Abstract
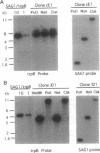
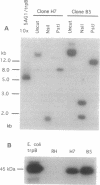
The protozoan parasite Toxoplasma gondii infects a wide range of vertebrate hosts and is an important opportunistic pathogen in immunocompromised humans. Although Toxoplasma is amenable to both biochemical and cellular experimental approaches, the molecular basis of its success as an intracellular parasite is poorly understood. To provide a system for molecular genetic analyses, we have developed a stable DNA transformation system for Toxoplasma based on complementation of its naturally occurring tryptophan auxotrophy. Complementation was accomplished by expressing the Escherichia coli trpB gene, encoding the beta subunit of tryptophan synthase (EC 4.2.1.20), the enzyme that catalyzes the formation of tryptophan from indole plus serine. Transformants were obtained by electroporation of a plasmid, called SAG1/trpB, containing the trpB gene flanked by Toxoplasma surface antigen 1 (SAG1) gene sequences and selection for growth on indole. Transformants were obtained with circular forms of the SAG1/trpB plasmid with efficiencies of 10(-4) per cell. Transformation with either circular or linear SAG1/trpB resulted in integration into the genome at distinct, nonhomologous sites. Trp+ transformants typically contained tandemly repeated copies of the SAG1/trpB plasmid and were stable in the absence of continued selection. The Trp phenotype provides a dominant selectable marker that should allow expression of foreign or altered genes in Toxoplasma and facilitate molecular analyses of genes important for intracellular survival.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg J. L., Perelman D., Kasper L. H., Ware P. L., Boothroyd J. C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988 Nov 15;141(10):3584–3591. [PubMed] [Google Scholar]
- Crawford I. P., Nichols B. P., Yanofsky C. Nucleotide sequence of the trpB gene in Escherichia coli and Salmonella typhimurium. J Mol Biol. 1980 Oct 5;142(4):489–502. doi: 10.1016/0022-2836(80)90259-4. [DOI] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Donald R. G., Roos D. S. Homologous recombination and gene replacement at the dihydrofolate reductase-thymidylate synthase locus in Toxoplasma gondii. Mol Biochem Parasitol. 1994 Feb;63(2):243–253. doi: 10.1016/0166-6851(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Eid J., Sollner-Webb B. Stable integrative transformation of Trypanosoma brucei that occurs exclusively by homologous recombination. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2118–2121. doi: 10.1073/pnas.88.6.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Goldberg M. E. Polypeptide-antibody binding mechanism: conformational adaptation investigated by equilibrium and kinetic analysis. Res Immunol. 1989 May;140(4):355–376. doi: 10.1016/0923-2494(89)90142-9. [DOI] [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Soldati D., Boothroyd J. C. Gene replacement in Toxoplasma gondii with chloramphenicol acetyltransferase as selectable marker. Science. 1993 Nov 5;262(5135):911–914. doi: 10.1126/science.8235614. [DOI] [PubMed] [Google Scholar]
- Laban A., Tobin J. F., Curotto de Lafaille M. A., Wirth D. F. Stable expression of the bacterial neor gene in Leishmania enriettii. Nature. 1990 Feb 8;343(6258):572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Van der Ploeg L. H. Homologous recombination and stable transfection in the parasitic protozoan Trypanosoma brucei. Science. 1990 Dec 14;250(4987):1583–1587. doi: 10.1126/science.2177225. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Remington J. S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992 Aug;15(2):211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- Mercier C., Lecordier L., Darcy F., Deslee D., Murray A., Tourvieille B., Maes P., Capron A., Cesbron-Delauw M. F. Molecular characterization of a dense granule antigen (Gra 2) associated with the network of the parasitophorous vacuole in Toxoplasma gondii. Mol Biochem Parasitol. 1993 Mar;58(1):71–82. doi: 10.1016/0166-6851(93)90092-c. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Szuro-Sudol A., Wellner D., Oca M. J., Granger A. M., Libby D. M., Rothermel C. D., Rubin B. Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989 Mar;57(3):845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Quantitative studies of the mutagenesis of Toxoplasma gondii. J Parasitol. 1979 Jun;65(3):364–370. [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn L. C., Pfefferkorn E. R. Toxoplasma gondii: genetic recombination between drug resistant mutants. Exp Parasitol. 1980 Dec;50(3):305–316. doi: 10.1016/0014-4894(80)90034-x. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Boothroyd J. C. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992 Sep 3;359(6390):82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- Sibley L. D. Interactions between Toxoplasma gondii and its mammalian host cells. Semin Cell Biol. 1993 Oct;4(5):335–344. doi: 10.1006/scel.1993.1040. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., LeBlanc A. J., Pfefferkorn E. R., Boothroyd J. C. Generation of a restriction fragment length polymorphism linkage map for Toxoplasma gondii. Genetics. 1992 Dec;132(4):1003–1015. doi: 10.1093/genetics/132.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldati D., Boothroyd J. C. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993 Apr 16;260(5106):349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Garrity L. F., Brandt C. R., Schobert C. S., Feng G. S., Taylor M. W., Carlin J. M., Byrne G. I. IFN-gamma-mediated antimicrobial response. Indoleamine 2,3-dioxygenase-deficient mutant host cells no longer inhibit intracellular Chlamydia spp. or Toxoplasma growth. J Immunol. 1993 Jun 15;150(12):5529–5534. [PubMed] [Google Scholar]
- ten Asbroek A. L., Ouellette M., Borst P. Targeted insertion of the neomycin phosphotransferase gene into the tubulin gene cluster of Trypanosoma brucei. Nature. 1990 Nov 8;348(6297):174–175. doi: 10.1038/348174a0. [DOI] [PubMed] [Google Scholar]