Individuals with congenital insensitivity to pain (CIP) have never felt pain despite possessing an anatomically normal peripheral nervous system and normal intelligence.1 In 2006/7, biallelic null mutations in the transmembrane voltage-gated sodium channel NaV1.7/SCN9A were discovered as a cause of CIP.2, 3 Recently, a new form of CIP was reported in two isolated unrelated cases. Both had recurrent injuries and self-mutilations secondary to feeling no pain, and identical de novo heterozygous p.L811P variants in the voltage-gated sodium channel NaV1.9, encoded by SCN11A.4 We have now identified a further case with the identical variant c.2432T>C (p.(L811P)) and report that all have a complex extended phenotype, see Table 1.
Table 1. A comparison of the phenotype of each individual with congenital insensitivity to pain due to SCN11A/NaV1.9 variant p.L811P.
| Phenotype | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Origin | German | Swedish | Scottish |
| Mutation in SCN11A/NaV1.9 | p.L811P, de novo | p.L811P, de novo | p.L811P, de novo |
| Neuro-cutaneous features | |||
| Peripheral pain felt | No | No | No |
| Self-inflicted injuries | Yes | Yes | Yes |
| Predilection of skin ulcers to cervical region | Yes | Yes | Yes |
| Slow healing wounds | Yes | Yes | Yes |
| Intolerance of moderate cold and moderate heat, but can sense temperature change | Yes | Yes | Yes |
| Sweats more than expected for ambient temperature or exercise done | Yes | Yes | Yes |
| Pruritus | Yes | Yes | Yes |
| Axon flare response | No | No | Yes |
| Can produce tears, can blush, but can't be tickled | Yes | Yes | Yes |
| Gastrointestinal features | |||
| Significant problems with failure to thrive secondary to intestinal dysmotility | Yes | Yes | Yes |
| Diarrhoea | Yes (episodes of diarrhoea/constipation) | Yes (continuous Imodium therapy) | Yes (during first 1–2 years of life) |
| Continuing problems with constipation | ? | No | Yes |
| Abdominal discomfort coinciding with constipation | ? | Yes | Yes |
| Perineal discomfort on passing motion or urine | No | Yes | Yes |
| Episodic abdominal pain (not coinciding with constipation) | No | No | Yes |
| Rectal pain on defecation (similar to paroxysmal extreme pain disorder due to NaV1.7 mutations) | No | Yes | Yes |
| Intermittent parenteral nutrition | Yes | Yes | No |
| Neurodevelopmental profile | |||
| Delayed motor milestones | Yes | Yes | Yes |
| Intelligence | Normal (born 2002) | Normal (born 2004) | Normal (born 2007) |
| Emotions, mood, personality | Normal | Normal | Normal |
| Neurologic features | |||
| Intact sense of smell | Yes | Yes | Yes |
| Persisting minor hypotonia and muscle weakness | Yes | Yes | Yes |
| Adopts postures at extremes of joint movement range when awake (giving an appearance similar to dystonia) | Yes | No | Yes |
| Adopts postures at extremes of joint movement range when asleep | Yes | No | Yes |
| Standard nerve conduction studies | Normal | Borderline | Normal |
| Sural nerve biopsy | Normal | Not done | Not done |
| Musculoskeletal features | |||
| Multiple painless fractures | Yes | Yes | Yes |
| Charcot-like arthropathy | Yes | Yes | Yes |
| Pharmacologic features | |||
| Response of pain episodes to carbamazepine (and some other sodium channel blockers, eg, lamotrigine) | Not done | Not done | No |
| Response of pruritus to standard anti-histamines | Not done | No | No |
The presenting feature in all three was failure to thrive secondary to intestinal dysmotility. Consequent upon this, all had multiple hospital admissions and investigations and two required parenteral nutrition. Abnormal gut peristalsis was found; however, intestinal biopsies were repeatedly normal. All have continuing problems with diarrhoea and/or constipation.
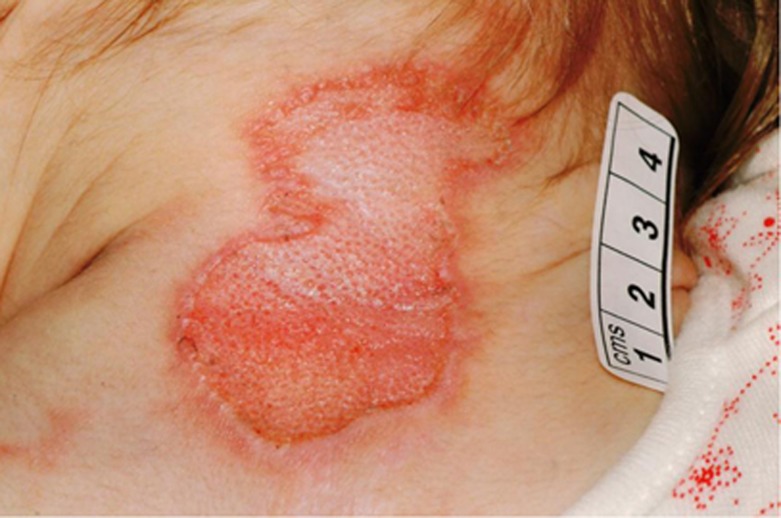
All three have severe pruritus, scratching themselves sufficiently to cause full-thickness skin loss in the cervical area during infancy (see Figure 1). Remarkably this was at the same location as seen in transgenic Scn11a-mutant mice.4 Ulceration and itching may be secondary to hyperhidrosis, as itching only reduced following the use of cyprohepatidine, which lead to a clear reduction in sweating. Hyperhidrosis persisted throughout life, and increased on exertion and raised ambient temperatures. All cried and blushed normally, and none had any abnormal cardiovascular findings nor emotional liability.
Figure 1.
Neck of case 3 with the SCN11A/NaV1.9 mutation p.L811P at the age of 1 year 5 months. A large contiguous healing region is shown. This was caused by the child scratching, mostly while awake. This area was excoriated for 6 months due to intense itching for which hyperhidrosis was a significant contributory factor.
Motor milestones were delayed, consequences of a persisting hypotonia and mild muscle weakness. No clinical or significant neurophysiological signs of peripheral neuropathy were observed. Two individuals adopted bizarre dystonia-like postures at the extremes of normal joint positions, awake and asleep.
All have slow wound healing, and patient 3 in particular had Staphylococci repeatedly cultured from skin lesions. This apparent selective reduced immunity to staphylococcal infections is also seen in the hereditary autonomic and sensory neuropathy types 4 and 5, caused by biallelic mutations in NTRK1 and NGF, respectively.
No peripheral pain was felt, for example, painless bone fractures and self-amputation of the tongue tip and lips, however, defecation produced significant discomfort. Furthermore, whereas temperature within the normal range could be perceived, variations in temperature such as a gust of cold wind were distinctly unpleasant. These were the only experiences of ‘pain' that the individuals describe.
NaV1.9 is strongly expressed in enteric plexus and nociceptor/temperature sensing neurons, which patient biopsies show are present. Therefore the phenotype is mainly caused by nerve dysfunction and not by its absence. The p.L811P variant causes a complex pattern of effects on neuronal subtype activity: it is excitatory in the enteric plexus (NaV1.9 knockout mice have increased intestinal activity5), in sweat glands leading to hyperhidrosis and in ano-rectal nociceptors showing these innervations are functioning; but it is inhibitory in most nociceptors and in infective inflammation (which also occur in sensory neuropathies where small unmyelinated nerves are absent). The NaV1.9–CIP phenotype is unique and clearly clinically distinguishable from NaV1.7–CIP, which is accompanied by anosmia but not gastrointestinal motility disturbances or muscle weakness. In contrast to these channelopathies, the NTRK1-associated hereditary autonomic and sensory neuropathy type 4 (also termed ‘congenital insensitivity to pain with anhidrosis') is characterized by variable degree of intellectual disability and lack of sweat gland innervation, resulting in anhidrosis and recurrent febrile episodes owing to poor thermoregulation. The complex pathophysiological basis of the NaV1.9–CIP is further illustrated by the report of families with a dominant episodic pain syndrome caused by missense SCN11A/NaV1.9 variants and by further missense variants leading to adult onset painful neuropathy.6, 7 All mutations including p.L811P show gain-of-function properties at a channel level and suggest neuron hyperexcitability.4, 6, 7 Yet the contrary physiological outcome depending on the variant, that is, increased versus abolished pain perception, remains puzzling. One explanation might be the effect size of the respective mutation on NaV1.9 function that determines the physiological consequences.
This recent insight in human NaV1.9 pathology suggests that channel agonists and antagonists could have multiple therapeutic potentials.
Information regarding the SCN11A variant c.2432T>C (p.Leu811Pro) (NM_014139.2) is available: OMIM Mutation ID 604385.0001 http://omim.org/entry/604385#0001; ClinVar: http://www.ncbi.nlm.nih.gov/clinvar/RCV000074494/
Patient consent had been obtained.
Acknowledgments
This work is dedicated to the memory of Dr John Tolmie who sadly passed away during its final preparation. John was a highly regarded geneticist as well as a clinician, an inspiring colleague and above all a great person.
CGW is a Principal Investigator of an MRC MICA grant including Neusentis. The remaining authors declare no conflict of interest.
References
- Görke W. The differential diagnosis of congenital analgesia and other diseases with diminished pain perception in childhood. Case report and review. Neuropediatrics. 1981;12:33–44. doi: 10.1055/s-2008-1059637. [DOI] [PubMed] [Google Scholar]
- Cox JJ, Reimann F, Nicholas AK, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444:894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg YP, MacFarlane J, MacDonald ML, et al. Loss-of-function mutations in the NaV1.7 gene underlie congenital indifference to pain in multiple human populations. Clin Genet. 2007;71:311–319. doi: 10.1111/j.1399-0004.2007.00790.x. [DOI] [PubMed] [Google Scholar]
- Leipold E, Liebmann L, Korenke GC, et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat Genet. 2013;45:1399–1404. doi: 10.1038/ng.2767. [DOI] [PubMed] [Google Scholar]
- Copel C, Clerc N, Osorio N, Delmas P, Mazet B. The NaV1.9 channel regulates colonic motility in mice. Front Neurosci. 2013;7:58. doi: 10.3389/fnins.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Han C, Estacion M, et al. Gain-of-function mutations in sodium channel NaV1.9 in painful neuropathy. Brain. 2014;137 (Pt 6:1627–1642. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Wen J, Yang W, et al. Gain-of-function mutations in SCN11A cause familial episodic pain. Am J Hum Genet. 2013;93:957–966. doi: 10.1016/j.ajhg.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]