients with early stage CRC using standardised detection assays. The aim of this study was to elucidate the role of CTC in patients with non-metastatic CRC.
Methods:
A total of 287 patients with potentially curable CRC were enrolled, including 239 patients with UICC stage I–III. CTC were measured in the blood using the CellSearch system preoperatively and on postoperative days 3 and 7. The complete patient group (UICC I–IV) and the non-metastatic cohort (UICC I–III) were analysed independently. Patients were followed for 28 (0–53) months. Prognostic factors for overall and progression-free survival were analysed using univariate and multivariate analyses.
Results:
CTC were detected more frequently in patients with metastatic disease. No clinicopathological variables were associated with CTC detection in non-metastatic patients. CTC detection (⩾1 CTC per 7.5 ml blood) in the blood was significantly associated with worse overall survival (49.8 vs 38.4 months; P<0.001) in the non-metastatic group (UICC I–III), as well as in the complete cohort (48.4 vs 33.6 months; P<0.001). On multivariate analysis CTC were the strongest prognostic factor in non-metastatic patients (hazard ratio (HR) 5.5; 95% confidence interval (CI) 2.3–13.6) as well as in the entire study group (HR 5.6; 95% CI 2.6–12.0).
Conclusions:
Preoperative CTC detection is a strong and independent prognostic marker in non-metastatic CRC.
Keywords: CTC, DTC, circulating tumour cells, colorectal cancer, minimal residual disease
Although tremendous efforts in diagnosis and treatment have been made, colorectal cancer (CRC) remains the fourth most common cause of cancer-related death with 1 million new cases and 500 000 deaths worldwide each year (Weitz et al, 2005; Cunningham et al, 2010; Brenner et al, 2014). In theory, early stage CRC is cured by surgical resection alone and UICC stage I and II patients are only offered adjuvant chemotherapy in case of risk factors (e.g., intraoperative tumour perforation). UICC stage III patients are routinely recommended adjuvant chemotherapy according to current guidelines (Engstrom et al, 2009; Labianca et al, 2010). However, up to 25% of these patients will develop recurrent disease and current selection criteria for high-risk patients remain insufficient (Weitz et al, 2005; Engstrom et al, 2009; O'Connor et al, 2011).
The prognostic impact of circulating tumour cells (CTC) in the blood of patients with CRC has been studied extensively and was recently confirmed by a meta-analysis (Rahbari et al, 2010). However, different non-standardised detection methods of CTC have made inter-study comparisons difficult, and only limited data exists about the prognostic role of CTC in patients with early stage CRC (Ito et al, 2002; Iinuma et al, 2006, 2011; Lloyd et al, 2006; Allen-Mersh et al, 2007; Sadahiro et al, 2007; Wang et al, 2007; Uen et al, 2008; Maestro et al, 2009; Wong et al, 2009; Lu et al, 2011; Thorsteinsson and Jess, 2011; Thorsteinsson et al, 2011). The Food and Drug Administration has approved the CellSearch system for CTC monitoring in patients with metastatic colorectal, prostate and breast cancers (Cohen et al, 2006, 2008, 2009; Miller et al, 2010; Sastre et al, 2012). Introduction of this semi-automatic assay allows for detection and enumeration of CTC in a standardised fashion. However, data on CTC as a predictive and prognostic marker in CRC patients have so far been exclusively available for patients with metastatic disease. Recently, it has been reported that detection of CTC in the blood of patients with non-metastatic breast cancer is an independent prognostic marker for overall and progression-free survival (OS and PFS, respectively) (Lucci et al, 2012).
It was the aim of the present prospective study to evaluate the prognostic value of CTC measured by the CellSearch system in patients with potentially curable disease, focussing on non-metastatic CRC.
Methods
Reporting of the present study was in accordance with the REMARK guidelines (McShane et al, 2005).
Patients
Patients with UICC stage I–IV CRC who underwent surgery with curative intent at the Department of General, GI and Transplant Surgery, University Hospital Heidelberg, Heidelberg, Germany between May 2009 and August 2012 were included in this study. In addition to patients with non-metastatic CRC (UICC I–III group), patients with limited, synchronous metastases were eligible for inclusion if their operation was done with curative intent and no macroscopic tumour (primary CRC and metastases) remained after surgery (UICC I–IV group). Patients were deemed ineligible if they refused participation, presented with unresectable CRC and/or liver metastases or had a history of any other malignancy during the past 5 years. Written informed consent was obtained prior to surgery. The study was approved by the ethics committee of the University of Heidelberg (323/3004). Details about our management of patients with primary CRC and colorectal liver metastases have been reported elsewhere (Reissfelder et al, 2009; Rahbari et al, 2012). Adjuvant treatment was done according to current treatment guidelines after obtaining interdisciplinary consensus for each patient.
Blood sample collection and CTC detection
TNM cancer staging was in accordance with the criteria set by the American Joint Commission on Cancer (AJCC, 2010). Blood samples from the central venous blood or from the peripheral blood were taken immediately prior to surgical incision after induction of general anaesthesia for all patients in the operating room. CTC detection in the central venous blood compartment is similar to the cell count obtained from peripheral blood, which has been validated by us in previous studies. Blood samples were drawn into and immediately transferred to cell preservative tubes (CellSave, Veridex LLC, Raritan, NJ, USA). Isolation of CTC with the CellTracks Autoprep System (Veridex LLC) and semi-automatic analysis via the CellTracks Analyzer II system (Veridex LLC) have been described previously (Allard et al, 2004; Rahbari et al, 2012). Two trained operators blinded to patient's data screened results independently, and differences in CTC count were resolved by discussion. All results for individual patients were masked from the analysing investigators by using a number system as a unique patient identifier. Operators of the CellSearch system were blinded for clinicopathological data of patient samples.
Blood samples were only analysed for patients, and no control group with healthy volunteers or patients with benign disease were included in this trial. CTC count in healthy volunteers has been studied extensively for the validation purposes of the CellSearch system (Allard et al, 2004; Cristofanilli et al, 2004; Miller et al, 2010). CTC detected by the CellSearch technique are extremely rare in healthy volunteers (<3.5% for a threshold ⩾1 CTC per 7.5 ml blood) and patients with benign disease (<7.5% for a threshold of ⩾1 CTC per 7.5 ml blood) (Miller et al, 2010). A control group was not included as it would not have added any substantial information to the aim of the present study.
Statistical analyses
All analyses were carried out separately for patients with non-metastatic CRC (UICC I–III group) and the entire cohort (UICC I–IV group). Patients with complete pathological response (T0, N0, M0) were included in the UICC I–III group. Categorical data were presented as absolute and relative frequencies. Continuous data were presented as median and range. For CTC data, the arithmetic mean and s.e.m. (s.d.) were reported. The association of CTC detection with clinicopathological variables was evaluated using the χ2-test. The primary end point of the present study was OS, which was calculated from the date of surgery to the date of death due to any cause or the date of last follow-up information. Progression-free survival was defined as time from the date of surgery until objective tumour progression or death. Survival curves were constructed according to the Kaplan–Meier method and compared using the log-rank test. As limited data were available on cutoff levels for the number of CTC to determine tumour cell-positive patients, all analyses were carried out for three cutoffs, that is, ⩾1 CTC, ⩾2 CTC and ⩾3 CTC. Variables that had significant associations with CTC detection on univariate analyses were included in multivariate analyses using Cox proportional hazards regression methodology. A P-value ⩽0.05 was considered to indicate a statistically significant difference. All P-values were two-sided. Statistical analyses were done with the SPSS software version 19 (SPSS, Chicago, IL, USA) and JMP program version 7 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 287 Patients with resectable CRC who underwent potentially curative therapy were included in this study, including 239 patients with non-metastatic disease (UICC stage I–III). Forty-eight patients underwent resection for CRC with synchronous metastases with curative intent. In the UICC stage I–III group, 157 male (65.7%) and 82 female (34.3%) patients were included; in the UICC stage I–IV group, there were 186 male (64.5%) and 101 female patients (35.2%). The majority of patients with metastatic disease had liver metastases, whereas metastases in the lungs and other locations were present in 6 (12.5%) and 7 (14.6%) patients, respectively. Three (6.2%) patients had multiple sites of metastases (Table 1).
Table 1. Clinicopathological characteristics of the study population.
| Stage I–III (n=239) | Stage I–IV (n=287) | |
|---|---|---|
| Age | 64 (27–96) | 64 (27–96) |
|
Sex | ||
| Male | 157 (65.7) | 186 (64.5) |
| Female | 82 (34.3) | 101 (35.2) |
|
Site of disease | ||
| Colon | 102 (42.7) | 134 (46.7) |
| Rectum | 137 (57.3) | 153 (53.3) |
|
T stage | ||
| T0 | 6 (2.5) | 6 (2.1) |
| T1 | 18 (7.5) | 20 (7.0) |
| T2 | 63 (26.4) | 65 (22.6) |
| T3 | 131 (54.8) | 161 (56.1) |
| T4 | 21 (8.8) | 35 (12.2) |
|
N Stage | ||
| N0 | 155 (64.9) | 170 (59.2) |
| N1 | 59 (24.7) | 76 (26.5) |
| N2 | 25 (10.5) | 41 (14.3) |
|
Resection margins | ||
| R0 | 223 (97.4) | 262 (96.3) |
| R1 | 6 (2.6) | 10 (3.7) |
| Tumour height (cm) | 7.3 (0–18) | 7.4 (0–18) |
|
Distribution of metastases | ||
| Liver | — | 32 (66.7) |
| Lung | — | 6 (12.5) |
| Other | — | 7 (14.6) |
| Multiple sites | — | 3 (6.2) |
| Neoadjuvant therapy | 81 (33.9) | 95 (33.1) |
| Neoadjuvant radiation | 81 (33.9) | 87 (30.0) |
| Adjuvant therapy | 57 (36.5) | 83 (43.7) |
| Follow-up (months) | 28 (0–53) | 28 (0–53) |
| CEA (>2.5 U l−1) | 54 (24) | 87 (32.0) |
| CA 19-9 (>37 U l−1) | 24 (10.7) | 39 (14.3) |
Abbreviation: CEA=carcinoembryonic antigen.
Detection rate of CTC in CRC is stage dependent
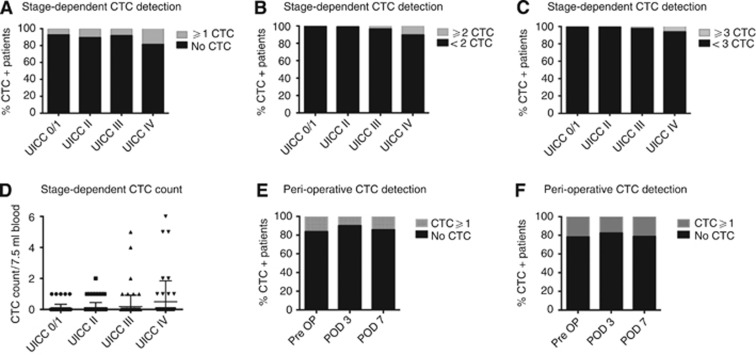
In the analysis of preoperative blood samples, ⩾1 CTC per 7.5 ml of blood were found in 30 patients (10.5%), ⩾2 CTC in 9 patients (3.1%) and ⩾3 more CTC in 5 patients (1.7%) (Supplementary Table 1). Detection rate of CTC was significantly correlated with the stage of disease comparing non-metastatic and metastatic patients with 3 (4.9%), 9 (10.5%), 7 (8.3%) and 9 (18.8%) patients with ⩾1 detected CTC in UICC stages I, II, III and IV, respectively (P=0.03). The stage-dependent CTC detection with increased detection rates for patients with UICC IV disease was confirmed for detection of ⩾2 (P=0.001) or ⩾3 (P=0.008) CTC per 7.5 ml of blood (Figure 1). Furthermore, patients' stage of disease was associated significantly with the number of CTC in peripheral blood detected intraoperatively. Postoperative blood samples with CTC analyses on postoperative days 3 and 7 were carried out in 51 and 28 patients, respectively. In the UICC I–III group ⩾1 CTC was detected on postoperative days 3 and 7 in 4 (10.0%) and 3 (14.3%) patients, whereas in the UICC stage I–IV group 9 (17.6%) and 6 (21.4%) patients had detectable CTC.
Figure 1.
Detection rate and count of CTC. (A–C) Stage-dependent detection rate of CTC with a threshold of ⩾1 (A), ⩾2 (B) and ⩾3 (C) CTC. (D) Stage-dependent detection count of CTC. (E and F) Perioperative detection of ⩾1 CTC in UICC stage I–III (E) and stage I–IV (F) patients.
Lack of association of primary tumour characteristics with CTC detection in non-metastatic CRC
The association of various clinicopathological characteristics with preoperative presence of CTC in the systemic circulation was analysed for three different cutoff values of CTC detection. No primary tumour characteristics or clinicopathological parameters predicted the presence of CTC for the non-metastatic (UICC stage I–III) group (Supplementary Table 2). There was, however, a trend towards higher detection rates in patients who had received any kind of neoadjuvant therapy (P=0.07) and a trend towards increased CTC detection in patients with locally advanced tumours (T3/4 vs T1/2; P=0.14). To further elucidate whether a certain kind of neoadjuvant therapy caused an increase in CTC, we performed further subgroup analyses. These revealed a significantly higher CTC detection rate in non-metastatic patients with neoadjuvant chemoradiotherapy (P=0.002). This association remained statistically significant for the entire study cohort, including patients with stage IV disease (P=0.03).
The analyses of the entire study group confirmed the significantly higher presence of CTC in patients with metastatic disease compared with non-metastatic patients (P=0.04). In addition, preoperative CEA level (P=0.03) and CA 19-9 level (P=0.01) were significantly associated with detection of ⩾1 CTC per 5 ml of blood in patients with UICC I–IV disease (Supplementary Table 2). The association of T stage and CTC detection failed to reach statistical significance in these patients (P=0.08).
Presence of CTC is a strong and independent predictor of OS and PFS in patients with non-metastatic CRC
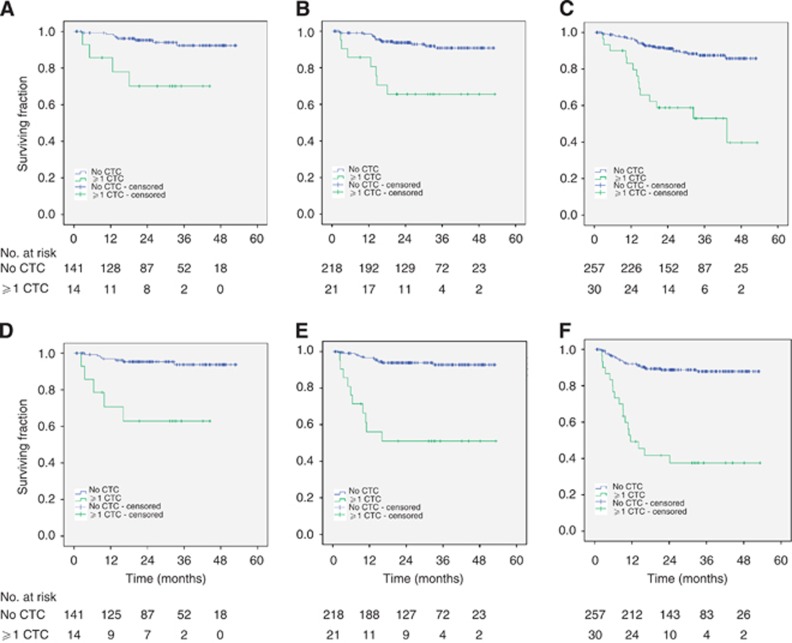
The mean follow-up time was 28 (0–53) months. During the follow-up period, 22 (9.2%) patients died and 23 (9.6%) patients were diagnosed with disease progression in the UICC I–III group. In the complete study group, 40 (13.9%) patients died and 45 (15.7%) patients had disease progression during follow-up (Figure 2).
Figure 2.
Overall survival and progression-free survival according to presence of at least 1 CTC stratified for the stage of disease. (A–C) Kaplan–Meier estimates of overall survival in patients with UICC stage I–II (A), stage I–III (B) and stage I–IV (C) disease. (D–F) Kaplan–Meier estimates of progression-free survival in patients with UICC stage I–II (D), stage I–III (E) and stage I–IV (F) disease.
To evaluate the prognostic value of preoperative CTC detection on OS and PFS in patients with non-metastatic CRC, univariate analyses were performed initially to evaluate known prognosticators in the present study cohort. In patients with non-metastatic disease, age (P=0.036) was associated with significantly shorter OS, whereas T stage (P=0.029) and N status (P=0.002) were predictors of poor PFS. Patients with non-metastatic disease who were found to have ⩾1 CTC per 7.5 ml blood preoperatively had significantly shorter OS (P<0.001) and PFS (P<0.001) on univariate analyses (Table 2). This association was also confirmed for the preoperative detection of ⩾2 and ⩾3 CTC (data not shown). Furthermore, the adverse prognostic impact of detection of ⩾1 CTC on OS (P<0.001) and PFS (P<0.001) was confirmed for the cohort of 158 patients who had no neoadjuvant treatment (Supplementary Figure 1).
Table 2. Univariate analyses of factors associated with overall survival and progression-free survival in non-metastatic CRC (UICC I–III).
|
Overall survival |
Progression-free survival |
|||
|---|---|---|---|---|
| Mean survival (95% CI) | P | Mean survival (95% CI) | P | |
|
Gender | ||||
| Male | 47.9 (45.7–50.2) | 0.220 | 47.7 (45.4–50.1) | 0.356 |
| Female | 50.4 (48.0–52.8) | 49.6 (46.8–52.4) | ||
|
Age (years) | ||||
| <65 | 50.4 (48.5–52.3) | 0.036 | 49.1 (46.7–51.4) | 0.305 |
| ⩾65 | 47.2 (44.5–50.0) | 47.6 (44.8–50.4) | ||
|
T stage | ||||
| T 1/2 | 49.8 (47.6–52.1) | 0.228 | 50.7 (48.7–52.7) | 0.029 |
| T 3/4 | 48.2 (44.8–50.5) | 46.9 (44.3–49.5) | ||
|
N stage | ||||
| N0 | 49.7 (47.7–51.6) | 0.294 | 49.8 (47.8–51.7) | 0.002 |
| N1 | 48.1 (44.4–51.8) | 48.8 (47.8–51.7) | ||
| N2 | 36.0 (31.1–41.0) | 31.2 (24.8–37.6) | ||
|
R status | ||||
| R0 | 49.4 (47.7–51.1) | 0.069 | 48.6 (46.8–50.5) | 0.28 |
| R1 | 37.7 (21.6–53.7) | 44.6 (30.4–58.9) | ||
|
Site of primary tumour | ||||
| Colon | 49.5 (47.1–51.9) | 0.510 | 49.3 (46.7–51.9) | 0.431 |
| Rectum | 48.2 (45.9–50.6) | 47.7 (45.2–50.2) | ||
|
Stage of disease | ||||
| UICC I–III | — | — | ||
| UICC I–IV | — | — | ||
|
CEA level (μg l−1) | ||||
| <2.5 | 49.2 (47.6–51.1) | 0.739 | 49.1 (47.1–51.0) | 0.447 |
| ⩾2.5 | 48.6 (44.7–52.4) | 47.5 (43.2–51.8) | ||
|
CA 19-9 level (μg l−1) | ||||
| <37 | 49.0 (47.2–50.8) | 0.970 | 48.9 (47.0–50.7) | 0.541 |
| ⩾37 | 49.2 (43.8–54.5) | 47.2 (40.8–53.6) | ||
|
Neoadjuvant therapy | ||||
| Yes | 49.7 (47.8–51.6) | 0.138 | 49.2 (47.1–51.3) | 0.288 |
| No | 47.2 (43.8–50.5) | 46.9 (43.3–50.4) | ||
|
CTC⩾1 | ||||
| No | 49.8 (48.3–51.4) | <0.001 | 50.1 (48.6–51.6) | <0.001 |
| Yes | 38.4 (29.4–47.4) | 30.8 (20.8–40.8) | ||
|
CTC⩾2 | ||||
| No | 49.3 (47.6–51.0) | 0.003 | 48.9 (47.1–50.6) | 0.003 |
| Yes | 28.4 (8.7–48.1) | 27.4 (6.9–47.9) | ||
|
CTC⩾3 | ||||
| No | 49.1 (47.4–50.8) | 0.021 | 48.7 (46.9–50.5) | 0.016 |
| Yes | 25.6 (0–56.8 ) | 25.3 (0.0–56.9) | ||
Abbreviations: CI=confidence interval; CEA=carcinoembryonic antigen; CTC=circulating tumour cells; UICC=Union for International Cancer Control.
Identification of patients with node-negative disease who are at risk for disease recurrence remains a clinical challenge. Our study population included 155 patients with node-negative CRC who had no evidence of distant metastases at the time of diagnosis. Of these, 14 (9%) had ⩾1 CTC per 7.5 ml blood preoperatively. These patients had significantly worse OS (34.1 vs 50.4 months; P=0.001) and PFS (30.8 vs 50.6 months; P<0.001).
On multivariate analyses, preoperative detection of ⩾1 CTC per 7.5 ml blood was confirmed as a strong and independent predictor of OS (HR 5.5; 95% CI 2.3–13.6; P<0.001) and PFS (HR 12.7; 95% CI 5.2–31.1; P<0.001) in patients with UICC stage I–III disease.
The analyses of the entire study cohort, including patients with UICC stage I–IV disease, revealed patients' age (P=0.04), N status (P=0.001), presence of distant metastases (P<0.001), elevated preoperative CEA level (P=0.001) and elevated preoperative CA 19-9 level (P<0.001) to be associated with significantly impaired OS, whereas a significant association with shortened PFS was found for patients' T stage (P=0.005), N stage (P<0.001), presence of distant metastases (P<0.001), elevated preoperative CEA level (P=0.001) and elevated preoperative CA 19-9 level (P<0.035) (Table 3). Patients with ⩾1 CTC per 7.5 ml blood preoperatively had significantly worse OS (P<0.001) and PFS (P<0.001). A strong association of preoperative CTC detection and OS well as PFS was also found for a cutoff of ⩾2 and ⩾3 CTC per 7.5 ml blood preoperatively. After exclusion of patients with neoadjuvant therapy, CTC detection was still associated with poor OS (P<0.001) and PFS (P<0.001).
Table 3. Univariate analyses of factors associated with overall survival and progression-free survival in the complete study cohort (UICC I–IV).
|
Overall survival |
Progression-free survival |
|||
|---|---|---|---|---|
| Mean survival (95% CI) | P | Mean survival (95% CI) | P | |
|
Gender | ||||
| Male | 46.7 (44.2–48.9) | 0.960 | 44.9 (42.4–47.5) | 0.536 |
| Female | 47.0 (43.9–50.0) | 46.4 (43.1–49.7) | ||
|
Age (years) | ||||
| <65 | 48.3 (46.1–50.5) | 0.044 | 45.1 (42.2–47.9) | 0.909 |
| ⩾65 | 45.0 (42.2–47.8) | 45.7 (42.8–48.6) | ||
|
T stage | ||||
| T 1/2 | 48.2 (45.5–50.9) | 0.178 | 49.3 (46.9–51.8) | 0.005 |
| T 3/4 | 46.0 (43.7–48.4) | 43.5 (40.7–46.2) | ||
|
N stage | ||||
| N0 | 49.2 (47.2–51.1) | 0.001 | 48.6 (46.5–50.8) | <0.001 |
| N1 | 45.1 (41.4–48.9) | 43.4 (39.1–47.7) | ||
| N2 | 37.6 (31.9–43.3) | 34.1 (27.5–40.6) | ||
|
R status | ||||
| R0 | 47.4 (45.5–49.2) | 0.064 | 45.7 (43.6–47.9) | 0.368 |
| R1 | 36.9 (25.4–48.4) | 37.7 (23.9–51.4) | ||
|
Site of primary tumour | ||||
| Colon | 45.9 (43.2–48.7) | 0.333 | 45.5 (42.5–48.5) | 0.986 |
| Rectum | 47.5 (45.2–49.9) | 45.4 (42.6–48.1) | ||
|
Stage of disease | ||||
| UICC I–III | 48.7 (47.0–50.5) | <0.001 | 48.5 (46.7–50.4) | <0.001 |
| UICC I–IV | 38.2 (33.0–43.4) | 30.3 (23.8–36.8) | ||
|
CEA level (μg l−1) | ||||
| <2.5 | 49.1 (47.3–51.0) | 0.001 | 48.1 (46.0–50.2) | 0.001 |
| ⩾2.5 | 42.5 (38.4–46.5) | 40.5 (36.0–45.1) | ||
|
CA 19-9 level (μg l−1) | ||||
| <37 | 48.2 (46.5–50.0) | 0.000 | 46.5 (44.4–48.6) | 0.035 |
| ⩾37 | 39.3 (33.0–45.7) | 40.4 (33.5–47.2) | ||
|
Neoadjuvant therapy | ||||
| No | 47.0 (44.9–49.2) | 0.852 | 46.5 (44.1–48.8) | 0.229 |
| Yes | 46.6 (43.3–49.8) | 43.6 (39.7–47.5) | ||
|
CTC⩾1 | ||||
| No | 48.4 (46.7–50.0) | <0.001 | 47.9 (46.0–49.7) | <0.001 |
| Yes | 33.6 (26.3–41.0) | 25.6 (17.6–33.6) | ||
|
CTC⩾2 | ||||
| No | 47.5 (45.7–49.2) | <0.001 | 46.5 (44.6–48.5) | <0.001 |
| Yes | 28.3 (16.4–40.2) | 16.3 (5.0–27.6) | ||
|
CTC⩾3 | ||||
| No | 47.2 (45.5–49.0) | 0.001 | 46.1 (44.1–48.1) | <0.001 |
| Yes | 24.7 (7.7–41.8) | 14.0 (0.0–29.2) | ||
Abbreviations: CI=confidence interval; CEA=carcinoembryonic antigen; CTC=circulating tumour cells; UICC=Union for International Cancer Control.
In these patients, ⩾1 CTC per 7.5 ml blood was associated with poor OS (P<0.001) and PFS (P<0.001). This association was confirmed for the preoperative detection of ⩾2 and ⩾3 CTC per 7.5 ml blood. In addition, age (P=0.036) was associated with poor OS, whereas T stage (P=0.029) and N status (P=0.002) were predictors of poor PFS in these patients with non-metastatic disease.
On multivariate analyses, including all variables with significant associations on univariate analyses, presence of ⩾1 CTC was revealed as an independent predictor of OS (HR 5.6; 95% CI 2.6–12.0; P<0.001) and PFS (HR 7.8; 95% CI 3.9–15.5; P<0.001). Remarkably, the prognostic impact of preoperative CTC detection was independent of the presence of distant metastases, which was revealed as a further independent prognostic factor of OS (HR 2.3; 95% CI 1.0–5.0; P=0.035) and PFS (HR 7.8; 95% CI 3.9–15.5; P=0.002) (Table 4).
Table 4. Cox proportional hazards models for overall survival and progression-free survival.
| Variable | Comparison | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|
|
UICC Stage I–III | ||||
| Overall survival | ||||
| Age (years) | ⩾65 vs<65 | 2.7 | 1.0–7.0 | 0.037 |
| CTC | ⩾1 vs 0 | 5.5 | 2.3–13.6 | <0.001 |
| Progression-free survival | ||||
| N stage | N0 | 0.003 | ||
| N1 | 1.4 | 0.5–4.3 | 0.510 | |
| N2 | 6.2 | 2.1–18.2 | 0.001 | |
| T stage | T 3/4 vs T 1/2 | 1.7 | 0.5–5.4 | 0.368 |
| CTC | ⩾1 vs 0 | 12.7 | 5.2–31.1 | <0.001 |
|
UICC Stage I–IV | ||||
| Overall survival | ||||
| Age (years) | ⩾65 vs<65 | 2.9 | 1.4–6.2 | 0.005 |
| N stage | N0 | 0.107 | ||
| N1 | 1.6 | 0.7–3.8 | 0.247 | |
| N2 | 2.5 | 1.0–5.9 | 0.035 | |
| Stage of disease | UICC IV vs III | 2.3 | 1.0–5.0 | 0.035 |
| CEA level (μg l−1) | ⩾2.5 vs <2.5 | 1.5 | 0.6–3.3 | 0.372 |
| CA 19-9 level (μg l−1) | ⩾37 vs <37 | 1.3 | 0.6–2.9 | 0.548 |
| CTC | ⩾1 vs 0 | 5.6 | 2.6–12.0 | <0.001 |
| Progression-free survival | ||||
| T stage | T 3/4 vs T 1/2 | 2.0 | 0.7–5.6 | 0.177 |
| N stage | N0 | 0.129 | ||
| N1 | 1.2 | 0.5–2.6 | 0.688 | |
| N2 | 2.3 | 1.0–5.4 | 0.059 | |
| Stage of disease | UICC IV vs III | 3.3 | 1.5–7.2 | 0.002 |
| CEA level (μg l−1) | ⩾2.5 vs <2.5 | 1.2 | 0.6–2.8 | 0.558 |
| CA 19-9 level (μg l−1) | ⩾37 vs <37 | 0.7 | 0.3–1.5 | 0.347 |
| CTC | ⩾1 vs 0 | 7.8 | 3.9–15.5 | <0.001 |
Abbreviations: CI=confidence interval; CEA=carcinoembryonic antigen; CTC=circulating tumour cells; UICC=Union for International Cancer Control.
Discussion
In the present study, we investigated whether preoperative detection of CTC in the systemic circulation can serve as a prognostic biomarker in CRC patients without distant metastases or with limited metastatic disease amenable to curative resection. Although we have previously demonstrated a prognostic value of CTC in patients with primary CRC in a meta-analysis, the included studies varied considerably with respect to the methods of CTC detection. Indeed, the predictive and prognostic value of CTC using standardised assays has only been demonstrated and validated for advanced metastatic CRC (Cohen et al, 2008, 2009; Seeberg et al, 2014). A recently published study from the MD Anderson Cancer Center revealed CTC detected by the CellSearch system as an independent prognostic marker for OS and PFS in patients with non-metastatic breast cancer (Lucci et al, 2012).
Here we show for the first time that preoperative CTC detection using the standardised CellSearch system is a strong and independent prognostic factor for disease progression and survival in non-metastatic CRC patients. Interestingly, the detection of CTC preoperatively did not correlate with any other clinicopathological factor in patients with non-metastatic disease. As we did not find any association between CTC detection and clinical parameters, this suggests CTC detection as an independent indicator of aggressive tumour biology in our patients, in line with the findings of Lucci et al (2012) in non-metastasised breast-cancer patients. In line with these findings, the detection of CTC in UICC stage I–III patients proofed to be the strongest predictor of OS and PFS, considering known prognostic factors, such as age or lymph node status. Collectively, these data suggest CTC as a potentially useful prognostic and predictive biomarker in non-metastatic CRC patients that may help to further stratify patient's risk status within different stages of disease. Furthermore, this should prompt further studies to dissect the molecular mechanisms of tumour cell dissemination.
The CellSearch system enables the detection as well as enumeration of CTC. Previous studies have suggested different cutoff levels to determine ‘CTC positivity'. Allard et al (2004) evaluated the CellSearch system in a study of 964 patients. Based on the mean CTC count in healthy subjects, these authors suggested the detection of ⩾2 CTC per 7.5 ml of blood as abnormal. Using a training and validation set of patients with metastatic CRC, Cohen et al (2008, 2009) defined unfavorable prognostic groups by detection of ⩾3 CTC per 7.5 ml of blood. All patients in these studies had metastatic disease, we report here the largest study of patients with non-metastatic CRC studied with the CellSearch system so far and the first to evaluate its prognostic value in these patients. Our results show a significantly lower detection rate of CTC in patients with non-metastatic CRC. Based on the relatively low proportion of patients with ⩾1 CTC per 7.5 ml blood who had a significantly worse outcome compared with patients without CTC, we recommend that risk stratification of non-metastatic CRC patients should be done at a threshold of ⩾1 CTC.
Adjuvant therapy in patients with node-negative CRC remains a controversial issue. Evidence from recent studies suggests that patients' selection for adjuvant therapy based on clinical factors might be inaccurate and molecular markers might be useful (O'Connor et al, 2011; Rahbari et al, 2011, 2014). Our study demonstrates that the CellSearch system provides prognostic information in CRC patients with UICC stage I/II disease and confirms previous data using CTC detection by CK20 PCR (Koch et al, 2006). These data suggest that detection of CTC using standardised assays should be considered to improve current staging of patients (i.e., cM0(i+)) and the selection of patients for adjuvant chemotherapy. However, several issues need to be addressed before integration of CTC detection into the TNM classification and treatment guidelines. First, our results on patients with non-metastatic CRC need to be validated in further cohorts from different institutions. Second, there is no proof so far that adjuvant therapy improves long-term outcome of patients with cM0(i+) disease. We recently showed that preoperative risk status using clinical parameters can predict efficacy of adjuvant chemotherapy after resection of CRC liver metastases (Rahbari et al, 2014). A randomised controlled trial would be needed to achieve level I evidence on the benefit of adjuvant therapy in CRC patients with cM0(i+) disease. However, such a trial will be almost impossible to perform owing to the low CTC detection rate in this group of patients, and an international patient registry might offer an alternative strategy to provide valuable information. Third, detection of CTC using the CellSearch technique is only investigating a subgroup of CM0(i+) patients. The CellSearch technique only detects CTC in the blood, which are positive for EPCAM and cytokeratin. However, there is evidence that not all CTC are positive for these markers, and it remains subject of future studies to evaluate the prognostic and predictive value of other CTC detection assays capturing a broader spectrum of CTC populations (Steinert et al, 2014). Furthermore, the clinical value of tumour cell detection in further body compartments such as the bone marrow, regional lymph nodes and mesenteric blood needs to be clarified to determine which compartment provides the most accurate information on patients' outcome (Rahbari et al, 2011, 2012).
Interestingly, even though CTC detection per se is a strong indicator of survival and disease burden, it remains unclear whether detected CTC are actually precursors of metastatic lesions (and possess the ability to form distant metastases) or whether CTC originate from metastases (or the primary tumour) and are just a measurement of overall disease burden. The molecular characterisation of single CTC will be an important step forward to answer these questions and to ultimately use CTC as liquid biopsies during different stages of disease progression for treatment decisions and monitoring purposes (Torino et al, 2013; Coget et al, 2014).
In conclusion, our study provides promising results for the use of CTC as a prognostic and predictive biomarker in patients with non-metastatic CTC. The lack of association between CTC detection and clinical parameters suggests CTC as an independent indicator of aggressive tumour biology. This should clearly be followed in larger trials with longer follow-up times. Our results warrant further validation within larger multi-institutional trials to test and clarify whether detection of CTC in the blood should be included into current treatment guidelines of non-metastatic CRC.
Acknowledgments
We thank Melanie Bernhardt for excellent technical assistance. This work was funded by German Research Foundation (DFG; WE 3548/4-1), University Hospital Heidelberg, Heidelberg, Germany.
Author contributions
UB, NNR, JW, MWB and MK designed the study. UB, NNR, SS, CR and CK collected the samples. UB and NNR analysed the data and NNR performed the statistical analyses. UB and NNR wrote the manuscript with assistance from MK and final approval from all authors.
Moritz Koch has served as an external scientific advisory board member of Veridex LLC. The other authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- AJCC . AJCC Publisher, Springer: New York, USA; 2010. AJCC Cancer Staging Manual, 7th edn. [Google Scholar]
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AGJ, Uhr JW, Terstappen LWMM. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- Allen-Mersh TG, McCullough TK, Patel H, Wharton RQ, Glover C, Jonas SK. Role of circulating tumour cells in predicting recurrence after excision of primary colorectal carcinoma. Br J Surg. 2007;94:96–105. doi: 10.1002/bjs.5526. [DOI] [PubMed] [Google Scholar]
- Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014;146:709–717. doi: 10.1053/j.gastro.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Coget J, Borrini F, Susman S, Sabourin J-C. Colorectal carcinomas in 2013: the search for powerful prognostic markers is still on the go! Cancer Biomarkers. 2014;14:145–150. doi: 10.3233/CBM-130378. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Alpaugh RK, Gross S, O'Hara SM, Smirnov DA, Terstappen LWMM, Allard WJ, Bilbee M, Cheng JD, Hoffman JP, Lewis NL, Pellegrino A, Rogatko A, Sigurdson E, Wang H, Watson JC, Weiner LM, Meropol NJ. Isolation and characterization of circulating tumor cells in patients with metastatic colorectal cancer. Clin Colorectal Cancer. 2006;6:125–132. doi: 10.3816/CCC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LWMM, Meropol NJ. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Punt CJA, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC, Doyle GV, Tissing H, Terstappen LWMM, Meropol NJ. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20:1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Atkin W, Lenz H-J, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- Engstrom PF, Arnoletti JP, Benson AB, 3rd, Chen Y-J, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, Fakih MG, Fleshman J, Jr, Fuchs C, Grem JL, Kiel K, Knol JA, Leong LA, Lin E, Mulcahy MF, Rao S, Ryan DP, Saltz L, Shibata D, Skibber JM, Sofocleous C, Thomas J, Venook AP, Willett C, National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- Iinuma H, Okinaga K, Egami H, Mimori K, Hayashi N, Nishida K, Adachi M, Mori M, Sasako M. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306. [PubMed] [Google Scholar]
- Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M, Mori M. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol. 2011;29:1547–1555. doi: 10.1200/JCO.2010.30.5151. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakanishi H, Hirai T, Kato T, Kodera Y, Feng Z, Kasai Y, Ito K, Akiyama S, Nakao A, Tatematsu M. Quantitative detection of CEA expressing free tumor cells in the peripheral blood of colorectal cancer patients during surgery with real-time RT-PCR on a LightCycler. Cancer Lett. 2002;183:195–203. doi: 10.1016/s0304-3835(02)00157-x. [DOI] [PubMed] [Google Scholar]
- Koch M, Kienle P, Kastrati D, Antolovic D, Schmidt J, Herfarth C, von Knebel Doeberitz M, Weitz J. Prognostic impact of hematogenous tumor cell dissemination in patients with stage II colorectal cancer. Int J Cancer. 2006;118:3072–3077. doi: 10.1002/ijc.21784. [DOI] [PubMed] [Google Scholar]
- Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO clinical practice guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v70–v77. doi: 10.1093/annonc/mdq168. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, McIver CM, Stephenson S-A, Hewett PJ, Rieger N, Hardingham JE. Identification of early-stage colorectal cancer patients at risk of relapse post-resection by immunobead reverse transcription-PCR analysis of peritoneal lavage fluid for malignant cells. Clin Cancer Res. 2006;12:417–423. doi: 10.1158/1078-0432.CCR-05-1473. [DOI] [PubMed] [Google Scholar]
- Lu C-Y, Uen Y-H, Tsai H-L, Chuang S-C, Hou M-F, Wu D-C, Juo S-HH, Lin S-R, Wang J-Y. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer. 2011;104:1178–1184. doi: 10.1038/bjc.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–695. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- Maestro LM, Sastre J, Rafael SB, Veganzones SB, Vidaurreta M, Martín M, Olivier C, DE La Orden VB, Garcia-Saenz JA, Alfonso R, Arroyo M, Diaz-Rubio E. Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res. 2009;29:4839–4843. [PubMed] [Google Scholar]
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- Miller MC, Doyle GV, Terstappen LWMM. Significance of circulating tumor cells detected by the CellSearch system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421. doi: 10.1155/2010/617421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou J-I, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C, Büchler MW, Koch M, Weitz J. Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol. 2012;19:2195–2202. doi: 10.1245/s10434-011-2178-1. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Bork U, Motschall E, Thorlund K, Büchler MW, Koch M, Weitz J. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2011;30:60–70. doi: 10.1200/JCO.2011.36.9504. [DOI] [PubMed] [Google Scholar]
- Rahbari NN, Reissfelder C, Schulze-Bergkamen H, Jäger D, Büchler MW, Weitz J, Koch M. Adjuvant therapy after resection of colorectal liver metastases: the predictive value of the MSKCC clinical risk score in the era of modern chemotherapy. BMC Cancer. 2014;14:174. doi: 10.1186/1471-2407-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissfelder C, Rahbari NN, Koch M, Ulrich A, Pfeilschifter I, Waltert A, Müller SA, Schemmer P, Büchler MW, Weitz J. Validation of prognostic scoring systems for patients undergoing resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:3279–3288. doi: 10.1245/s10434-009-0654-7. [DOI] [PubMed] [Google Scholar]
- Sadahiro S, Suzuki T, Maeda Y, Yurimoto S, Yasuda S, Makuuchi H, Kamijo A, Murayama C. Detection of carcinoembryonic antigen messenger RNA-expressing cells in peripheral blood 7 days after curative surgery is a novel prognostic factor in colorectal cancer. Ann Surg Oncol. 2007;14:1092–1098. doi: 10.1245/s10434-006-9289-0. [DOI] [PubMed] [Google Scholar]
- Sastre J, Maestro ML, Gómez-España A, Rivera F, Valladares M, Massuti B, Benavides M, Gallén M, Marcuello E, Abad A, Arrivi A, Fernández-Martos C, González E, Tabernero JM, Vidaurreta M, Aranda E, Díaz-Rubio E. Circulating tumor cell count is a prognostic factor in metastatic colorectal cancer patients receiving first-line chemotherapy plus bevacizumab: a Spanish Cooperative Group for the Treatment of Digestive Tumors study. Oncologist. 2012;17:947–955. doi: 10.1634/theoncologist.2012-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg LT, Waage A, Brunborg C, Hugenschmidt H, Renolen A, Stav I, Bjørnbeth BA, Brudvik KW, Borgen EF, Naume B, Wiedswang G. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg. 2014;261:164–171. doi: 10.1097/SLA.0000000000000580. [DOI] [PubMed] [Google Scholar]
- Steinert G, Schölch S, Niemietz T, Iwata N, García SA, Behrens B, Voigt A, Kloor M, Benner A, Bork U, Rahbari NN, Büchler MW, Stoecklein NH, Weitz J, Koch M. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 2014;74:1694–1704. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer—a review. Eur J Surg Oncol. 2011;37:459–465. doi: 10.1016/j.ejso.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Thorsteinsson M, Söletormos G, Jess P. Low number of detectable circulating tumor cells in non-metastatic colon cancer. Anticancer Res. 2011;31:613–617. [PubMed] [Google Scholar]
- Torino F, Bonmassar E, Bonmassar L, De Vecchis L, Barnabei A, Zuppi C, Capoluongo E, Aquino A. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev. 2013;39:759–772. doi: 10.1016/j.ctrv.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Uen Y-H, Lu C-Y, Tsai H-L, Yu F-J, Huang M-Y, Cheng T-L, Lin S-R, Wang J-Y. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol. 2008;15:2120–2128. doi: 10.1245/s10434-008-9961-7. [DOI] [PubMed] [Google Scholar]
- Wang J-Y, Lin S-R, Wu D-C, Lu C-Y, Yu F-J, Hsieh J-S, Cheng T-L, Koay L-B, Uen Y-H. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res. 2007;13:2406–2413. doi: 10.1158/1078-0432.CCR-06-2054. [DOI] [PubMed] [Google Scholar]
- Weitz J, Koch M, Debus J, Höhler T, Galle PR, Büchler MW. Colorectal cancer. Lancet. 2005;365:153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- Wong SCC, Chan CML, Ma BBY, Hui EP, Ng SSM, Lai PBS, Cheung MT, Lo ESF, Chan AKC, Lam MYY, Au TCC, Chan ATC. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res. 2009;15:1005–1012. doi: 10.1158/1078-0432.CCR-08-1515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.