Abstract
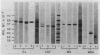
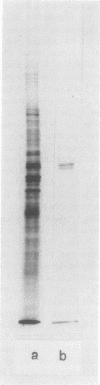
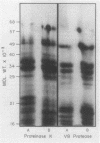
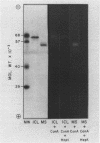
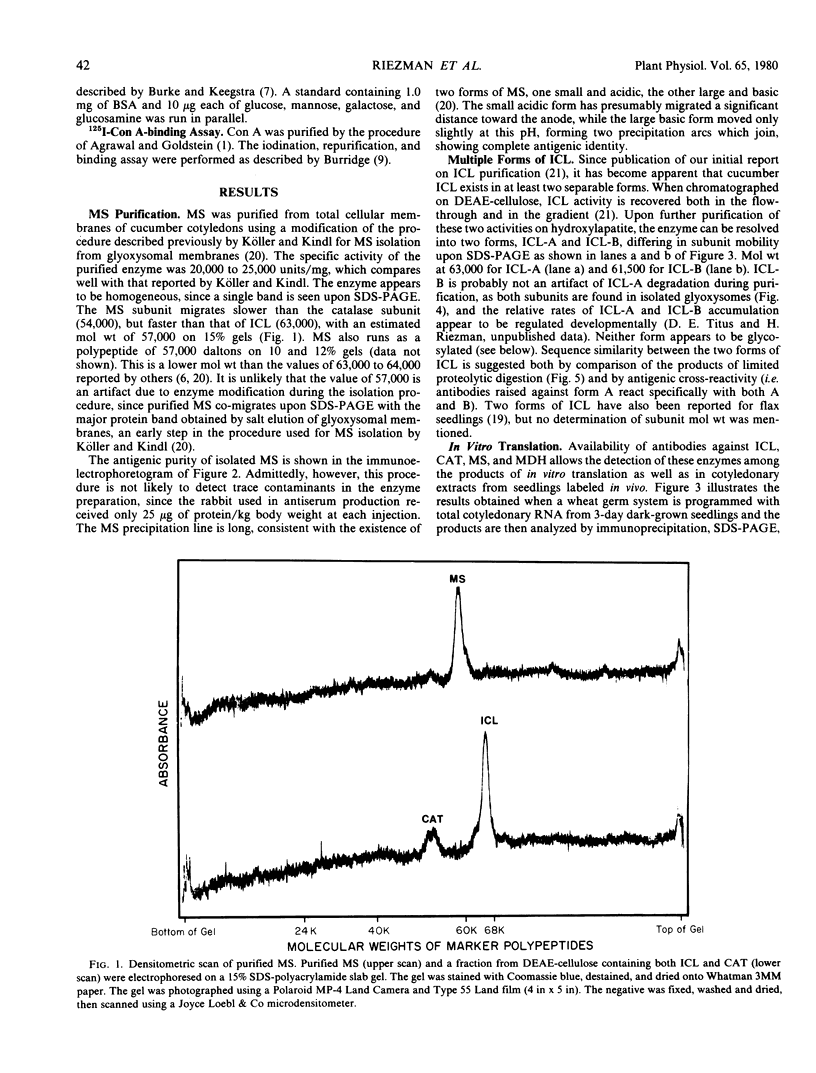
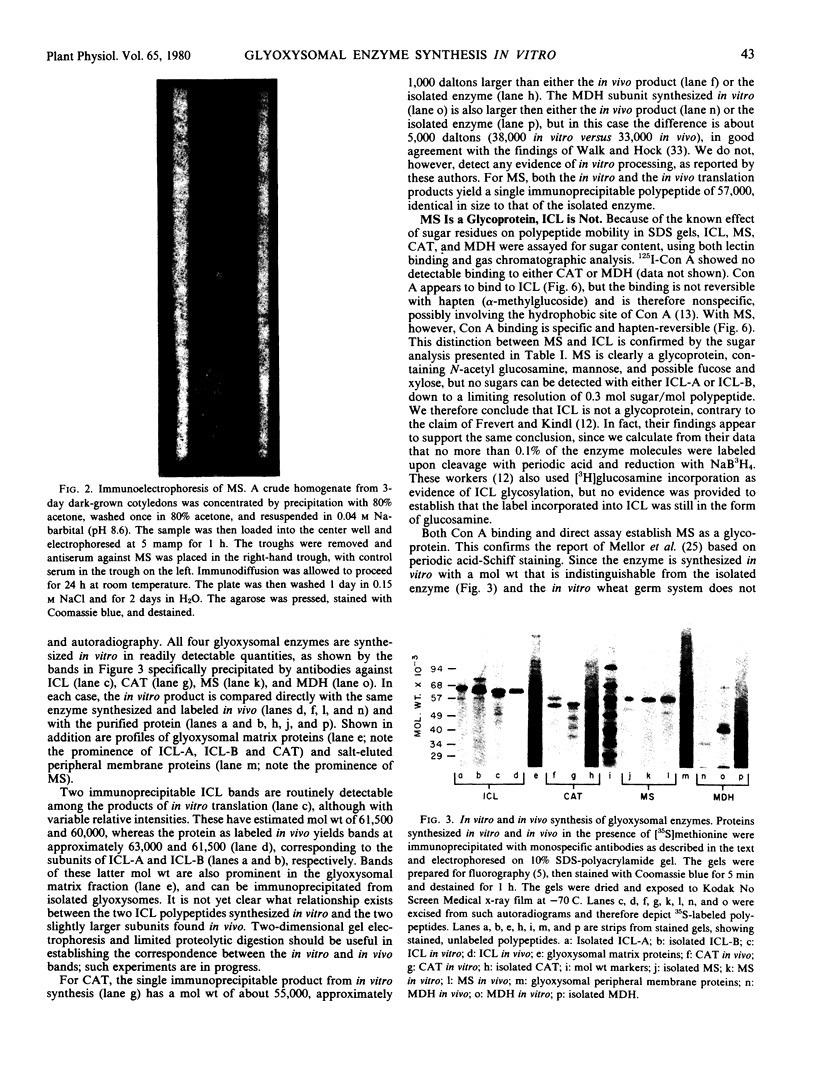
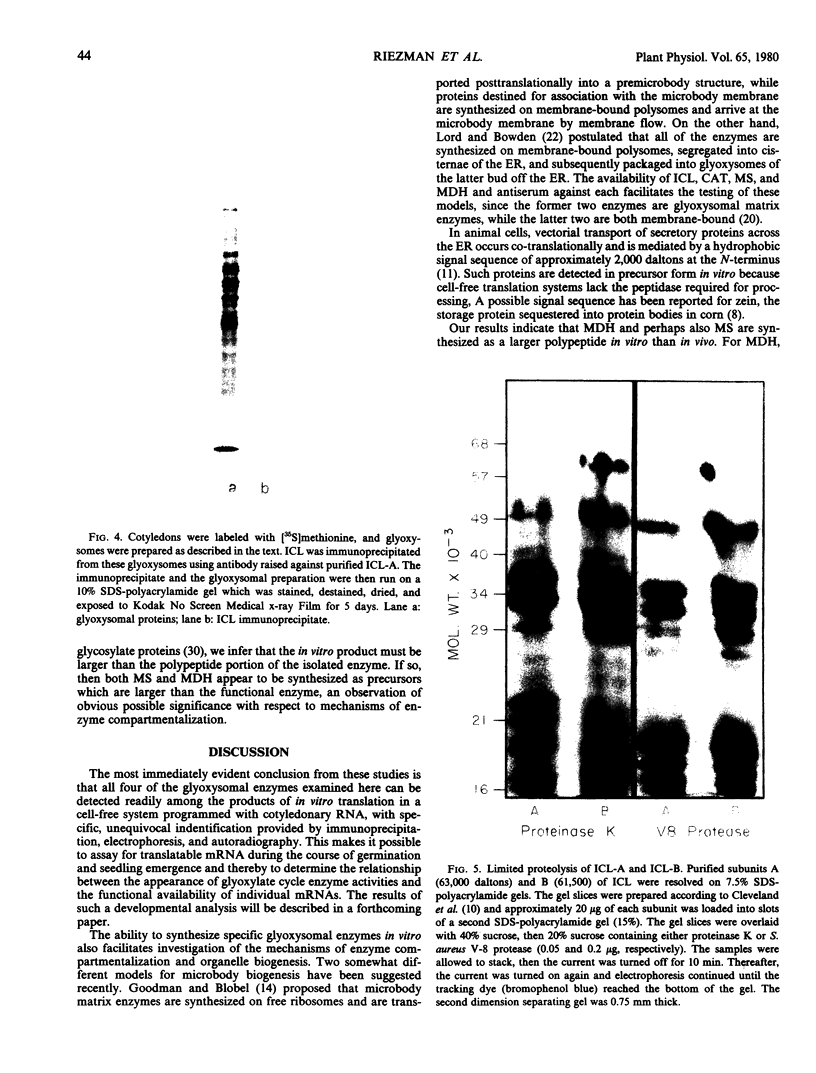
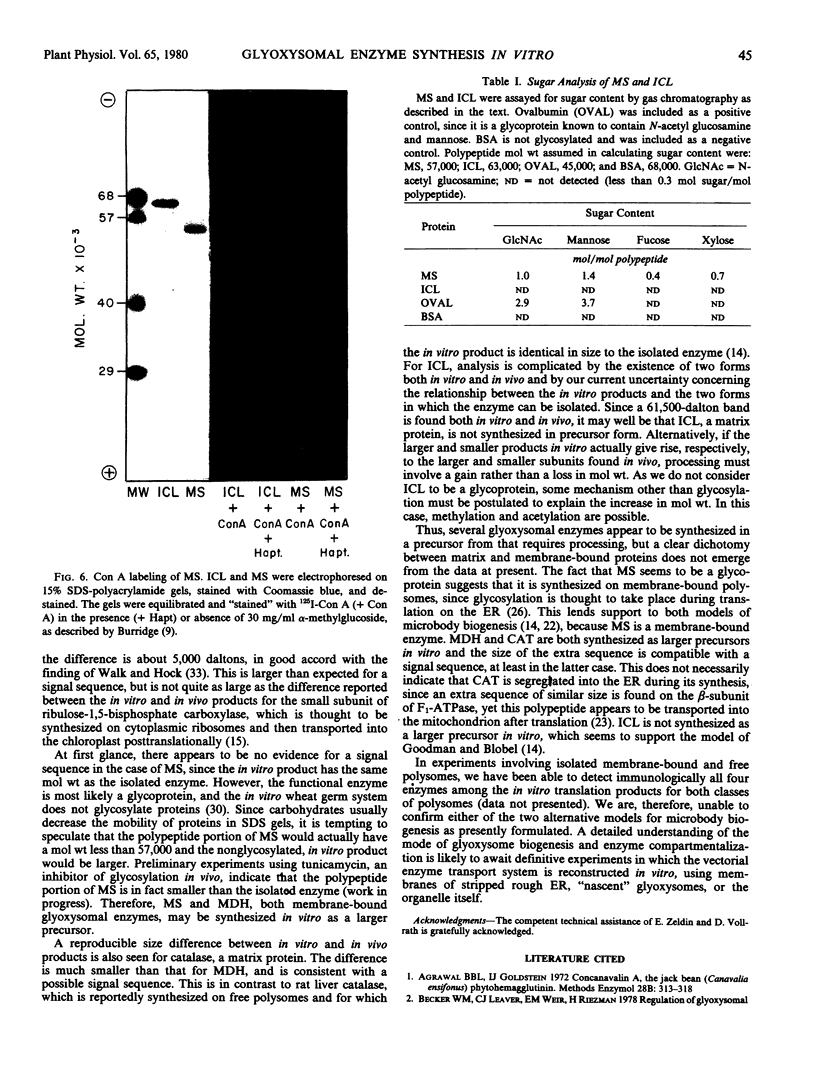
Monospecific antibodies raised against four glyoxysomal enzymes (isocitrate lyase, catalase, malate synthase, and malate dehydrogenase) have been used to detect these proteins among the products of in vitro translation in a wheat germ system programmed with cotyledonary RNA from cucumber seedlings. In vitro immunoprecipitates were compared electrophoretically with the same enzymes labeled in vivo and also with the purified proteins. Isocitrate lyase yields two bands on sodium dodecyl sulfate-polyacrylamide gels, as synthesized both in vitro (61.5K and 60K products) and in vivo (63K and 61.5K polypeptides). Both the 63K and 61.5K subunits can also be demonstrated for the isolated enzyme. The two subunits are antigenically cross-reactive and yield similar electrophoretic profiles upon partial proteolytic digestion. A larger subunit is seen in vitro than in vivo for both malate dehydrogenase (38K versus 33K) and catalase (55K versus 54K); this suggests a need for processing which is often a characteristic of proteins that must be transported across or into membranes. Malate synthase has a molecular weight of 57K both in vitro and in vivo, but the isolated enzyme is a glycoprotein, containing N-acetyl glucosamine, mannose, and possibly also fucose and xylose. This indicates that the polypeptide portion of the isolated enzyme is smaller than the in vitro product and suggests processing of malate synthase also. None of the other three enzymes appears to be glycosylated. The implications of these size differences for the compartmentalization of matrix and membrane-bound glyoxysomal enzymes are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bowden L., Lord J. M. Purification and comparative properties of microsomal and glyoxysomal malate synthase from castor bean endosperm. Plant Physiol. 1978 Feb;61(2):259–265. doi: 10.1104/pp.61.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Keegstra K. Carbohydrate structure of Sindbis virus glycoprotein E2 from virus grown in hamster and chicken cells. J Virol. 1979 Feb;29(2):546–554. doi: 10.1128/jvi.29.2.546-554.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr B., Burr F. A., Rubenstein I., Simon M. N. Purification and translation of zein messenger RNA from maize endosperm protein bodies. Proc Natl Acad Sci U S A. 1978 Feb;75(2):696–700. doi: 10.1073/pnas.75.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- Khan F. R., Saleemuddin M., Siddiqi M., McFadden B. A. Purification and properties of isocitrate lyase from flax seedlings. Arch Biochem Biophys. 1977 Sep;183(1):13–23. doi: 10.1016/0003-9861(77)90413-1. [DOI] [PubMed] [Google Scholar]
- Köller W., Kindl H. Glyoxylate cycle enzymes of the glyoxysomal membrane from cucumber cotyledons. Arch Biochem Biophys. 1977 May;181(1):236–248. doi: 10.1016/0003-9861(77)90502-1. [DOI] [PubMed] [Google Scholar]
- Lamb J. E., Riezman H., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 2. Isolation and Immunological Detection of Isocitrate Lyase and Catalase. Plant Physiol. 1978 Nov;62(5):754–760. doi: 10.1104/pp.62.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Bowden L. Evidence that glyoxysomal malate synthase is segregated by the endoplasmic reticulum. Plant Physiol. 1978 Feb;61(2):266–270. doi: 10.1104/pp.61.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Palacios R., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. II. Quantification and immunoprecipitation of polysomes. J Biol Chem. 1972 May 25;247(10):3296–3304. [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972 Sep 25;29(3):572–578. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walk R. A., Hock B. Cell-free synthesis of glyoxysomal malate dehydrogenase. Biochem Biophys Res Commun. 1978 Mar 30;81(2):636–643. doi: 10.1016/0006-291x(78)91583-8. [DOI] [PubMed] [Google Scholar]