Abstract
Purpose
To compare 25-hydroxyvitamin D (25OHD) levels in patients with neovascular age-related macular degeneration (NVAMD) with patients with nonneovascular age-related macular degeneration and control patients.
Methods
Medical records of all patients diagnosed with age-related macular degeneration and tested for serum 25OHD level at a single medical center were reviewed. Control patients were selected from patients diagnosed with pseudophakia but without age-related macular degeneration. The lowest 25OHD level available for each patient was recorded.
Results
Two hundred sixteen patients with nonneovascular age-related macular degeneration, 146 with NVAMD, and 100 non–age-related macular degeneration control patients were included. The levels of 25OHD (mean ± SD) were significantly lower in NVAMD patients (26.1 ± 14.4 ng/mL) versus nonneovascular age-related macular degeneration (31.5 ± 18.2 ng/mL, P = 0.003) and control (29.4 ± 10.1 ng/mL, P = 0.049) patients. The prevalence of vitamin D insufficiency (<30 ng/mL 25OHD), deficiency (<20 ng/mL), and severe deficiency (<10 ng/mL) were highest in the NVAMD group. The highest quintile of 25OHD was associated with a 0.35 (95% confidence interval, 0.18– 0.68) odds ratio for NVAMD.
Conclusion
This is the largest study to compare 25OHD levels in patients with the different clinical forms of age-related macular degeneration. Mean 25OHD levels were lower and vitamin D deficiency was more prevalent in NVAMD patients. These associations suggest that further research is necessary regarding vitamin D deficiency as a potentially modifiable risk factor for the development of NVAMD.
Keywords: vitamin D, 25-hydroxyvitamin D, vitamin D deficiency, age-related macular degeneration, neovascular AMD, nonneovascular AMD
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness in elderly patients and prevention remains a critical issue to reduce the burden of the disease. Modifiable risk factors for the development and progression of AMD include cigarette smoking, hypertension, abdominal obesity, physical activity, and dietary fat intake.1–5 Supplementation with high-dose antioxidants, and recently macular carotenoids, have been proven to reduce the progression from intermediate nonneovascular age-related macular degeneration (NNVAMD) to advanced AMD.6,7 Because the pathogenesis of AMD is known to be related to inflammation and angiogenesis, factors that influence these processes may affect the progression from NNVAMD to neovascular age-related macular degeneration (NVAMD).8–10
Vitamin D deficiency has been linked with a number of systemic disorders ranging from multiple sclerosis to tuberculosis.11-12 The biologically active form of vitamin D (1,25-dihydroxyvitamin D) has demonstrated anti-inflammatory and antineovascular properties. Active vitamin D has been shown to inhibit the proliferation of T-helper and cytotoxic T cells while also reducing the production of proinflammatory cytokines IL-2, IL-6, IL-8, and IL-12.12-16 Active vitamin D also demonstrates antineovascular properties because it inhibits endothelial cell proliferation and decreases levels of hypoxia inducible factor 1, which promotes the expression of vascular endothelial growth factor.17,18 Furthermore, active vitamin D has been shown to protect against oxidative stress in vitro possibly by inducing glucose-6-phosphate dehydrogenase (G6PD).19 At the tissue level, genes involved in the production and metabolism of active vitamin D, including CYP27B1 and the vitamin D receptor, are expressed in vitreous, retina, retinal pigment epithelium, and choroidal tissues.20 These findings suggest a plausible biologic link between dysregulation of the normal homeostatic role of vitamin D and the known pathobiology of AMD. The serum level of 25-hydroxyvitamin D (25OHD) is the primary indicator of vitamin D status in the body
Three population-based studies have investigated the relationship between vitamin D levels and the presence of NNVAMD versus non-AMD with conflicting results.21-23 Another three studies, each with limited cohorts, have included data about NVAMD versus NNVAMD patients. Morrison et al 20 compared levels of 25OHD in 50 pairs of discordant siblings with NVAMD versus NNVAMD, and found an insignificant trend toward higher levels in the sibling with the lower AMD severity (P = 0.22). Similarly, Seddon et al 24 found that a lower dietary intake of vitamin D correlated with worse AMD disease. However, in a retrospective cohort study of the Medicare 5% data comparing a population of vitamin D deficient patients versus matched controls, Day et al 25 found no difference in the incident rates of NNVAMD or NVAMD.
Our aim was to compare 25OHD levels in a large cohort of patients with NNVAMD, NVAMD, and controls. Given the antineovascular and anti-inflammatory properties of vitamin D, we hypothesized that lower 25OHD levels and vitamin D deficiency are more associated with NVAMD, versus NNVAMD and control patients.
Methods
Cohorts
After obtaining approval from the Duke University Institutional Review Board, electronic medical records were searched from July 1997 through November 2011 to identify all patients older than 55 years at Duke University Medical Center tested for vitamin D level and diagnosed with NNVAMD (International Classification of Diseases version 9 [ICD-9] code [362.50, 362.51] and NVAMD [362.52]). Patients were included in the NNVAMD group if they were ever advised to use Age-related Eye Disease Study (AREDS) supplementation and if they were without evidence of NVAMD in either eye. Patients were included in the NVAMD group if they had ever been treated, by any method, for a choroidal neovascular membrane not attributed to non-AMD conditions such as pathologic myopia, ocular histoplasmosis, or idiopathic choroidal neovascular membrane. Geographic atrophy (GA) was noted if GA involving the fovea was documented on clinical examination, and these patients were included as a subgroup within NNVAMD patients. A group of 100 patients with no evidence of AMD documented on prior examination were selected, in a masked fashion, from patients with a diagnosis of pseudophakia (v43.1) and whose 25OHD level had been measured; of a random group of 226 patients, a group of 100 were selected to match the NNVAMD group regarding age, sex, and race. After the entire group of controls was selected, further chart review was performed to collect 25OHD levels and additional demographic and medical information on each patient.
Record Review
The first and lowest available 25OHD levels were recorded for each patient and a corresponding creatinine level. All recorded 25OHD levels were determined by the chemiluminescence method at the Duke laboratory using the LIAISON assay (DiaSorin, Stillwater, MN). Patients whose 25OHD level was tested solely with the liquid chromatography–tandem mass (LC-MS/MS) method were included in a separate analysis. Medical records and ICD-9 codes were reviewed for smoking status and the diagnosis of cardiovascular disease, hypertension, or osteoporosis; the most recent medication list was used to determine the total number of systemic (nonophthalmic) prescription medications as a measure of overall morbidity.26 All available medication lists were used to assess if the patient had ever been on vitamin D supplementation. Age at the time of lowest 25OHD level was recorded for each patient, as well as the body mass index measurement nearest to this time point documented in the medical record.
Statistics
Pairwise comparisons of mean 25OHD levels between groups were assessed using a 2-tailed t-test. To control for the seasonal variation in 25OHD levels, an analysis was performed where 25OHD concentrations were adjusted for month of blood acquisition using the local regression (LOESS) procedure (PROC LOESS in SAS version 9.2; SAS Institute, Cary, NC).27 The prevalence of vitamin D insufficiency (25OHD level <30 ng/mL), deficiency (<20 ng/mL), and severe deficiency (<10 ng/mL) was compared between groups using chi-squared analysis. The entire range of lowest 25OHD levels of the NNVAMD, NVAMD, and control groups were then separated into quintiles. Odds ratios (ORs) and 95% confidence intervals (CIs) for NVAMD versus NNVAMD by quintile of serum 25OHD were calculated, and logistic regression was used to adjust for variables where the P value for the differences between groups was less than 0.20. A P value of <0.05 was determined to be significant.
Results
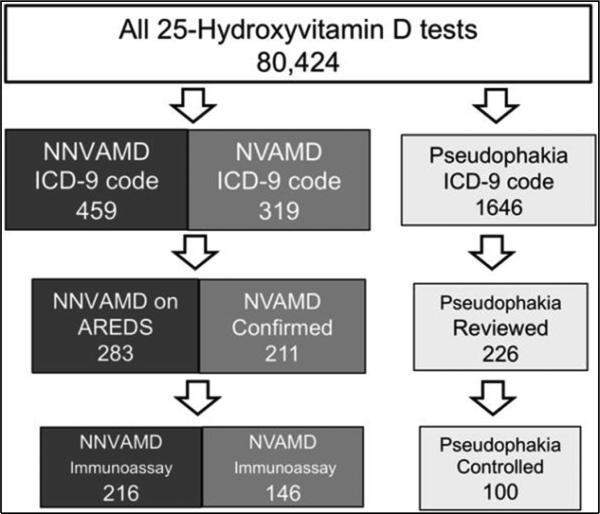
Database review of all patients tested for 25OHD level revealed 459 patients diagnosed with NNVAMD (ICD-9 code 362.50 or 362.51), 319 with NVAMD (ICD-9 code 362.52), and 1,646 patients with pseudophakia (ICD-9 code v43.1) (Figure 1). In the NNVAMD group, 283 (61.6%) patients were identified to be taking or were prescribed AREDS supplementation and 38 (17.6%) were identified to have fovea-involving GA. Of the 319 patients in the NVAMD group, 211 (66%) were confirmed to have NVAMD. A random cohort of 100 patients from the pseudophakia group matched by age, sex, and race to the NNVAMD population were selected as controls. A total of 462 patients both met the inclusion criteria and had 25OHD levels tested by the chemiluminescence method, and were part of the final analysis, including 216 NNVAMD, 146 NVAMD, and 100 control patients.
Fig. 1.
Medical records of all patients tested for 25-hydroxyvitamin D (25OHD) and diagnosed by ICD-9 code for AMD were reviewed. Patients were included in the NNVAMD group if recommended AREDS supplementation and with NVAMD if patient had choroidal neovascularization in any eye not attributable to an alternate cause; patients whose 25OHD level was tested by LC-MS/MS assay were included in a separate analysis. The control group was selected from patients diagnosed with pseudophakia and tested for 25OHD level. A total of 226 medical records at random were reviewed, of which a control group was selected to match the NNVAMD group for sex, race, and age.
Patients (Table 1) were found to be mostly female and white. NVAMD patients were significantly older than control patients (P = 0.035) and had a higher prevalence of smoking than NNVAMD and control patients (P = 0.043). The body mass index trended to be higher in NVAMD patients compared with that of NNVAMD patients (P = 0.102); the P value for differences between groups for all other variables were greater than 0.20.
Table 1.
Patient Characteristics
|
NNVAMD
|
|||||
|---|---|---|---|---|---|
| Control (N=100) | All (n=216) | GA (n=38) | NVAMD (n=146) | Significance of Difference* | |
| Demographics | |||||
| Female (%) | 78.0 | 75.5 | 84.2 | 78.1 | None |
| White (%) | 92.0 | 95.8 | 94.7 | 93.8 | None |
| Age, mean (SD) | 78.4 (8.6) | 79.1 (9.2) | 81.7 (7.7) | 80.9 (9.4) | + |
| Medical factors | |||||
| BMI (mean), kg/m2 | 25.8 | 26.1 | 26.1 | 27.2 | None |
| Hypertension, % | 79.6 | 78.9 | 81.6 | 83.5 | None |
| Cardiovascular, % | 27.8 | 35.1 | 26.3 | 31.9 | None |
| Creatinine, mean (SD) | 1.1 (0.5) | 1.1 (1.0) | 1.3 (1.3) | 1.1 (0.6) | None |
| Medications, mean (SD) | 5.7 (2.4) | 6.0 (3.2) | 6.2 (2.9) | 6.1 (3.1) | None |
| On vitamin D Supplements, % | 82.7 | 71.7 | 85.7 | 77.2 | None |
| Smoking, % | 22.7 | 26.9 | 27.0 | 36.6 | + # |
Pairwise comparisons completed to compare NVAMD versus NNVAMD, NVAMD versus control, and control versus NNVAMD Patients. None is noted if P > 0.05 for all comparisons.
P < 0.05 NVAMD versus control patients.
P < 0.05 NVAMD versus NNVAMD patients
$ P > 0.05 for all differences between control and NNVAMD patients.
BMI, body mass index.
Serum 25OHD Status and Age-Related Macular Degeneration
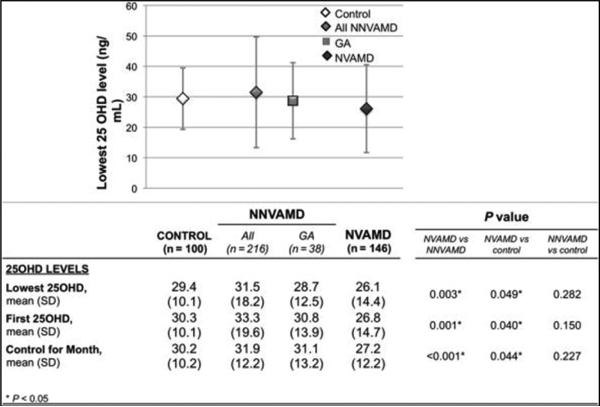
In 94% of patients, the lowest 25OHD level was also the first level measured, and thus the lowest values are presented in all further analyses. The distribution of the 25OHD values is presented in Figure 2. Mean levels were significantly lower in NVAMD patients versus NNVAMD (P = 0.003) and control patients (P = 0.049); the differences remained significant after controlling individually for differences in age (P = 0.006), body mass index (P = 0.028), and smoking status (P = 0.011), and also when controlling for these all these variables in multiple regression (P = 0.033).
Fig. 2.
Distribution of 25-hydroxyvitamin D levels according to group: mean of the lowest level graphed. Error bars represent SD
There was no season where there was a statistically significant difference between groups regarding proportion of 25OHD tests in that season (P = 1.00). When controlling for seasonal variation using the LOESS analysis, mean levels remained significantly lower in NVAMD versus NNVAMD (P = 0.003) and control patients (P = 0.049). The difference in means between NNVAMD and controls was not significant.
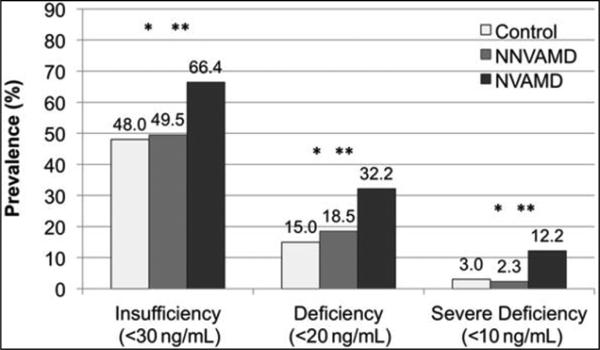
The prevalence of vitamin D insufficiency, deficiency, and severe deficiency (Figure 3) were all highest in the NVAMD versus NNVAMD and control patients. Although NVAMD patients were only 1.3 times (95% CI, 1.03–1.72, P = 0.002) more insufficient compared with NNVAMD, they were approximately 5.3 times (95% CI, 1.6– 19.0, P < 0.001) more severely deficient.
Fig. 3.
Prevalence of vitamin D insufficiency, deficiency, and severe deficiency according to group. *P P P > 0.05 for all differences between control and NNVAMD patients
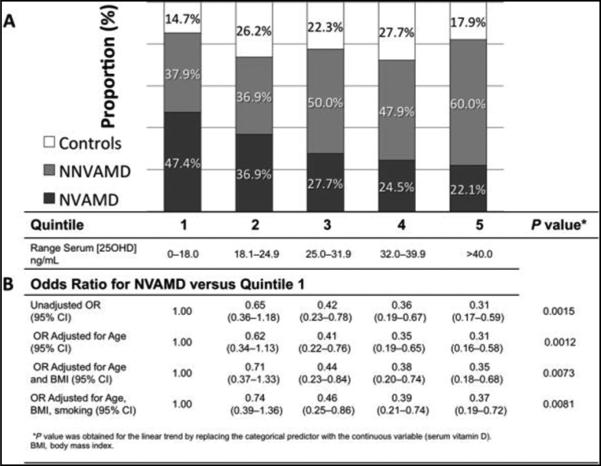
This relationship is further highlighted when 25OHD levels of NNVAMD, NVAMD, and control patients were separated into quintiles (Figure 4) and the proportion of NVAMD patients was highest in the lowest quintile of 25OHD levels. Figure 4B presents the odds of having NVAMD in each higher quintile versus quintile 1. The age and body mass index-adjusted OR for NVAMD in the highest versus lowest quintile was 0.35 (95% CI, 0.18–0.68). The odds for NVAMD decreased with increasing 25OHD level (P = 0.0073). There was no significant difference between ORs in each quintile versus quintile 1 for NNVAMD compared with controls (data not shown).
Fig. 4.
Quintile analysis of 25-hydroxyvitamin D levels: A. overall proportions of patients of each group in each quintile. B. Odds ratios and 95% CIs for NVAMD by quintiles of serum vitamin D level
Geographic Atrophy
Thirty-eight patients in the NNVAMD group were identified to have GA affecting the fovea and central vision. Geographic atrophy patients trended older than non-GA NNVAMD patients (P = 0.059). Mean levels of 25OHD levels in GA patients were not significantly different than NVAMD patients (28.7 vs. 26.1 ng/mL, P = 0.31) or than non- GA NNVAMD patients (28.7 vs. 32.1 ng/mL, P = 0.30). Rates of vitamin D deficiency (25OHD < 20 ng/mL) in GA patients were not significantly different than NVAMD patients (24 vs. 32%, P = 0.43) or NNVAMD patients (24 vs. 17%, P = 0.36).
Liquid Chromatography–Tandem Mass Method
A separate analysis was performed for the 67 NNVAMD patients and 65 NVAMD patients whose 25OHD level was tested only by the LC-MS/MS assay (see Supplemental Table). Liquid chromatography–tandem mass measurements of 25OHD are not directly comparable with the levels generated by the chemiluminescence method, which has been generally preferred.12,28 In these patients, mean levels were not different between groups; however, significantly more NVAMD patients were severely deficient (<10 ng/mL 25OHD) compared with NNVAMD patients (P = 0.043); there was also a trend for more NVAMD patients being vitamin D deficient (10–25 ng/mL 25OHD) (P = 0.091).
Discussion
Our analysis of patients at a single academic institution tested for 25OHD level and diagnosed with AMD demonstrated lower mean levels of 25OHD and higher rates of vitamin D deficiency in NVAMD patients versus NNVAMD and controls. A quintile analysis of all patients demonstrated an adjusted OR of 0.35 for NVAMD in the fifth quintile compared with the first quintile and demonstrated the lowest proportion of NVAMD in patients with the highest levels of 25OHD. This is the largest study to date to compare 25OHD levels in NVAMD versus NNVAMD and the first to find a statistically significant difference in these groups. Conclusions are limited because it was not possible to make a temporal connection between vitamin D testing and AMD diagnosis in this retrospective chart review.
Our findings are in line with previous studies that have compared vitamin D status in AMD patients (Table 2). Morrison et al 20 examined 25OHD levels in 50 pairs of discordant siblings, and found a nonsignificant trend toward lower levels in the affected siblings with NVAMD 24.7 ± 10.5 ng/mL vs. 27.5 ± 10.7 ng/mL, P = 0.22). Seddon et al,24 in a population of monozygotic twins with discordant AMD status, found that a higher dietary intake of vitamin D correlated with a lower drusen burden and an earlier stage of advanced AMD; however, 25OHD levels were not measured. Graffe et al performed a case control study measuring 25OHD levels on 31 patients with AMD (26 with late-stage AMD) versus 34 age-matched controls and found a higher prevalence of vitamin D deficiency in AMD patients (71%) versus control patients (44.1%) (P = 0.03). Day et al performed a retrospective cohort study of the Medicare 5% claims database comparing a population of vitamin D deficient patients versus matched controls. The authors found no difference in the incident rates of NNVAMD or NVAMD; however, the study was driven by ICD-9 coding and did not factor in the possible effect of vitamin D supplementation after the diagnosis of vitamin D deficiency. Furthermore, the diagnosis of vitamin D deficiency was not confirmed by actual vitamin D levels.25
Table 2.
Summary of Existing Clinical Studies Investigating the Relationship Between Vitamin D Status and AMD
| Study | Population (n) | Result |
|---|---|---|
| Parekh et al22 | NHANES III (7,752 civilians) | For all patients, 0.64 odds of early AMD in highest quintile of 25OHD level |
| Millen et al21 | CAREDS/WHIOS (1,313 post menopausal women) | For all patients, 0.72 odds of AMD in highest quintile, For all patients <75 years of age, 0.52 odds of AMD in highest quintile |
| Golan et al23 | Israel Maccabi Health care Services database (1,045 AMD and 8,124 controls) | No difference in mean 25OHD levels between AMD and controls (24.1 vs 24.13 ng/mL, P = 0.925 |
| Day et al25 | Medicare 5% database (6,966 vitamin D deficient, 6,966 controls) | No difference in incident rates of AMD in vitamin D deficient patients versus controls (8.90 vs. 9.14%, P = 0.62 |
| Morrison et al20 | Discordant sibling cohort, 100 pts | Trend toward lower levels in NVAMD versus NNVAMD sibling (24.7 + 10.5 ng/mL vs 27.5 + 10.7 ng/mL, P = 0.22) |
| Seddon et al24 | Discordant twins cohort, 60 pts | Higher vitamin D intake in twin with less advanced AMD (200.3+/- 18.5 vs 170.9 +/− 16.9 IU/day, P = 0.01 |
| Graffe et al35 | 31 adutls with AMD (26 with late-stage AMD) 34 age-matched controls |
Vitamin D def (<20 ng/mL) more prevalent in AMD patients (71.0%) vs. controls (44.1%) (P = 0.03). Those with vitamin D deficiency had an adjusted OR of 3.29 for development of late-stage NVAMD |
CAREDS, Carotenoids in Age-Related Eye Disease Study; IU, international units; NHANES III, Third National Health and Nutrition Examination Survey; WHIOS, Women's Health Initiative Observational Study; pt, patients
We found no significant difference between NNVAMD and controls regarding mean 25OHD level or proportion with vitamin D deficiency. Although this is in accordance with findings from Golan et al, which analyzed patients in a health care maintenance organization in Israel, our findings contrast larger population-based studies of the NHANES III and CAREDS populations that found a protective of effect of higher 25OHD levels for NNVAMD versus non-AMD patients.21–23 It should be noted that AMD diagnosis in the study by Golan et al was purely driven by ICD-9 coding, whereas the studies of the NHANES III and CAREDS populations used fundus photography and a certified reading center. In each of these three studies, the number of NVAMD patients has been small, thus limiting any definitive conclusions about the effect of vitamin D status on NVAMD versus NNVAMD.
Ours is the first study to report on 25OHD levels in patients with GA. This subset of NNVAMD patients demonstrated mean 25OHD levels and proportions of vitamin D deficiency above the remaining NNVAMD patients and below NVAMD patients. These differences were not statistically significant. The association between vitamin D deficiency and GA is unclear from our data.
The quintile analysis of 25OHD levels has been used in multiple epidemiologic studies to investigate the effect of 25OHD levels on a wide range of medical conditions.29,30 Based on ORs in the highest quintile of 25OHD levels, the protective effect of higher 25OHD levels for NVAMD versus NNVAMD in our study was stronger than what was identified for NNVAMD versus non-AMD in the NHANES and CAREDS populations.21,22 The protective effect in our study for NVAMD initially becomes significant in the third quintile (adjusted OR, 0.44; 95% CI, 0.23–0.84) when 25OHD levels are >25.0 ng/mL; this roughly correlates to 25OHD levels thought to constitute sufficiency that ranges between 20 and 30 ng/mL.12 As extrarenal production of active vitamin D (1,25-dihydroxyvitamin D) is driven by the availability of 25OHD, lower systemic levels of 25OHD may directly diminish the amount of active vitamin D in the macula.
Included in a separate analysis are 127 patients with AMD whose 25OHD levels were measured by the LC-MS/MS method (see Supplemental Table). This assay, although being able to provide fractionated measurements of 25OHD, has been found to be less reproducible across institutions; total 25OHD levels are not comparable with the chemiluminescence assay, and the LC-MS/MS assay generally provides higher total 25OHD levels and lower rates of vitamin D deficiency when both tests are performed on the same population.28 When comparing NVAMD versus NNVAMD patients, similar relationships existed in this smaller population of patients tested by the LC-MS/MS assay as seen in larger population tested by the chemiluminescence assay.
It is biologically plausible that active vitamin D may affect the development of NVAMD more than that of NNVAMD. Beyond its anti-inflammatory properties, active vitamin D also demonstrates antineovascular properties as it has been shown to inhibit endothelial cell proliferation and reduce levels of hypoxia inducible factor 1 in in vivo models of diabetic retinopathy in mice. Initial investigations into the role of vitamin D in retinal vascular disorders have found that lower 25OHD levels are correlated with worse levels of diabetic retinopathy 31,32; a study of patients with Type 1 diabetes identified that 25OHD levels correlated to severity of diabetic retinopathy more than body mass index, blood pressure, the presence of microalbuminuria and neuropathy.33 Active vitamin D has been found to interrupt key signaling pathways in angiogenesis, which may explain its antitumorigenic properties as well. Finally, active vitamin D has been shown to improve barrier function of cultured human corneal epithelial cells by increasing expression of occludin; its affect on RPE cells is yet unclear.34
Being a retrospective review, there are several limitations in this study. Given the variability of timing of 25OHD testing and eye examinations, there was no temporal connection between time of vitamin D testing and the diagnosis of a patient's ocular disease. Often, patients identified to be deficient at their initial testing were retested multiple times and found to have higher levels of 25OHD; this likely reflects the effect of vitamin D supplementation, and it is not known the effect this may have had on AMD. Furthermore, diagnoses of AMD relied on provider assessments because fundus photographs were not present for all patients. To mitigate this, only NNVAMD patients taking or prescribed AREDS were included to ensure that only AMD patients, presumably with at least intermediate or AREDS category 3, would be included in the study. Also, patients in our analysis were mostly female and white reflecting the general population of patients whose 25OHD level is more likely to be measured; thus, only limited conclusions could be made about other populations. It was not possible to ascertain the indication for vitamin D testing in this retrospective chart review. Finally, no conclusions can be made about causation, and larger prospective studies are required to assess whether vitamin D deficiency is potentially a modifiable risk factor for the development of NVAMD.
In summary, this comparison of 25OHD levels in large cohorts of NNVAMD, NVAMD, and control patients demonstrated lower mean values and higher rates of vitamin D deficiency in NVAMD versus NNVAMD and controls. There are many limitations of the study as a retrospective review including that no temporal connection was made between the timing of AMD diagnosis and vitamin D testing. The results are compelling, though, because of the biologic plausibility of the association. Further studies that correlate 25OHD levels at specific time points in the course of AMD will help elucidate whether vitamin D deficiency is a risk factor for the development of NVAMD.
Supplementary Material
Acknowledgments
Supported in part by NIH Grant 2P308716-06 (K.W.L).
Footnotes
Presented in part at the Annual meeting of the American Society of Retina Specialists, Las Vegas, NV, August 2012; and at the Annual Meeting of the American Academy of Ophthalmology, Chicago, IL, November 2012.
None of the authors have any conflicting interests to disclose
References
- 1.Mitchell P, Wang JJ, Smith W, Leeder SR. Smoking and the 5-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 2002;120:1357–1363. doi: 10.1001/archopht.120.10.1357. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 3.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peeters A, Magliano DJ, Stevens J, et al. Changes in abdominal obesity and age-related macular degeneration: the Atherosclerosis Risk in Communities Study. Arch Ophthalmol. 2008;126:1554–1560. doi: 10.1001/archopht.126.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams PT. Prospective study of incident age-related macular degeneration in relation to vigorous physical activity during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50:101–106. doi: 10.1167/iovs.08-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AREDS 2 Research Group Lutein + zeaxanthin and omega-3 fattty acids for age related macular degeneration: the age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 8.Despriet DD, Klaver CC, Witteman JC, et al. Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA. 2006;296:301–309. doi: 10.1001/jama.296.3.301. [DOI] [PubMed] [Google Scholar]
- 9.Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997;81:154–162. doi: 10.1136/bjo.81.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson LV, Ozaki S, Staples MK, et al. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000;70:441–449. doi: 10.1006/exer.1999.0798. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin North Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. Table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–336. doi: 10.1146/annurev-pharmtox-010510-100611. [DOI] [PubMed] [Google Scholar]
- 15.Lee V, Rekhi E, Hoh Kam J, Jeffery G. Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol Aging. 2012;33:2382–2389. doi: 10.1016/j.neurobiolaging.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert DM, Scheef EA, Wang S, et al. Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci. 2007;48:2327–2334. doi: 10.1167/iovs.06-1210. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shoshan M, Amir S, Dang DT, et al. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther. 2007;6:1433–1439. doi: 10.1158/1535-7163.MCT-06-0677. [DOI] [PubMed] [Google Scholar]
- 19.Bao BY, Ting HJ, Hsu JW, Lee YF. Protective role of 1 alpha, 25-dihydroxyvitamin D3 against oxidative stress in nonmalignant human prostate epithelial cells. Int J Cancer. 2008;122:2699–2706. doi: 10.1002/ijc.23460. [DOI] [PubMed] [Google Scholar]
- 20.Morrison MA, Silveira AC, Huynh N, et al. Systems biology-based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum Genomics. 2011;5:538–568. doi: 10.1186/1479-7364-5-6-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millen AE, Voland R, Sondel SA, et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch Ophthalmol. 2011;129:481–489. doi: 10.1001/archophthalmol.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parekh N, Chappell RJ, Millen AE, et al. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol. 2007;125:661–669. doi: 10.1001/archopht.125.5.661. [DOI] [PubMed] [Google Scholar]
- 23.Golan S, Shalev V, Treister G, et al. Reconsidering the connection between vitamin D levels and age-related macular degeneration. Eye (Lond) 2011;25:1122–1129. doi: 10.1038/eye.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118:1386–1394. doi: 10.1016/j.ophtha.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day S, Acquah K, Platt A, et al. Association of vitamin D deficiency and age-related macular degeneration in medicare beneficiaries. Arch Ophthalmol. 2012;130:1070–1071. doi: 10.1001/archophthalmol.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins AJ, Kroenke K, Unutzer J, et al. Common comorbidity scales were similar in their ability to predict health care costs and mortality. J Clin Epidemiol. 2004;57:1040–1048. doi: 10.1016/j.jclinepi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 27.SAS/STAT® 9.2 User's Guide. 2nd ed. PROC LOESS Statement. SAS Institute Inc.; Cary, NC: 1999. [Google Scholar]
- 28.Lai JK, Lucas RM, Banks E, Ponsonby AL. Variability in vitamin D assays impairs clinical assessment of vitamin D status. Intern Med J. 2012;42:43–50. doi: 10.1111/j.1445-5994.2011.02471.x. [DOI] [PubMed] [Google Scholar]
- 29.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 30.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 31.Patrick PA, Visintainer PF, Shi Q, et al. Vitamin D and retinopathy in adults with diabetes mellitus. Arch Ophthalmol. 2012;130:756–760. doi: 10.1001/archophthalmol.2011.2749. [DOI] [PubMed] [Google Scholar]
- 32.Payne JF, Ray R, Watson DG, et al. Vitamin D insufficiency in diabetic retinopathy. Endocr Pract. 2012;18:185–193. doi: 10.4158/EP11147.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur H, Donaghue KC, Chan AK, et al. Vitamin D deficiency is associated with retinopathy in children and adolescents with type 1 diabetes. Diabetes Care. 2011;34:1400–1402. doi: 10.2337/dc11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin Z, Pintea V, Lin Y, et al. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52:7359–7364. doi: 10.1167/iovs.11-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graffe A, Milea D, Annweiler C, et al. Association between hypovitaminosis D and late stages of age-related macular degeneration: a case-control study. J Am Geriatr Soc. 2012;60:1367–1369. doi: 10.1111/j.1532-5415.2012.04015.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.