Abstract
Iron-cofactored superoxide dismutase (SodB) of Helicobacter pylori plays an indispensable role in the bacterium's colonization of the stomach. Previously, we demonstrated that FecA1, a Fe3+-dicitrate transporter homolog, contributes to SodB activation by supplying ferrous iron (Fe2+) to SodB, and fecA1-deletion mutant strains have reduced gastric mucosal-colonization ability in Mongolian gerbils, suggesting that FecA1 is a possible target for the development of a novel eradication therapy. This study aimed to identify novel FecA1-binding compounds in silico and then examined the effect of a predicted FecA1-binding compound on H. pylori SodB activity in vitro. Specifically, we demonstrated that nordihydroguaiaretic acid (NDGA) is a predicted FecA1-binding compound. NDGA reduced intracellular Fe2+ levels in H. pylori and reduced SodB activity. Additionally, NDGA increased H2O2 sensitivity of H. pylori and increased the metronidazole (Mtz) sensitivity. The present study demonstrated that NDGA repressed SodB activity associated with the gastric mucosal-colonization via inhibition of intracellular Fe2+ uptake by FecA1, suggesting that NDGA might be effective for the development of a novel eradication therapy.
1. Introduction
Helicobacter pylori infection is one of the most common infectious diseases worldwide. Chronic infection of H. pylori plays a causative role in gastritis, peptic ulcer, and gastric carcinomas [1]. Excessive reactive oxygen species (ROS) are generated by neutrophils and macrophages in H. pylori colonized human stomachs [2]. Although the generation of ROS is an important host immune response against persistent pathogens, H. pylori counteracts oxidative stress using a variety of enzymes to establish a chronic infection [3, 4]. Therefore, persistent and excessive ROS induces oxidative stress injuries to the gastric mucosa.
The H. pylori genome encodes iron-cofactored superoxide dismutase (SodB) [5, 6]. Mutants with a deletion of sodB show a reduced gastric mucosal-colonization ability in mice, suggesting that SodB plays an indispensable role in the colonization of host gastric mucosa owing to its defensive role against excessive ROS [7]. Ferrous ion (Fe2+) is required for the activation of SodB. We recently demonstrated that the deletion mutant of the fecA1 gene, a Fe3+-dicitrate transporter homolog, shows reduced SodB activity [8]. Our previous findings suggested that the Fe2+-supply system associated with SodB activation proceeds as follows: Fe3+-dicitrate transport is enhanced by FecA1, and then intracellular ferric ion (Fe3+) is reduced to Fe2+ by Fe3+-reductase (ribBA), providing Fe2+ to SodB [8, 9]. In addition, fecA1-deletion mutant strains show reduced gastric mucosal-colonization ability in Mongolian gerbils [8]. These results indicated that fecA1-deletion mutant strains were eradicated by excessive ROS in host gastric mucosa via disruption of SodB activity. In fact, FecA1 associated with SodB activation is an important determinant of the establishment of chronic infections.
Recently, we demonstrated that SodB overexpression is caused by amino acid mutations of ferric uptake regulator (Fur), which is associated with the development of metronidazole (Mtz) resistance [10]. The deletion of fecA1 in Mtz-resistant strains (KS0048 and KS0145) results in increased Mtz sensitivity, suggesting that FecA1 is also associated with the development of Mtz resistance [8].
Our previous results indicated that FecA1 is a possible target for the development of a selective H. pylori eradication therapy mediated by excessive ROS accumulation in the human stomach and for avoiding the development of Mtz resistance associated with SodB overexpression. In the present study, we identified a compound that demonstrated an inhibitory effect on SodB activity via the inhibition of FecA1.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
H. pylori strains ATCC700392, KS0048, and KS0145 were used in this study. The KS strains were clinically isolated and maintained at −80°C in Brucella broth (Becton–Dickinson, Franklin Lakes, NJ, USA) containing 25% (vol/vol) glycerol. The KS0048 and KS0145 strains were used as the Mtz-resistant strains with SodB overexpression owing to Fur amino acid mutations. ATCC700392 pHel3::sodB strain was used as the SodB-overexpressing strain of H. pylori ATCC700392, and ATCC700392 pHel3 ctrl strain was used as the control strain of ATCC700392 pHel3::sodB strain [10]. It was confirmed that the SodB activity in ATCC700392 pHel3::sodB was higher as compared with that of ATCC700392 pHel3 ctrl strain [10]. ATCC700392ΔfecA1, KS0048ΔfecA1, and KS0145ΔfecA1 strains were used as fecA1-deletion mutant strains of H. pylori ATCC700392, KS0048, and KS0145, respectively [8]. It was confirmed that the SodB activities in fecA1-deletion mutant strains were significantly decreased as compared with wild-type strains [8]. These strains were maintained at −80°C in Brucella broth (Becton–Dickinson, Franklin Lakes, NJ, USA) containing 25% (vol/vol) glycerol. The bacteria were cultured on Brucella agar containing 7% sheep blood and 7% fetal bovine serum for 2 days at 37°C under microaerobic conditions maintained with AnaeroPack MicroAero (Mitsubishi Gas, Tokyo, Japan).
2.2. In Silico Screening of Chemical Compounds with Binding Affinity to FecA1
The FecA1 amino acid sequence of H. pylori (UniProt ID: O25395) was inserted into the COPICAT (Comprehensive Predictor of Interactions between Chemical Compounds and Target Proteins) program, which is an in silico screening system to predict the comprehensive interactions between target proteins and chemical compounds [11]. A chemical compound that is likely to interact with FecA1 was predicted.
2.3. Measurement of Intracellular Fe2+ Levels
The bacteria normalized to an OD600 of 0.5 were incubated with or without nordihydroguaiaretic acid (NDGA) for 3 hr. The bacteria were washed three times with HBSS and then 10 μM RhoNox-1 (from 1 mM stock solution in DMSO) was added [12]. After incubation for 1 hr at 37°C, the bacteria were washed with HBSS and then normalized to an OD600 of 0.1. Fluorescence intensity was measured using 560 nm excitation and 595 nm emission.
2.4. Measurement of SOD Activity
The bacteria normalized to an OD600 of 0.5 were incubated with or without NDGA for 3 hr. The bacteria were centrifuged, were washed three times with PBS, and then were sonicated (1.5 min at 25% power). The bacterial lysates were centrifuged, and the SOD activity was measured using the SOD Assay Kit (Dojindo, Kumamoto, Japan) following the manufacturer's guidelines [8]. Protein concentrations of the bacterial lysates were measured by BCA assays (Thermo Scientific, Rockford, IL, USA).
2.5. Measurement of the MICs of Mtz
The bacteria normalized to an OD600 of 0.5 were incubated with or without 50 μM NDGA for 3 hr. The bacteria (at an OD600 of 0.1) were inoculated on an agar plate containing Mtz in serial twofold dilutions (0.5–128 μg/mL). All plates were incubated at 37°C under microaerobic conditions, and the minimum inhibitory concentration (MIC) values were determined [8].
2.6. Disk Assays for H2O2 Susceptibility
The bacteria normalized to an OD600 of 0.5 were incubated with or without 50 μM NDGA for 3 hr. The bacteria were centrifuged and washed three times with PBS. The bacteria, normalized to an OD600 of 0.1, were plated for confluent growth on Nissui Helicobacter agar (Nissui, Tokyo, Japan). Sterile 5 mm disks saturated with 10 μL of 5 M hydrogen peroxide (H2O2) were placed on the plates. After 3 days, the zone of inhibition around the disks was measured [8].
2.7. Statistical Analysis
All values were expressed as means ± SD. The statistical significance of differences between three or more groups was evaluated using the Tukey test. Differences were considered to be significant for values of P < 0.05.
3. Results
3.1. Screening of Chemical Compounds with Binding Affinity to FecA1 of H. pylori
To search for chemical compounds with binding affinity to FecA1 of H. pylori, we used the COPICAT web-based software system [11]. Using the FecA1 protein (UniProt ID: O25395) as the COPICAT input, nordihydroguaiaretic acid (NDGA), sevoflurane, and enflurane were a predicted FecA1-binding compound. Considering the clinical application and toxicity of these compounds, sevoflurane and enflurane were excluded. We focused on the effect of NDGA on the intracellular Fe2+ levels and SodB activity in H. pylori (Figure 1).
Figure 1.
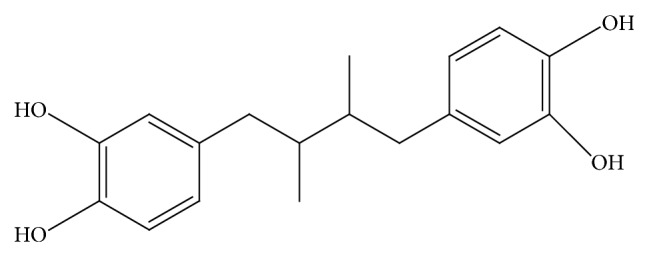
Chemical structure of nordihydroguaiaretic acid (NDGA). Other name of NDGA is Masoprocol. Chemical structural formula of NDGA was described using the ChemBioDraw13.0.
3.2. Effect of NDGA on Intracellular Fe2+ Levels and SodB Activity in H. pylori
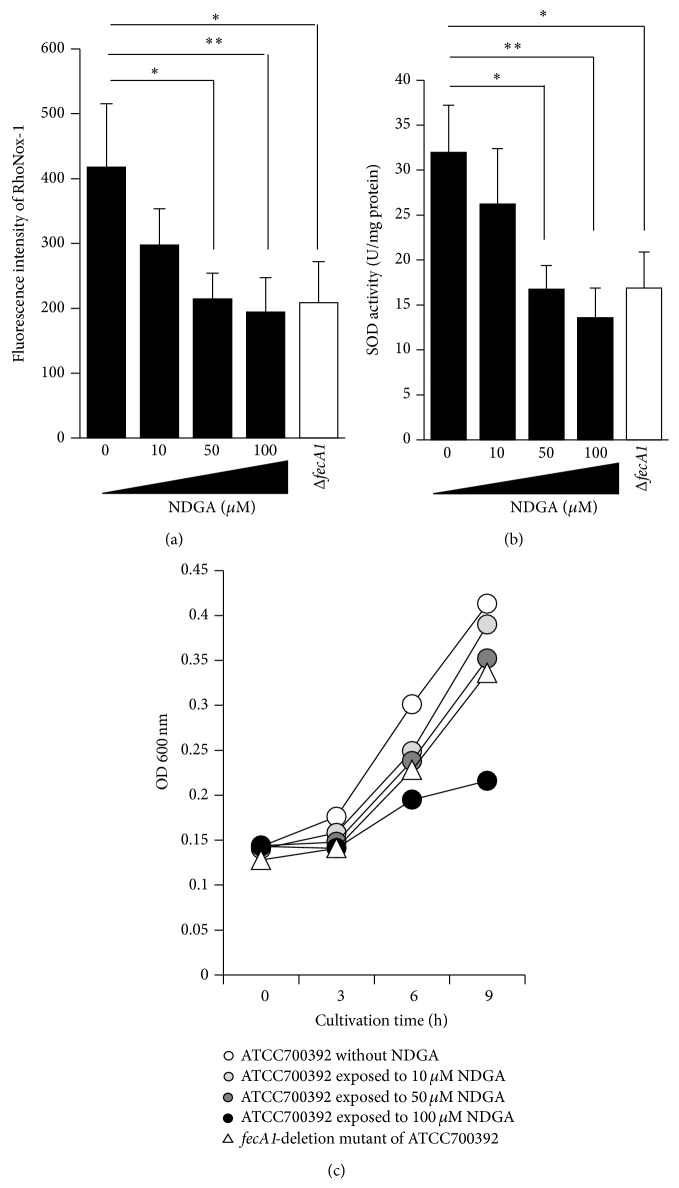
We examined the effect of NDGA on intracellular Fe2+ levels using a turn-on fluorescent probe, RhoNox-1, for the selective detection of Fe2+ [12]. The intracellular Fe2+ levels were significantly lower in NDGA-treated strains than in control strains, and this effect was dose-dependent (Figure 2(a)). Previously, we demonstrated that fecA1-deletion mutant strains show lower SodB activity than the wild-type strain [8]. Therefore, we investigated the effect of NDGA on SodB activity. The SodB activity was significantly decreased after exposure to NDGA in a dose-dependent manner (Figure 2(b)). Treatment with 50 μM NDGA decreased the SodB activity to the same level observed in the fecA1-deletion mutant strains (ΔfecA1) (Figure 2(b)). To evaluate the effect of NDGA on the bacterial viability, we estimated growth rates after NDGA exposure by measuring optical density (OD600 nm). Although the bacterial growth was suppressed after exposure to 100 μM NDGA, it was hardly influenced by exposure to 10 and 50 μM NDGA (Figure 2(c)). Similarly, deletion of the fecA1 gene had little influence on the bacterial growth (Figure 2(c)). These results suggested that 50 μM NDGA was a potent selective inhibitor of SodB activity in H. pylori via the repression of intracellular Fe2+ levels, without bacteriostatic activity.
Figure 2.
Effect of NDGA on intracellular Fe2+ levels, SOD activity, and bacterial growth. (a) Intracellular Fe2+ levels in ATCC700392 exposed to NDGA and fecA1-deletion mutant of ATCC700392 (ΔfecA1) were measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test). (b) The SodB activities in ATCC700392 exposed to NDGA and fecA1-deletion mutant of ATCC700392 (ΔfecA1) were measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test). (c) The growth rates of ATCC700392 without NDGA (white circle), ATCC700392 exposed to 10 μM NDGA (gray circle), ATCC700392 exposed to 50 μM NDGA (deep-gray circle), ATCC700392 exposed to 100 μM NDGA (black circle), and fecA1-deletion mutant of ATCC700392 (white triangle) were measured by measuring optical density (OD 600 nm).
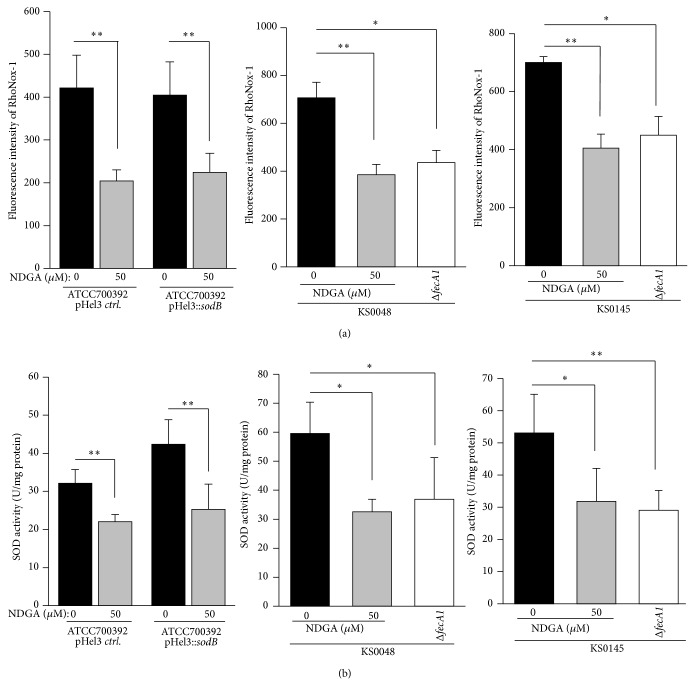
Recently, we demonstrated that SodB activities of Mtz-resistant strains (KS0048 and KS0145) increase due to Fur amino acid mutations [10]. Therefore, we investigated whether NDGA repressed the enhanced SodB activities of KS0048 and KS0145. The intracellular Fe2+ levels of the SodB-overexpressing strain (ATCC700392 pHel3::sodB) as well as that of the control strain (ATCC700392 pHel3 ctrl) decreased significantly for exposure to 50 μM NDGA (Figure 3(a)). Similarly, the intracellular Fe2+ levels of KS0048 and KS0145 decreased significantly to levels comparable to the fecA1-deletion mutant strains (KS0048ΔfecA1 and KS0145ΔfecA1, resp.) (Figure 3(a)). The SodB activities of these strains decreased significantly to the same levels as those of fecA1-deletion mutant strains (KS0048ΔfecA1 and KS0145ΔfecA1, resp.) (Figure 3(b)). These results suggested that NDGA also repressed the enhanced SodB activities of KS0048 and KS0145.
Figure 3.
The intracellular Fe2+ levels and the SOD activities of Mtz-resistant strains under exposure to 50 μM NDGA. (a) The intracellular Fe2+ levels of control strain (ATCC700392 pHel3 ctrl.), SodB-overexpressing strain (ATCC700392 pHel3::sodB), KS0048, fecA1-deletion mutant of KS0048 (KS0048ΔfecA1), KS0145, and fecA1-deletion mutant of KS0145 (KS0145ΔfecA1) were measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test). (b) The SOD activities of control strain (ATCC700392 pHel3 ctrl.), SodB-overexpressing strain (ATCC700392 pHel3::sodB), KS0048, fecA1-deletion mutant of KS0048 (KS0048ΔfecA1), KS0145, and fecA1-deletion mutant of KS0145 (KS0145ΔfecA1) were measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test).
3.3. Effect of NDGA on Mtz Susceptibility of H. pylori
Previously, we demonstrated that fecA1-deletion mutants of KS0048 and KS0145 have decreased MICs of Mtz [8]. Therefore, we examined whether Mtz resistance of KS0048 and KS0145 was reversed by NDGA treatment. The MICs of Mtz for KS0048 and KS0145 decreased from 32 to 8 and from 128 to 16 μg/mL, respectively (Table 1). In particular, the Mtz resistance of ATCC700392 pHel3::sodB was completely reversed by treatment with 50 μM NDGA (MIC < 8 μg/mL) (Table 1).
Table 1.
Effect of NDGA on MICs (μg/mL) of Mtz-resistant strains.
| Strain numbers | Without NDGA | With 50 μM NDGA |
|---|---|---|
| ATCC700392 | 32 | 4 |
| pHel3::sodB | ||
| KS0048 | 32 | 8 |
| KS0145 | 128 | 16 |
3.4. Effect of NDGA on H. pylori H2O2 Sensitivity
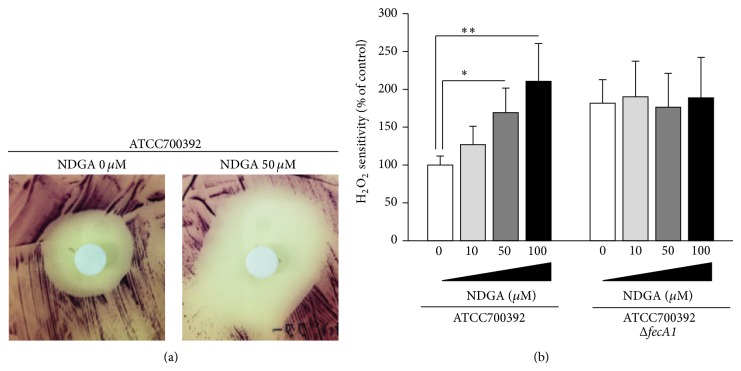
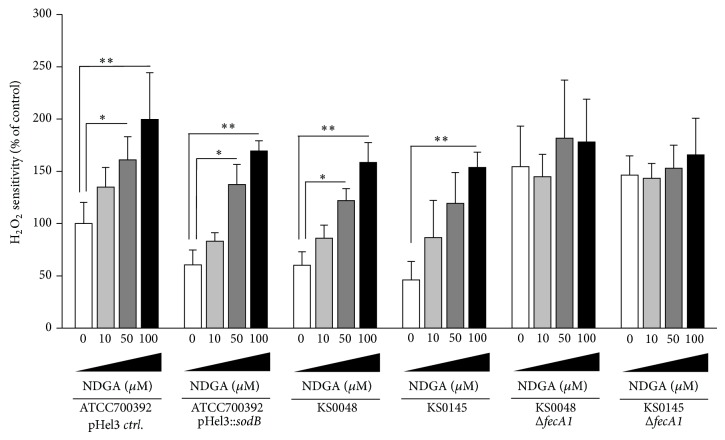
To investigate whether NDGA repressed the antioxidant ability of H. pylori, we examined the H2O2 sensitivity of H. pylori by disk assays. The H2O2 sensitivity of H. pylori was significantly increased by NDGA treatment in a dose-dependent manner (Figures 4(a) and 4(b)). On the other hand, enhanced H2O2 sensitivity of fecA1-deletion mutant strains (ATCC700392ΔfecA1) was not affected by NDGA treatment (Figure 4(b)). Similarly, the H2O2 sensitivity of SodB-overexpressing strains (ATCC700392 pHel3::sodB, KS0048, and KS0145) was significantly increased after exposure to NDGA in a dose-dependent manner (Figure 5). Enhanced H2O2 sensitivity by deletion of fecA1 genes (KS0048ΔfecA1 and KS0145ΔfecA1) was not affected by NDGA treatment (Figure 5). From these results, it was thought that NDGA disrupted the SodB-dependent antioxidant ability of H. pylori via FecA1 inhibition.
Figure 4.
Effect of NDGA on H2O2 sensitivity. (a) Representative disk assays for H2O2 sensitivity of ATCC700392 exposed to 50 μM NDGA. (b) The H2O2 sensitivity of ATCC700392 and fecA1-deletion mutant of ATCC700392 (ATCC700392ΔfecA1) under exposure to NDGA was measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test).
Figure 5.
The H2O2 sensitivity of Mtz-resistant strains under exposure to NDGA. The H2O2 sensitivities of control strain (ATCC700392 pHel3 ctrl.), SodB-overexpressing strain (ATCC700392 pHel3::sodB), KS0048, KS0145, fecA1-deletion mutant of KS0048 (KS0048ΔfecA1), and fecA1-deletion mutant of KS0145 (KS0145ΔfecA1) were measured by the method described under Materials and Methods. Results are means ± SD of three independent assays. * P < 0.05; ** P < 0.01 (Tukey test).
4. Discussion
Previously, we demonstrated that fecA1-deletion mutant strains show reduced host colonization owing to the inactivation of SodB, suggesting that H. pylori was eradicated by excessive ROS mediated by host immune responses [8]. In this in vitro study, we showed that NDGA treatment inhibited the SodB activity and increased the H2O2 sensitivity to the same levels observed in fecA1-deletion mutant strains. Therefore, it was expected that NDGA might reduce the host-colonization ability of H. pylori.
Although the growth of H. pylori was suppressed under 100 μM NDGA exposure, it was minimally influenced under 50 μM NDGA, suggesting that high concentrations of NDGA showed bacteriostatic activity (Figure 2(c)). Since the lower intracellular Fe2+ levels under 100 μM NDGA exposure were the same as those observed for 50 μM NDGA exposure, the bacteriostatic activity and the repression of intracellular Fe2+ levels are caused by different mechanisms.
The present study showed that NDGA repressed the SodB activity without bactericidal activity by itself (Figure 2). As a result, NDGA enhanced the bactericidal activity by ROS exposure (Figure 4). These results suggested that NDGA is effective for selective eradication therapy of pathogenic bacteria of inducing inflammatory response without affecting intestinal microbiota that does not induce an inflammatory response. Therefore, H. pylori eradication therapy with NDGA is expected to contribute to the development of selective eradication therapy of H. pylori by excessive ROS in host gastric mucosa without affecting intestinal microbiota. H. pylori eradication therapy often induces side effects such as diarrhea and soft stools. It was reported that 10–30% of ulcer patients receiving eradication therapy experienced diarrhea and soft stools [13, 14]. The reason for these side effects was assumed to be a disturbance in the composition of intestinal microbiota by antibiotics. Therefore, H. pylori eradication therapy with NDGA is expected to be effective in reducing the side effects such as diarrhea and soft stools.
Recently, the prevalence of Mtz resistance has increased in Asia and Europe [15–17]. It is suggested that the widespread use of Mtz may contribute to the increasing prevalence of Mtz resistance [16, 18]. Mtz is a prodrug, and its bactericidal activity is dependent on the generation of superoxide radicals mediated by the reduction of its nitro group to nitro anion radicals [19, 20]. Therefore, enhanced SodB activity is associated with the development of Mtz resistance [10]. In the present study, it was shown that NDGA repressed the SodB activity and then increased Mtz sensitivity in Mtz-resistant strains with SodB overexpression (Figure 3(b) and Table 1). From these results, it is expected that Mtz and NDGA combination therapy is effective for eradicating Mtz-resistant strains and preventing the development of Mtz resistance.
NDGA is the main metabolite of the creosote bush, Larrea tridentata, which is commonly known as chaparral or greasewood in the United States and as gobernadora or hediondilla in Mexico [21]. The creosote bush has been widely used in Mexican traditional herbal medicine [22]. It is known that NDGA is a potent scavenger of ROS such as peroxynitrite (ONOO−), singlet oxygen (1O2), hydroxyl radicals (•OH), superoxide anion (O2 −•), H2O2, and hypochlorous acid (HOCl) [23]. Because the H2O2 sensitivity of H. pylori exposed to NDGA was significantly increased, it was thought that the SodB-inactivation caused by NDGA is independent of its ROS-scavenging activity. The present study is the first demonstration that NDGA suppresses the SodB activity of H. pylori via repression of intracellular Fe2+ by FecA1 in vitro. Recent studies have indicated the inhibitory effect of NDGA against N-methyl-N-nitrosourea-initiated and H. pylori-promoted gastric carcinogenesis in Mongolian gerbils [24]. This inhibitory effect of NDGA might be associated with antioxidant activity and inhibitory effects on the progression of gastritis [24]. Therefore, it is expected that NDGA might be effective for both the eradication of H. pylori and the prevention of gastric carcinogenesis.
From our in vitro findings, it is expected that NDGA would bind to FecA1 in vivo. To investigate whether NDGA binds to FecA1 in vivo, further experiments in which fluorescence intensity and fluorescence localization are examined by immunohistochemical analysis using H. pylori infection Mongolian gerbil treated with fluorescently labeled NDGA are needed.
The reported acute NDGA LD50 ranges between 800 and 500 mg/kg body weight orally in rodents [22]. Although administration of 3% NDGA results in renal toxicity in rats, 0.5% and 1% NDGA did not exhibit toxicity [22]. Further studies are needed to determine the effect and toxicity of 50 μM NDGA in vivo. In addition, it is important to analyze the binding mode of NDGA with FecA1 in vitro, which would contribute to the chemical modification of NDGA that mediates the toxicity.
In conclusion, NDGA repressed the SodB activity associated with the gastric mucosal-colonization ability via repression of intracellular Fe2+ by FecA1 and increased the H2O2 sensitivity and the Mtz sensitivity of H. pylori in vitro.
Acknowledgments
The authors are grateful to Kouichi Takahashi, Ph.D. (National Center for Geriatrics and Gerontology), for valuable advice of chemical structure. This work was supported by a grant-in-aid for Challenging Exploratory Research from Japan Society for the Promotion of Science (no. 26670065, to Hidekazu Suzuki), the Translational Research Network Program from Ministry of Education, Culture, Sports, Science and Technology of Japan (to Hidekazu Suzuki), the Princess Takamatsu Cancer Research grants (to Hidekazu Suzuki), and a grant from the Smoking Research Foundation (to Hidekazu Suzuki).
Conflict of Interests
During the last 2 years, the author Hidekazu Suzuki received scholarship funds for the research from Astellas Pharm Inc., Astra-Zeneca K.K., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd., and received service honoraria from Astellas Pharm Inc., Astra-Zeneca K.K., Eisai Co., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., and Zeria Pharmaceutical Co., Ltd.
References
- 1.Suzuki H., Hibi T., Marshall B. J. Helicobacter pylori: present status and future prospects in Japan. Journal of Gastroenterology. 2007;42(1):1–15. doi: 10.1007/s00535-006-1990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagchi D., Bhattacharya G., Stohs S. J. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori . Free Radical Research. 1996;24(6):439–450. doi: 10.3109/10715769609088043. [DOI] [PubMed] [Google Scholar]
- 3.Allen L.-A. H. Phagocytosis and persistence of Helicobacter pylori . Cellular Microbiology. 2007;9(4):817–828. doi: 10.1111/j.1462-5822.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang G., Alamuri P., Maier R. J. The diverse antioxidant systems of Helicobacter pylori . Molecular Microbiology. 2006;61(4):847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- 5.Spiegelhalder C., Gerstenecker B., Kersten A., Schiltz E., Kist M. Purification of Helicobacter pylori superoxide dismutase and cloning and sequencing of the gene. Infection and Immunity. 1993;61(12):5315–5325. doi: 10.1128/iai.61.12.5315-5325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pesci E. C., Pickett C. L. Genetic organization and enzymatic activity of a superoxide dismutase from the microaerophilic human pathogen, Helicobacter pylori . Gene. 1994;143(1):111–116. doi: 10.1016/0378-1119(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 7.Seyler R. W., Jr., Olson J. W., Maier R. J. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infection and Immunity. 2001;69(6):4034–4040. doi: 10.1128/iai.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsugawa H., Suzuki H., Matsuzaki J., Hirata K., Hibi T. FecA1, a bacterial iron transporter, determines the survival of Helicobacter pylori in the stomach. Free Radical Biology and Medicine. 2012;52(6):1003–1010. doi: 10.1016/j.freeradbiomed.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Worst D. J., Gerrits M. M., Vandenbroucke-Grauls C. M. J. E., Kusters J. G. Helicobacter pylori ribBA-mediated riboflavin production is involved in iron acquisition. Journal of Bacteriology. 1998;180(6):1473–1479. doi: 10.1128/jb.180.6.1473-1479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsugawa H., Suzuki H., Satoh K., et al. Two amino acids mutation of ferric uptake regulator determines Helicobacter pylori resistance to metronidazole. Antioxidants and Redox Signaling. 2011;14(1):15–23. doi: 10.1089/ars.2010.3146. [DOI] [PubMed] [Google Scholar]
- 11.Sakakibara Y., Hachiya T., Uchida M., et al. COPICAT: a software system for predicting interactions between proteins and chemical compounds. Bioinformatics. 2012;28(5):745–746. doi: 10.1093/bioinformatics/bts031. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama T., Okuda K., Nagasawa H. A highly selective turn-on fluorescent probe for iron(ii) to visualize labile iron in living cells. Chemical Science. 2013;4(3):1250–1256. doi: 10.1039/c2sc21649c. [DOI] [Google Scholar]
- 13.Asaka M., Sugiyama T., Kato M., et al. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6(3):254–261. doi: 10.1046/j.1523-5378.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Miwa H., Ohkura R., Murai T., et al. Impact of rabeprazole, a new proton pump inhibitor, in triple therapy for Helicobacter pylori infection—comparison with omeprazole and lansoprazole. Alimentary Pharmacology and Therapeutics. 1999;13(6):741–746. doi: 10.1046/j.1365-2036.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunn B. E., Cohen H., Blaser M. J. Helicobacter pylori . Clinical Microbiology Reviews. 1997;10(4):720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J. J., Reddy R., Lee M., et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. Journal of Antimicrobial Chemotherapy. 2001;47(4):459–461. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Wouden E. J., Van Zwet A. A., Thijs J. C., et al. Rapid increase in the prevalence of metronidazole-resistant Helicobacter pylori in the Netherlands. Emerging Infectious Diseases. 1997;3(3):385–389. doi: 10.3201/eid0303.970320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsugawa H., Suzuki H., Muraoka H., et al. Enhanced bacterial efflux system is the first step to the development of metronidazole resistance in Helicobacter pylori . Biochemical and Biophysical Research Communications. 2011;404(2):656–660. doi: 10.1016/j.bbrc.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Reyes E., Kalyanaraman B., Mason R. P. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Molecular Pharmacology. 1980;17(2):239–244. [PubMed] [Google Scholar]
- 20.Rao D. N., Mason R. P. Generation of nitro radical anions of some 5-nitrofurane, 2- and 5-nitroimidazoles by norepinephrine, dopamine, and serotonin. A possible mechanism for neurotoxicity caused by nitroheterocyclic drugs. Journal of Biological Chemistry. 1987;262(24):11731–11736. [PubMed] [Google Scholar]
- 21.Lambert J. D., Zhao D., Meyers R. O., Kuester R. K., Timmermann B. N., Dorr R. T. Nordihydroguaiaretic acid: hepatotoxicity and detoxification in the mouse. Toxicon. 2002;40(12):1701–1708. doi: 10.1016/s0041-0101(02)00203-9. [DOI] [PubMed] [Google Scholar]
- 22.Arteaga S., Andrade-Cetto A., Cárdenas R. Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. Journal of Ethnopharmacology. 2005;98(3):231–239. doi: 10.1016/j.jep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Floriano-Sánchez E., Villanueva C., Medina-Campos O. N., et al. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radical Research. 2006;40(5):523–533. doi: 10.1080/10715760500419365. [DOI] [PubMed] [Google Scholar]
- 24.Toyoda T., Tsukamoto T., Mizoshita T., et al. Inhibitory effect of nordihydroguaiaretic acid, a plant lignan, on Helicobacter pylori-associated gastric carcinogenesis in Mongolian gerbils . Cancer Science. 2007;98(11):1689–1695. doi: 10.1111/j.1349-7006.2007.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]