Abstract
The neuropeptide galanin is widely expressed in both the central and peripheral nervous systems. However there is limited understanding of how individual galanin receptor (GalR1, 2, and 3) subtypes mediate the physiological activity of galanin in vivo. To address this issue we utilized NAX-5055 a systemically available, metabolically stable galanin analog. NAX-5055 displays a preference for GalR1 receptors and possesses potent anticonvulsant activity in vivo, suggesting that NAX-5055 engages central galanin receptors. To determine if NAX-5055 also modulates the activity of peripheral galanin receptors, we evaluated the effect of NAX-5055 on blood glucose and insulin levels in mice. Acute and repeated (once daily for four days) systemic administration of NAX-5055 (4 mg/kg) significantly increased blood glucose levels compared to vehicle treated mice. However, a hyperglycemic response was not observed following systemic administration of NAX-805-1 a scrambled analog of NAX-5055, with critical receptor binding residues, Trp2 and Tyr9, reversed. These results suggest chemical modifications independent of the galanin backbone of NAX-5055 are not responsible for the hyperglycemic response. The effect of NAX-5055 on glucose homeostasis was further evaluated with a glucose tolerance test (GTT). Mice administered either acute or repeated (once daily for four days) injections of NAX-5055 (4mg/kg) displayed impaired glucose handling and reduced insulin response to an acute glucose (1g/kg) challenge. Here we have shown that systemic administration of a centrally active GalR1-preferring galanin analog produces acute hyperglycemia and an inhibition of insulin release in vivo and that these effects are not attenuated with repeated administration. NAX-5055 thus provides a new pharmacological tool to further the understanding of function of both central and peripheral GalR1 receptors in vivo.
Keywords: Galanin, NAX-5055, peripheral galanin receptors, Glucose tolerance, Insulin
INTRODUCTION
Galanin is a 29 (30 in humans) amino acid neuropeptide widely expressed throughout the central and peripheral nervous system with similar distribution in mice, rats, and humans (Köhler and Chan-Palay, 1990; Lang et al., 2007; Robertson et al., 2011; Tatemoto et al., 1983). Immunohistochemical studies have shown that galanin colocalizes with a number of classical neurotransmitters including glutamate, acetylcholine, serotonin, and GABA. Due to this broad expression pattern there has been diverse interest in the physiology of galanin. To date galanin has been linked to the regulation of a number of neuronal functions including learning and memory, neuronal excitability, neuroprotection, and neuroendocrine regulation (Crawley, 2008; Hobson et al., 2008; Mazarati et al., 1998). The ability of galanin to modulate these physiological processes has led to an interest in the development of galanin-based therapies for a number of common disorders including epilepsy, neuropathic pain, Alzheimer’s disease, and diabetes (Crawley, 2008; Kask et al., 1997; Lerner et al., 2008; Mechenthaler, 2008; Robertson et al., 2011). Galanin is known to bind to three distinct G-protein coupled receptors GalR1, GalR2, and GalR3 (Branchek et al., 2000). Both GalR1 and GalR3 receptors selectively couple to the inhibitory G-protein Gi (Parker et al., 1995; Tang et al., 2012). In contrast, GalR2 receptors demonstrate a more complicated signaling cascade due to interaction with multiple different G-proteins, including Gi and Gq/11, resulting in either a inhibitory or excitatory cellular response (Lang and Kofler, 2011). The understanding of how each of these receptor subtypes contributes to the different physiological actions of galanin has been limited by the lack of systemically active, receptor-selective, galanin agonists and reliable galanin receptor antibodies (Florén et al., 2000; Lu and Bartfai, 2009; Robertson et al., 2011). The development of better tools to investigate the effects of galanin both in vivo and in vitro will facilitate the potential development of neuropeptide-based therapeutics.
Previous attempts to generate systemically active galanin agonists have produced two compounds: galmic and galnon (Bartfai et al., 2004; Saar et al., 2002; Sollenberg et al., 2005). However, both compounds were shown to interact with non-galanin receptors in the micromolar (μM) range thereby limiting their use as specific and selective pharmacological tools (Florén et al., 2005; Lu et al., 2005). To address this issue, Bulaj and colleagues successfully designed a series of truncated galanin analogs in which nonessential amino acid residues are replaced by cationic and/or lipoamino acid residues (Bulaj et al., 2008). One analog from this group, NAX-5055, was demonstrated to possess a 15-fold preference for GalR1 over GalR2 with nanomolar (nM) binding affinity in a time-resolved fluorescence binding assay (Bulaj et al., 2008; White et al., 2009).
Although the galaninergic system provides an interesting set of molecular targets for therapeutic development, modulation of this system is complicated by the broad physiological activities of galanin. As a result, it is important to increase our understanding of how the different galanin receptor subtypes mediate the activity of galanin in a region and cell-type specific manor. Of particular interest is the observation that GalR1 mRNA and immunoreactivity are highly expressed in both the ventral hippocampus and β cells of the islets of Langerhans (Bhandari et al., 2010; Gustafson et al., 1996; Jungnickel and Gundlach, 2005; Parker et al., 1995). Within the ventral hippocampus galanin has been show to potently inhibit the release of neurotransmitters including acetylcholine and glutamate; thus suggesting a role for galanin in mediating cellular excitability in a region known to generate seizure activity (Mazarati et al., 2000; Yoshitake et al., 2011; Zini et al., 1993). Increasing the concentration of central galanin through either direct injection or genetic overexpression has been reported to delay acquisition of kindling and reduces the severity and duration of seizures following electronically or chemically evoked status epilepticus (Kanter-Schlifke et al., 2007; Schlifke et al., 2006). Further, we have recently shown that the GalR1 preferring analog NAX-5055 has potent anticonvulsant activity in a number of seizure and epilepsy animal models, suggesting that the GalR1 receptor is a promising molecular target for seizure control (White et al., 2009).
In the peripheral nervous system, galanin is known to regulate insulin secretion and blood glucose levels in many species including rodents and canines (Ahrén, 2000; Leibowitz, 2005; McDonald et al., 1994; Tatemoto et al., 1983). Galanin is expressed in sympathetic nerve terminals that innervate the endocrine pancreas and is colocalized with norepinephrine (Dunning et al., 1986; Mei et al., 2006). Stimulation of the mixed pancreatic nerve bundle results in the release of galanin at sufficient levels to inhibit insulin secretion, however this effect is impaired in galanin knockout mice (Ahrén et al., 2004; Dunning and Taborsky, 1989). Further, galanin has been shown to reduce glucose-stimulated insulin release from cultured β islet cells of the pancreas (Gregersen et al., 1991). Consistent with the inhibition of insulin secretion, galanin infusion increases blood glucose levels in canines, but not in humans (Dunning and Taborsky, 1989; McDonald et al., 1994). Although this hyperglycemic response could be mediated by the reduction of insulin secretion, there is additional evidence that glucagon levels are also increased following galanin infusion, which may play a role in the effect of galanin on circulating glucose levels (Dunning et al., 1986; Dunning and Taborsky, 1989). Recent work investigating the expression levels of the galanin receptors on mouse β-cells showed detectable mRNA levels for all three receptors, with GalR1 demonstrating the highest expression levels (Barreto et al., 2011). However, it still remains unclear how the different galanin receptors mediate the galanin response in the endocrine pancreas. Previous attempts to use galanin-based pharmacology to study the effects of galanin on the endocrine pancreas have failed due to lack of specificity and off-target effects of the available pharmacological tools galnon and galmic (Quynh et al., 2005).
Modulation of galanin and its receptors provide targets for therapeutic intervention in a number of common disorders including epilepsy and diabetes. However, given the broad expression and modulatory effects on multiple cell types it is critical to understand how pharmacological intervention in this system alters different physiological circuits. The present study was designed to determine if targeting GalR1 receptors with the GalR1-preferring agonist NAX-5055 would affect glucose and insulin regulation at doses that reliably produce anticonvulsant activity.
METHODS
Animals
Adult male CF-1 mice weighing at least 18g (Charles River, Kingston, WA) were used for all experiments in this study. Animals were housed in a temperature-, humidity-, and light-controlled (12 h light:dark cycle) facility. For all studies not involving a food restriction, mice were group-housed and provided free access to food (LabDiet) and water. For studies involving a fast, mice were initially group-housed and given full access to food and water until the beginning of the fasting period. At the beginning of the fasting period mice were housed individually and food restricted for 6 hr. During the fast, mice were allowed free access to water. All experimental procedures were performed in accordance with the guidelines established by the National Institutes of Health and received approval from the University of Utah’s Animal Care and Use Committee. At the completion of all experimental procedures mice were humanly sacrificed by CO2 asphyxiation.
Peptide preparation
The galanin analog NAX-5055 was originally designed and synthesized at the University of Utah as previously described (Bulaj et al., 2008), and later synthesized in bulk by NeoMPS (San Diego, CA). An inactive scrambled analog of NAX-5055 (referred to as NAX-805-1), in which amino acid residues Tyr2 and Trp9 were reversed, was also designed and synthesized at the University of Utah (White et al., 2009). Solutions were prepared by dissolving synthesized peptides in 0.9% saline containing 1% Tween 20 (Sigma-Aldrich, St. Louis, MO). Solutions were made fresh daily. Prior to each experiment, peptide concentration was confirmed by UV absorbance of tyrosine and tryptophan residues (Cary 50 Bio UV Spectrophotometer, Varian). Dosing solutions containing peptide were administered to mice via intraperitoneal (i.p.) injection in a volume of 0.01 mL/g body weight.
For all studies mice were randomly selected from their home cage and placed into experimental groups with no intentional bias. Mice were removed from the colony, weighed, treated, and returned to the colony (in their home cage) or sacrificed between the hours of 8:00 AM and 5:00 PM. For all experiments involving multiple dosing regimens, mice were administered a single i.p. injection of either NAX-5055 (4 mg/kg), NAX-805-1 (4 mg/kg) or vehicle (0.9% saline with 1% Tween 20) once daily for 4 consecutive days. The injection of peptide or vehicle was dependent on the randomly assigned experimental group as described below. Glucose and/or insulin levels were obtained one hour post-peptide injection, corresponding to the time to peak effect (TPE) for the anticonvulsant activity of NAX-5055 (Bulaj et al., 2008).
Acute and repeated administration studies
Studies designed to compare the effects of acute and repeated administration of galanin analogs utilized the same experimental groups. These groups included a vehicle control group where animals received vehicle (0.9% saline containing 1% Tween 20) injections only for 4 consecutive days; an acute peptide group where animals received vehicle injections for the first 3 days followed by a single injection of NAX-5055 on the 4th day, and a repeated peptide group where animals received NAX-5055 injections for all 4 days. In studies comparing the effect of NAX-805-1 a fourth group was used in which animals where treated with NAX-805-1 for the first 3 days followed by a single injection of NAX-5055 on the 4th day.
Repeated administration of NAX-5055 or NAX-805-1
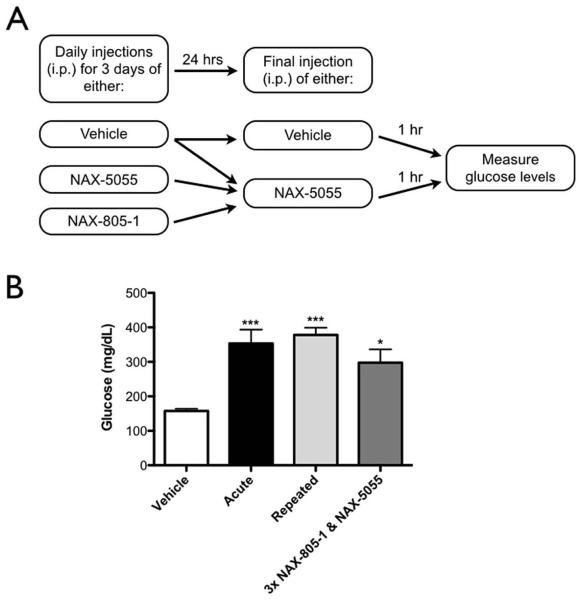
To compare the effect of repeated treatment with either NAX-5055 or NAX-805-1 on blood glucose levels, mice were split into one of four treatment groups as described above and depicted in Figure 2A (n = 8, per group). One hour after the last injection on day 4, glucose levels were obtained for all animals in all four groups.
Figure 2.
Repeated treatment with NAX-5055 does not attenuate the hyperglycemic effect seen with NAX-5055.
A) Schematic of the experimental treatment group design.
B) Glucose levels following acute or repeated treatment with NAX-5055 or NAX-805-1; groups described in detail in Methods. All groups that received at least one injection of NAX-5055 showed a significant increase in blood glucose levels (one-way ANOVA, ***p < 0.001, *p < 0.05, n = 8 per group).
Acute and repeated administration of NAX-5055
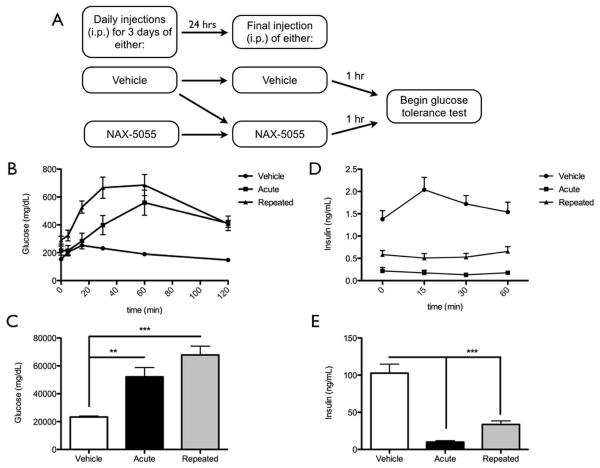
To compare the effects of acute versus repeated treatment with NAX-5055 on glucose or insulin levels mice were split into one of three groups as described above and depicted in figure 4A. Mice treated with this dosing regimen were also subjected to a glucose tolerance test (described in detail below) wherein glucose and/or insulin levels were determined (Figure 3). Given the amount of blood (approximately 200 μL per animal) that was required to obtain enough plasma to run duplicate samples using an insulin ELISA, blood samples were taken from different sets of mice and then pooled for insulin determination. For determination of glucose levels, 6 animals were used in the vehicle and acute NAX-5055 treatment groups, 8 animals were used from the repeated NAX-5055 treatment group and all animals were subjected to a GTT on the same day. For determination of insulin levels, 16 mice per group were used. Two separate glucose tolerance tests were conducted using 8 animals per group per day. There was no significant difference in insulin levels from vehicle-treated mice so data from both days were pooled.
Figure 4.
Mice given a single acute or repeated treatment with NAX-5055 show exaggerated hyperglycemia and decreased insulin secretion following a systemic glucose challenge.
A) Schematic of the experimental treatment group design.
B) Glucose levels in vehicle (n = 6), acute (n = 6), and repeatedly (n = 8) treated mice across the duration of the glucose tolerance test.
C) AUC measurements for all groups demonstrate both acute and repeated treatment groups have significantly increased blood glucose levels compared to vehicle treated mice (One-way ANOVA of AUC, **p < 0.01, ***p < 0.001).
D) Insulin levels in vehicle (n = 16), acute (n = 16), and repeatedly (n = 16) treated mice across the duration of the glucose tolerance test.
E) AUC measurements for all groups demonstrate both acute and repeated treatment groups have significantly decreased plasma insulin levels compared to vehicle treated mice (One-way ANOVA of AUC, ***p < 0.001).
Figure 3.
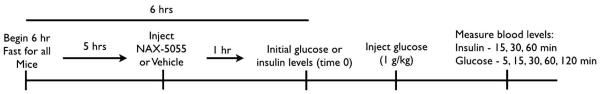
Schematic representation of the time course of events for the glucose tolerance test
Glucose and Insulin Measurements
Blood samples were obtained from a cut made approximately 1 cm from the tip of the tail in gently restrained mice. Glucose levels were measured using a Bayer Contour blood glucose meter (9545C, Tarrytown, NY) and Contour blood glucose test strips (7098C, Tarrytown, NY). Measurements were made directly from the tail clip. Insulin levels were measured from plasma. Blood was collected from tail clips in lithium heparin Microtainer® tubes (Becton Dickinson), and immediately placed on wet ice until samples from all animals had been collected. Blood samples were spun using a tabletop microcentrifuge (Labnet Spectrafuge™ 16M) for 5 min at 14,000 rpm. Plasma was removed from the samples and frozen at −80 °C until insulin levels could be determined using Ultra sensitive mouse insulin ELISA kits (Crystal Chem, Inc., Chicago, IL). Assays were run according to the manufacturers’ protocol. All samples were run in duplicate.
Glucose Tolerance Test
For the glucose tolerance test (GTT) mice were fasted for 6 hrs prior to the start of the test. As determined by their assigned treatment group (Fig. 4A), mice were given their fourth and final injection of either vehicle or NAX-5055 five hours after the start of the food fast and 1hr prior to the beginning to the test, so that the GTT would begin at the TPE for NAX-5055. At the beginning of the GTT (corresponding to time point 0), blood samples were collected for insulin measurement and glucose level determination. Immediately following the collection of these samples mice were administered a 1 g/kg i.p. injection of D-glucose (Sigma-Aldrich, St. Louis, MO). Glucose levels were sampled at 0, 5, 15, 30, 60, and 120 min post glucose injection. For insulin level determination, blood samples were drawn at 0, 15, 30, and 60 min following the glucose injection (Figure 2). All mice were immediately sacrificed after the final blood samples were obtained.
Data Analysis
All data and results are reported as means ± standard error of the mean (SEM). Statistical significance was determined by unpaired t-tests when comparing only two groups (Figure 1) and one-way ANOVA when comparing 3 or more groups (Figures 2 & 4). To determine significance between groups involving multiple comparisons, Bonferroni post hoc tests were used to compare significance between all groups and Dunetts post hoc tests were used to compare experimental treatments to vehicle controls. Area under the curve (AUC) was calculated, using the trapezoid rule, for individual curves generated during the time course of the GTT for both glucose and insulin measurements. A one-way ANOVA of the calculated AUC values was used to determine any significant differences. All statistics were done using GraphPad Prism Software (La Jolla, CA).
Figure 1.
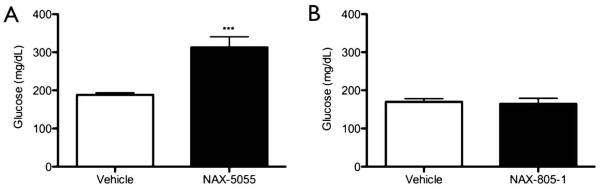
Systemic administration of the galanin analog NAX-5055 produces hyperglycemia.
A.) Mice were given a single i.p. injection of NAX-5055 (4 mg/kg) and glucose levels were measured 1 hr after injection. Treated mice showed a significant increase in blood glucose levels (unpaired t-test, ***p < 0.001, n = 8).
B.) Mice were given a single i.p. injection of the inactive analog NAX-805-1 (4 mg/kg), which lacks affinity for the galanin receptors GalR1 or GalR2. NAX-805-1 did not produce a significant change in blood glucose 1 hr after injection (unpaired t-test, p > 0.05, n = 4).
RESULTS
Glucose response to NAX-5055
We have previously demonstrated that the GalR1 preferring galanin analog NAX-5055 (4 mg/kg) protects against acute seizure. To determine if an anti-seizure dose of NAX-5055 also affects glucose homeostasis we measured blood glucose levels 1hr after a single systemic injection of vehicle or NAX-5055 (4 mg/kg). Mice administered NAX-5055 displayed increased glucose levels compared to controls (mean ± SEM: vehicle, 188.2 ± 5.3 mg/dL; NAX-5055, 312.9 ± 27.9 mg/dL, p < 0.001; n=14, per group) (Figure 1A).
To determine if the observed increase in glucose levels following systemic administration resulted from the interaction of the active Gal(1-13) fragment of NAX-5055 with galanin receptors, or as a side effect of the chemical modifications added to increase bioavailability we utilized a scrambled peptide version of NAX-5055; i.e., NAX-805-1. This peptide was designed with the residues Trp2 and Tyr9 reversed. Time-resolved fluorescent binding studies with NAX-805-1 confirm that this peptide does not bind with high affinity to either GalR1 or GalR2 receptors (data not shown). No significant increase in blood glucose levels were observed in mice treated with NAX-805-1 (4 mg/kg) 1 hr post-administration (mean ± SEM: vehicle, 169.8 ± 8.3 mg/dL; NAX-805-1, 164.5 ± 14.6 mg/dL, p = 0.76; n = 4 per group) (Figure 1B). These results suggest the active galanin fragment of NAX-5055 interacts with galanin receptors involved in glucose homeostasis following systemic injection, eliciting a hyperglycemic response in mice.
Glucose response to repeated NAX-5055
Both the GalR1 and GalR2 receptors have been shown to rapidly internalize following chronic agonist exposure in vitro. To determine if repeated activation of galanin receptors in vivo would attenuate the observed hyperglycemia, we repeatedly dosed mice with NAX-5055 and evaluated blood glucose levels 1hr after the last dose. For this study, four treatment groups were employed as outlined in Figure 2A and described in detail in the Methods section. Mice given either a single or repeated administration of NAX-5055 showed elevated glucose levels compared to vehicle-treated mice (mean ± SEM: vehicle = 157.5 ± 6.5 mg/dL; acute NAX-5055 = 353.5 ± 39.9 mg/dL; repeated NAX-5055 = 378.0 ± 20.8 mg/dL, p < 0.001, n = 8 per group) (Figure 2B). In addition, mice repeatedly treated with NAX-805-1 followed by a single administration of NAX-5055 also displayed elevated glucose levels compared to vehicle treated mice (mean ± SEM: vehicle = 157.5 ± 6.5 mg/dL; NAX-805-1 = 297.5 ± 39.0 mg/dL, n = 8, per group) (Figure 2B). There was no significant difference between the glucose levels of the acute, repeated or scrambled peptide treatment groups (p > 0.5) (Figure 2B). These data suggest that repeated exposure of galanin receptors to the agonist NAX-5055 in vivo does not diminish the hyperglycemia associated with acute NAX-5055 administration.
Glucose tolerance test following NAX-5055 administration
Based on the robust hyperglycemia induced by NAX-5055 under basal conditions, we sought to further investigate the effect of NAX-5055 on glucose and insulin levels in response to a glucose tolerance test (GTT) (Figure 3). We first measured glucose levels in mice treated with either acute or repeated doses of NAX-5055. At the 0 min time point (immediately before glucose injection) both the acute and repeatedly treated mice displayed elevated glucose levels compared to vehicle controls; however a significant difference was only observed between the vehicle and repeated treatment groups (mean ± SEM: vehicle = 152.8 ± 5.3 mg/dL; acute = 215.5 ± 32.7 mg/dL; repeated = 290.8 ± 28.1 mg/dL, p = 0.0051) (Figure 4B). All mice showed increased blood glucose levels following glucose injection, but varied in their time to peak effect and the magnitude of the hyperglycemia observed. In vehicle-treated animals, glucose levels peaked at 15 min after glucose injection and returned to baseline after 60 min. However, in both the acute and repeated treatment group, glucose levels increased immediately after glucose injection, but did not reach peak levels until the 60 min time point (Figure 4B). Total AUC for the glucose response curve was significantly increased for the acute and repeated treatment groups compared to the vehicle treated controls (p < 0.001). No significant difference in AUC of the glucose response curve was observed between mice given acute or repeated NAX-5055 treatment (Figure 4C).
Given that the mechanism of action for galanin receptor activation on pancreatic β-cells is proposed to be inhibition of insulin release, we also measured insulin levels following either acute or repeated treatment with NAX-5055 (Figure 3). At time point 0 in the GTT (immediately before glucose injection) both the acute and repeated treatment groups displayed significantly decreased insulin levels compared to vehicle controls (mean ± SEM: vehicle = 1.4 ± 0.19 ng/mL; acute = 0.22 ± 0.08 ng/mL; repeated = 0.58 ± 0.09 ng/mL, p < 0.001, n = 16 per group) (Figure 4D). Following glucose injection, only the vehicle treated animals showed any glucose-induced increase in insulin levels; insulin levels peaked at 15 minutes and rapidly returned to pre-glucose injection levels. The total area under the insulin response curve demonstrates a significant decrease in insulin release for both the acute and repeated treatment groups compared to vehicle treated controls (p < 0.001) (Figure 4E). In contrast, there was no significant difference between acute and repeated groups (p > 0.05).
DISCUSSION
This study is the first to demonstrate that a systemically available GalR1-preferring galanin analog can regulate glucose and insulin levels in vivo, in a manner similar to that proposed for endogenous galanin (Ahrén, 2000; McDonald et al., 1994). Acute administration of NAX-5055 resulted in a significant increase in glucose levels under both basal conditions and following an acute glucose challenge. In contrast, insulin levels were significantly reduced following acute NAX-5055 administration under basal conditions. Furthermore insulin levels of NAX-5055 treated mice were not altered in response to an acute glucose challenge. The effects of NAX-5055 on both glucose and insulin levels persisted following a four-day repeated dosing regimen.
The observed increase in basal glucose levels following a single administration of NAX-5055 is consistent with previous studies showing hyperglycemia in canine, rat and mouse following intravenous galanin infusion and stimulation of mixed pancreatic nerves, which is associated with galanin release (Lindskog and Ahrén, 1987; Manabe et al., 1986; McDonald et al., 1985). The effect of galanin on glucose homeostasis has largely been attributed to the inhibition of insulin release from β-cells within the pancreas (Gregersen et al., 1991; Lindskog and Ahrén, 1991; 1987). Our results support this hypothesis as insulin levels are also significantly reduced following acute NAX-5055 administration. Some suggested mechanisms for the inhibition of insulin by galanin include opening of ATP–sensitive K+ channels, reduction in cAMP levels by inhibition of adenylyl cyclase and direct inhibition of exocytosis (de Weille et al., 1988; Sharp, 1996; Tang et al., 2012). Activation of ATP-sensitive K+ channels and inhibition of cAMP levels has also been seen in the central nervous system following galanin receptor activation and is suggested to play a role in inhibiting neurotransmitter release (Hawes et al., 2006; Zini et al., 1993). It should be noted that intravenous galanin administration not only affects insulin secretion, but it may also increase glucagon levels as seen in both the canine and mouse, but not in rat (Dunning et al., 1986; Lindskog and Ahrén, 1987; Silvestre et al., 1987). During the GTT, both acute and repeated NAX-5055 treated mice showed increased glucose levels; however those receiving repeated NAX-5055 injections displayed higher glucose levels, particularly at the 15 and 30 minute time points. Although we did not directly measure glucagon levels in this study it is tempting to speculate that the robust increase in glucose following administration of NAX-5055 may result from both an inhibition of insulin as well as an increase in glucagon secretion.
All three galanin receptors are expressed within islet cells of the pancreas (Barreto et al., 2011; 2009), however it is unclear how each receptor subtype directly modulates islet function. Galanin and its receptors are also expressed on sympathetic nerve terminals and within the hypothalamus, providing another juncture where galanin-based pharmacological intervention could modulate glucose homeostasis (Branchek et al., 2000; Leibowitz, 2005; Potter and Smith-White, 2005). NAX-5055 displays a 15-fold preference for GalR1 with binding affinity in the nanomolar range (Bulaj et al., 2008), suggesting the physiological activity of NAX-5055 is primarily through GalR1 receptors. Preliminary evidence from our laboratory has shown that GalR2 preferring analogs administered systemically in similar dose ranges do not result in hyperglycemia, suggesting GalR2 receptors are likely not involved in NAX-5055 induced effects. However, we cannot rule out the potential contribution of GalR3 receptors to the observed disruption in glucose homeostasis. Indeed both GalR1 and GalR3 are known to couple to the Gi/o subfamily of G-proteins, resulting in similar downstream effects (Parker et al., 1995; Smith et al., 1998). Further, signaling though the Go2, a member of the Gi/o subfamily, mediates the inhibition of insulin release from β-cells, implicating this pathway in galanin’s effect on glucose homeostasis (Tang et al., 2012). Taken together these observations suggest that GalR1 receptors, and potentially GalR3 receptors play an important role in mediating the effects of galanin on glucose and insulin levels.
The finding that there is no significant attenuation of the hyperglycemic response to NAX-5055 following repeated treatment is particularly interesting. In the past, these types of studies have been difficult to perform with native neuropeptides in vivo because of their propensity for rapid degradation following systemic administration (Bulaj et al., 2008). Work with immortalized cell lines in vitro has suggested that both GalR1 and GalR2 receptors are prone to internalization following prolonged agonist exposure (Wang et al., 1998; Xia et al., 2008; 2004). Further, internalized GalR1 receptors have been shown to be trafficked to lysosomes for degradation and GalR1 activation-dependent inhibition of cAMP has been suggested to decrease receptor expression (Hawes et al., 2006; Xia et al., 2008). However, in our in vivo studies with mice, the hyperglycemic response elicited with single administration of NAX-5055 showed no attenuation to the response seen following four days of repeated dosing. These results suggest that peripheral galanin receptors are responsive to NAX-5055 following both acute and repeated exposure, and suggest that peripheral GalR1 receptors are not likely internalized following sub-chronic dosing with NAX-5055. This activity is dependent on the interaction of NAX-5055 with galanin receptors given that repeated treatment with the scrambled analog NAX-805-1, which does not bind with galanin receptors, did not modify the NAX-5055-induced hyperglycemic response. A limited supply of NAX-805-1 prevented us from directly testing the effects of repeated treatment with this peptide on blood glucose levels. However, we demonstrated that NAX-805-1 has no effect on glucose levels following acute administration and repeated NAX-805-1 administration does not attenuate or exaggerate the hyperglycemic response associated with acute NAX-5055 administration. Based on these observations and binding studies demonstrating NAX-805-1 has no affinity for galanin receptors, it is likely that NAX-805-1 would have no effect on blood glucose levels following repeated administration.
C57/Bl6J mice are often used to test the activity of galanin related compounds in the glucose tolerance test (Dunning et al., 1986; Zorrilla et al., 2007). For this study we chose to use the CF-1 mouse in order to directly relate our findings to the anticonvulsant studies that we have previously conducted with NAX-5055 using this mouse strain (Bulaj et al., 2008). Similar to work done with the C57Bl6J mice, glucose administration in vehicle-treated CF-1 mice elevated glucose and insulin levels rapidly; i.e., both peaked within 15 min of glucose injection. Both glucose and insulin levels returned to baseline levels by the completion of the test, confirming glucose sensitivity in this strain of mice. In contrast, the glucose tolerance test conducted in the acutely and repeatedly treated NAX-5055 mice demonstrated an altered response to the glucose challenge, consistent with previous GTT studies with native galanin (Dunning et al., 1986; Lindskog and Ahrén, 1987). The proposed mechanism of action of galanin in this test is the inhibition of insulin release (Ahrén et al., 2004; Olkowicz et al., 2007). Here we have shown that NAX-5055 completely abolishes any glucose stimulated increase in insulin release for the duration of the test. These observations suggest that NAX-5055 is an effective and potent inhibitor of insulin release in CF-1 mice. Although not statistically significant, the insulin levels observed following repeated treatment are slightly higher than those in the acute treatment group. Thus, it is possible that there is a slight tolerance to the inhibition of insulin release following repeated treatment with NAX-5055; however, further study is required to fully test this hypothesis.
The altered response to the glucose challenge following both acute and repeated NAX-5055 treatment is evident in the persistent increase in blood glucose levels and lack of insulin response following systemic glucose injection. These findings are consistent with other studies showing galanin-induced glucose insensitivity (Ahrén and Lindskog, 1992). Interestingly, studies utilizing the galanin knockout mouse and the GalR1 knockout mouse have also demonstrated similar glucose insensitivity to the glucose tolerance test; i.e., mice lacking galanin or GalR1 displayed impaired insulin secretion and glucose disposal (Ahrén et al., 2004; Zorrilla et al., 2007). It remains unclear why a similar physiological response to glucose stimulation is observed following both increased and decreased galanergic activity. One possibility is that galanin and/or the GalR1 receptor has an important role in the development of normal β-cell innervations or physiological function and thus normal cellular activity is compromised in the presence of the mutation. A second possibility is that there are compensatory changes in galanin receptor complement or other neuropeptide systems that may alter the response of β-cells to either galanin or glucose stimulation. NAX-5055 and similar galanin receptor selective analogs may help provide further insight into the role of galanin and the galanin receptors in the physiological response of β-cells to glucose stimulation.
The hyperglycemia and inhibition of insulin release observed with NAX-5055 raises concerns about the use of systemically active GalR1 preferring analogs for the treatment of neurological diseases in humans. However, the effect of galanin on glucose and insulin levels in humans is inconsistent. Similar to mice and dogs, humans express galanin in nerves innervating islet cells of the pancreas (Ahrén et al., 1991). Infusion of human galanin during an oral glucose test in normal humans at doses shown to inhibit insulin release in dogs did not change circulating glucose or insulin levels (McDonald et al., 1994). However, in isolated human islets cells, infusion of porcine galanin significantly inhibited insulin release (Ahrén et al., 1991). These differences between in vivo and in vitro studies suggest that galanin’s regulation of insulin levels in humans may extend beyond direct effects on β-cells. Regardless, additional investigation would be required before any firm conclusions regarding the effect of an exogenously administered GalR1 agonist on insulin secretion and hyperglycemia in humans can be made.
The regulation of galanin expression is also directly affected by glucose in humans, such that circulating galanin levels positively correlate with increased glucose levels in an oral glucose test in healthy controls (Legakis et al., 2007). Further, adults with type-2 diabetes and children with type-1 diabetes mellitus show elevated galanin (Celi et al., 2005; Legakis et al., 2005). Thus there is not a direct correlation between the effects of galanin in animal model systems and in humans suggesting the effects we report here for NAX-5055 may not translate to humans. In addition, it is still unclear how each galanin receptor mediates the effects of galanin on β-cell function. The data shown here suggest that GalR1 is involved in regulating glucose and insulin homeostasis; however, a non-GalR1 agonist may not display this same effect. Indeed preliminary evidence with GalR2-preferring analogs demonstrates that targeting this receptor results in anticonvulsant activity without negatively affecting blood glucose levels (unpublished results). Increased understanding of the role of each galanin receptor in the different physiological functions of galanin in both the peripheral and central nervous systems will help mitigate concerns about the clinical use of galanin-based therapeutics.
This study is the first to show that a GalR1-preferring galanin analog acts in the endocrine pancreas in a way similar to galanin infusions in vitro. Evidence that β-cells contain mRNA to encode all three galanin receptor subtypes enhances the need to further understand the role of each receptor subtype in mediating the neuromodulatory function of galanin in vivo. In conclusion, we have shown here that the GalR1 receptor agonist NAX-5055 is a potent regulator of both glucose and insulin in the mouse under basal and glucose-stimulated conditions. NAX-5055 thus provides the scientific community with a useful pharmacological tool to increase our understanding of how the different galanin receptor subtypes mediate the activity of galanin.
Highlights for: Regulation of Glucose and Insulin release following acute and repeated treatment with the synthetic galanin analog NAX-5055.
NAX-5055 is a synthetic galanin, GalR1 preferring agonist with anti-seizure effects
We examine the effect of NAX-5055 on glucose and insulin regulation in vivo.
NAX-5055 increases blood glucose and decreases blood insulin levels in vivo.
Results suggest GalR1 receptors important in regulating insulin release in vivo.
NAX-5055 provides a tool to study central & peripheral galanin receptor physiology
ACKNOWLEDGEMENTS
Dr. Grzegorz Bulaj for developing and sharing NAX-5055, without which this study would not be possible. Robert Cooksey and Deborah Jones for assistance in designing and administering the glucose tolerance test.
SOURCES OF SUPPORT: National Institute of Health – R21-NS059669 & U01-NS066911
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrén B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. doi:10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Ar’Rajab A, Böttcher G, Sundler F, Dunning BE. Presence of galanin in human pancreatic nerves and inhibition of insulin secretion from isolated human islets. Cell Tissue Res. 1991;264:263–267. doi: 10.1007/BF00313963. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Lindskog S. Galanin and the regulation of islet hormone secretion. Int. J. Pancreatol. 1992;11:147–160. doi: 10.1007/BF02924180. [DOI] [PubMed] [Google Scholar]
- Ahrén B, Pacini G, Wynick D, Wierup N, Sundler F. Loss-of-function mutation of the galanin gene is associated with perturbed islet function in mice. Endocrinology. 2004;145:3190–3196. doi: 10.1210/en.2003-1700. doi:10.1210/en.2003-1700. [DOI] [PubMed] [Google Scholar]
- Barreto SG, Bazargan M, Zotti M, Hussey DJ, Sukocheva OA, Peiris H, Leong M, Keating DJ, Schloithe AC, Carati CJ, Smith C, Toouli J, Saccone GTP. Galanin receptor 3--a potential target for acute pancreatitis therapy. Neurogastroenterol. Motil. 2011;23:e141–51. doi: 10.1111/j.1365-2982.2010.01662.x. doi:10.1111/j.1365-2982.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- Barreto SG, Woods CM, Carati CJ, Schloithe AC, Jaya SR, Toouli J, Saccone GTP. Galanin inhibits caerulein-stimulated pancreatic amylase secretion via cholinergic nerves and insulin. Am J Physiol Gastrointest Liver Physiol. 2009;297:G333–9. doi: 10.1152/ajpgi.00078.2009. doi:10.1152/ajpgi.00078.2009. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Lu X, Badie-Mahdavi H, Barr AM, Mazarati A, Hua X-Y, Yaksh T, Haberhauer G, Ceide SC, Trembleau L, Somogyi L, Kröck L, Rebek J. Galmic, a nonpeptide galanin receptor agonist, affects behaviors in seizure, pain, and forced-swim tests. PNAS. 2004;101:10470–10475. doi: 10.1073/pnas.0403802101. doi:10.1073/pnas.0403802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari M, Thomas AC, Hussey DJ, Li X, Jaya SP, Woods CM, Schloithe AC, Mayne GC, Carati CJ, Toouli J, Ormandy CJ, Saccone GTP. Galanin mediates the pathogenesis of cerulein-induced acute pancreatitis in the mouse. Pancreas. 2010;39:182–187. doi: 10.1097/MPA.0b013e3181bdc152. doi:10.1097/MPA.0b013e3181bdc152. [DOI] [PubMed] [Google Scholar]
- Branchek TA, Smith KE, Gerald C, Walker MW. Galanin receptor subtypes. Trends Pharmacol Sci. 2000;21:109–117. doi: 10.1016/s0165-6147(00)01446-2. [DOI] [PubMed] [Google Scholar]
- Bulaj G, Green BR, Lee H-K, Robertson CR, White K, Zhang L, Sochanska M, Flynn SP, Scholl EA, Pruess TH, Smith MD, White HS. Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem. 2008;51:8038–8047. doi: 10.1021/jm801088x. doi:10.1021/jm801088x. [DOI] [PubMed] [Google Scholar]
- Celi F, Bini V, Papi F, Santilli E, Ferretti A, Mencacci M, Berioli MG, De Giorgi G, Falorni A. Circulating acylated and total ghrelin and galanin in children with insulin-treated type 1 diabetes: relationship to insulin therapy, metabolic control and pubertal development. Clin. Endocrinol. (Oxf) 2005;63:139–145. doi: 10.1111/j.1365-2265.2005.02313.x. doi:10.1111/j.1365-2265.2005.02313.x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Galanin impairs cognitive abilities in rodents: relevance to Alzheimer’s disease. Cell Mol Life Sci. 2008;65:1836–1841. doi: 10.1007/s00018-008-8158-3. doi:10.1007/s00018-008-8158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille J, Schmid-Antomarchi H, Fosset M, Lazdunski M. ATP-sensitive K+ channels that are blocked by hypoglycemia-inducing sulfonylureas in insulin-secreting cells are activated by galanin, a hyperglycemia-inducing hormone. PNAS. 1988;85:1312–1316. doi: 10.1073/pnas.85.4.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning BE, Ahrén B, Veith RC, Böttcher G, Sundler F, Taborsky GJ. Galanin: a novel pancreatic neuropeptide. Am J Physiol. 1986;251:E127–33. doi: 10.1152/ajpendo.1986.251.1.E127. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Taborsky GJ. Galanin release during pancreatic nerve stimulation is sufficient to influence islet function. Am J Physiol. 1989;256:E191–8. doi: 10.1152/ajpendo.1989.256.1.E191. [DOI] [PubMed] [Google Scholar]
- Florén A, Land T, Langel U. Galanin receptor subtypes and ligand binding. Neuropeptides. 2000;34:331–337. doi: 10.1054/npep.2000.0808. doi:10.1054/npep.2000.0808. [DOI] [PubMed] [Google Scholar]
- Florén A, Sollenberg U, Lundström L, Zorko M, Stojan J, Budihna M, Wheatley M, Martin NP, Kilk K, Mazarati A, Bartfai T, Lindgren M, Langel U. Multiple interaction sites of galnon trigger its biological effects. Neuropeptides. 2005;39:547–558. doi: 10.1016/j.npep.2005.09.005. doi:10.1016/j.npep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Gregersen S, Hermansen K, Langel U, Fisone G, Bartfai T, Ahrén B. Galanin-induced inhibition of insulin secretion from rat islets: effects of rat and pig galanin and galanin fragments and analogues. European Journal of Pharmacology. 1991;203:111–114. doi: 10.1016/0014-2999(91)90797-t. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Smith KE, Durkin MM, Gerald C, Branchek TA. Distribution of a rat galanin receptor mRNA in rat brain. Neuroreport. 1996;7:953–957. doi: 10.1097/00001756-199603220-00025. [DOI] [PubMed] [Google Scholar]
- Hawes JJ, Narasimhaiah R, Picciotto MR. Galanin attenuates cyclic AMP regulatory element-binding protein (CREB) phosphorylation induced by chronic morphine and naloxone challenge in Cath.a cells and primary striatal cultures. J Neurochem. 2006;96:1160–1168. doi: 10.1111/j.1471-4159.2005.03613.x. doi:10.1111/j.1471-4159.2005.03613.x. [DOI] [PubMed] [Google Scholar]
- Hobson S-A, Bacon A, Elliot-Hunt CR, Holmes FE, Kerr NCH, Pope R, Vanderplank P, Wynick D. Galanin acts as a trophic factor to the central and peripheral nervous systems. Cell Mol Life Sci. 2008;65:1806–1812. doi: 10.1007/s00018-008-8154-7. doi:10.1007/s00018-008-8154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel SR-F, Gundlach AL. [125I]-Galanin binding in brain of wildtype, and galanin- and GalR1-knockout mice: strain and species differences in GalR1 density and distribution. Neuroscience. 2005;131:407–421. doi: 10.1016/j.neuroscience.2004.11.023. doi:10.1016/j.neuroscience.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Kanter-Schlifke I, Toft Sørensen A, Ledri M, Kuteeva E, Hökfelt T, Kokaia M. Galanin gene transfer curtails generalized seizures in kindled rats without altering hippocampal synaptic plasticity. Neuroscience. 2007;150:984–992. doi: 10.1016/j.neuroscience.2007.09.056. doi:10.1016/j.neuroscience.2007.09.056. [DOI] [PubMed] [Google Scholar]
- Kask K, Berthold M, Bartfai T. Galanin receptors: involvement in feeding, pain, depression and Alzheimer’s disease. Life Sci. 1997;60:1523–1533. doi: 10.1016/s0024-3205(96)00624-8. [DOI] [PubMed] [Google Scholar]
- Köhler C, Chan-Palay V. Galanin receptors in the post-mortem human brain. Regional distribution of 125I-galanin binding sites using the method of in vitro receptor autoradiography. Neurosci Lett. 1990;120:179–182. doi: 10.1016/0304-3940(90)90032-5. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther. 2007;115:177–207. doi: 10.1016/j.pharmthera.2007.05.009. (null) doi:10.1016/j.pharmthera.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Lang R, Kofler B. The galanin peptide family in inflammation. Neuropeptides. 2011;45:1–8. doi: 10.1016/j.npep.2010.10.005. doi:10.1016/j.npep.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Legakis I, Mantzouridis T, Mountokalakis T. Positive correlation of galanin with glucose in type 2 diabetes. Diabetes Care. 2005;28:759–760. doi: 10.2337/diacare.28.3.759. [DOI] [PubMed] [Google Scholar]
- Legakis IN, Mantzouridis T, Mountokalakis T. Positive correlation of galanin with glucose in healthy volunteers during an oral glucose tolerance test. Horm. Metab. Res. 2007;39:53–55. doi: 10.1055/s-2006-957346. doi:10.1055/s-2006-957346. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF. Regulation and effects of hypothalamic galanin: relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides. 2005;39:327–332. doi: 10.1016/j.npep.2004.12.022. doi:10.1016/j.npep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Lerner JT, Sankar R, Mazarati AM. Galanin and epilepsy. Cell Mol Life Sci. 2008;65:1864–1871. doi: 10.1007/s00018-008-8161-8. doi:10.1007/s00018-008-8161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog S, Ahrén B. Galanin: effects on basal and stimulated insulin and glucagon secretion in the mouse. Acta Physiol. Scand. 1987;129:305–309. doi: 10.1111/j.1748-1716.1987.tb08073.x. [DOI] [PubMed] [Google Scholar]
- Lindskog S, Ahrén B. Studies on the mechanism by which galanin inhibits insulin secretion in islets. European Journal of Pharmacology. 1991;205:21–27. doi: 10.1016/0014-2999(91)90765-i. [DOI] [PubMed] [Google Scholar]
- Lu X, Bartfai T. Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn-Schmied Arch Pharmacol. 2009;379:417–420. doi: 10.1007/s00210-009-0394-z. doi:10.1007/s00210-009-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Lundström L, Langel U, Bartfai T. Galanin receptor ligands. Neuropeptides. 2005;39:143–146. doi: 10.1016/j.npep.2004.12.012. doi:10.1016/j.npep.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Manabe T, Yoshimura T, Kii E, Tanaka Y, Ohshio G, Tobe T, Akaji K, Yajima H. Galanin-induced hyperglycemia: effect on insulin and glucagon. Endocr. Res. 1986;12:93–98. doi: 10.1080/07435808609023655. [DOI] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, Langel U, Wasterlain CG. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald TJ, Dupré J, Tatemoto K, Greenberg GR, Radziuk J, Mutt V. Galanin inhibits insulin secretion and induces hyperglycemia in dogs. Diabetes. 1985;34:192–196. doi: 10.2337/diab.34.2.192. [DOI] [PubMed] [Google Scholar]
- McDonald TJ, Tu E, Brenner S, Zabel P, Behme M, Panchal C, Hramiak I, Barnett WB, Miller D, Dupré J. Canine, human, and rat plasma insulin responses to galanin administration: species response differences. Am J Physiol. 1994;266:E612–7. doi: 10.1152/ajpendo.1994.266.4.E612. [DOI] [PubMed] [Google Scholar]
- Mechenthaler I. Galanin and the neuroendocrine axes. Cell Mol Life Sci. 2008;65:1826–1835. doi: 10.1007/s00018-008-8157-4. doi:10.1007/s00018-008-8157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Q, Mundinger TO, Lernmark K, Taborsky GJ. Increased galanin expression in the celiac ganglion of BB diabetic rats. Neuropeptides. 2006;40:1–10. doi: 10.1016/j.npep.2005.08.005. doi:10.1016/j.npep.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Olkowicz M, Ruczyński J, Cybal M, Konstański Z, Petrusewicz J, Kamińska B, Rekowski P. New galanin(1-15) analogues modified in positions 9, 10 and 11 act as galanin antagonists on glucose-induced insulin secretion. J. Physiol. Pharmacol. 2007;58:859–872. [PubMed] [Google Scholar]
- Parker EM, Izzarelli DG, Nowak HP, Mahle CD, Iben LG, Wang J, Goldstein ME. Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res. Mol. Brain Res. 1995;34:179–189. doi: 10.1016/0169-328x(95)00159-p. [DOI] [PubMed] [Google Scholar]
- Potter EK, Smith-White MA. Galanin modulates cholinergic neurotransmission in the heart. Neuropeptides. 2005;39:345–348. doi: 10.1016/j.npep.2004.12.006. doi:10.1016/j.npep.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Quynh NTT, Islam MS, Islam SM, Florén A, Bartfai T, Langel U, Östenson C-G. Effects of galnon, a non-peptide galanin-receptor agonist, on insulin release from rat pancreatic islets. Biochem Biophys Res Commun. 2005;328:213–220. doi: 10.1016/j.bbrc.2004.12.150. doi:10.1016/j.bbrc.2004.12.150. [DOI] [PubMed] [Google Scholar]
- Robertson CR, Flynn SP, White HS, Bulaj G. Anticonvulsant neuropeptides as drug leads for neurological diseases. Nat Prod Rep. 2011;28:741–762. doi: 10.1039/c0np00048e. doi:10.1039/c0np00048e. [DOI] [PubMed] [Google Scholar]
- Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K, Hellberg S, Pooga M, Tolf B-R, Shi TS, Hökfelt T, Wasterlain C, Bartfai T, Langel U. Anticonvulsant activity of a nonpeptide galanin receptor agonist. PNAS. 2002;99:7136–7141. doi: 10.1073/pnas.102163499. doi:10.1073/pnas.102163499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlifke I, Kuteeva E, Hokfelt T, Kokaia M. Galanin expressed in the excitatory fibers attenuates synaptic strength and generalized seizures in the piriform cortex of mice. Exp Neurol. 2006;200:398–406. doi: 10.1016/j.expneurol.2006.02.124. doi:10.1016/j.expneurol.2006.02.124. [DOI] [PubMed] [Google Scholar]
- Sharp GW. Mechanisms of inhibition of insulin release. Am J Physiol. 1996;271:C1781–99. doi: 10.1152/ajpcell.1996.271.6.C1781. [DOI] [PubMed] [Google Scholar]
- Silvestre RA, Miralles P, Monge L, Moreno P, Villanueva ML, Marco J. Effects of galanin on hormone secretion from the in situ perfused rat pancreas and on glucose production in rat hepatocytes in vitro. Endocrinology. 1987;121:378–383. doi: 10.1210/endo-121-1-378. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Sollenberg U, Bartfai T, Langel U. Galnon--a low-molecular weight ligand of the galanin receptors. Neuropeptides. 2005;39:161–163. doi: 10.1016/j.npep.2004.12.019. doi:10.1016/j.npep.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Tang G, Wang Y, Park S, Bajpayee NS, Vi D, Nagaoka Y, Birnbaumer L, Jiang M. Go2 G protein mediates galanin inhibitory effects on insulin release from pancreatic β cells. PNAS. 2012;109:2636–2641. doi: 10.1073/pnas.1200100109. doi:10.1073/pnas.1200100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K, Rökaeus A, Jörnvall H, McDonald TJ, Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- Wang S, Clemmons A, Strader C, Bayne M. Evidence for hydrophobic interaction between galanin and the GalR1 galanin receptor and GalR1-mediated ligand internalization: fluorescent probing with a fluorescein-galanin. Biochemistry. 1998;37:9528–9535. doi: 10.1021/bi9731955. doi:10.1021/bi9731955. [DOI] [PubMed] [Google Scholar]
- White HS, Scholl EA, Klein BD, Flynn SP, Pruess TH, Green BR, Zhang L, Bulaj G. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2009;6:372–380. doi: 10.1016/j.nurt.2009.01.001. doi:10.1016/j.nurt.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Dun X-P, Hu P-S, Kjaer S, Zheng K, Qian Y, Solén C, Xu T, Fredholm B, Hokfelt T, Xu Z-QD. Postendocytotic traffic of the galanin R1 receptor: a lysosomal signal motif on the cytoplasmic terminus. PNAS. 2008;105:5609–5613. doi: 10.1073/pnas.0801456105. doi:10.1073/pnas.0801456105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Kjaer S, Zheng K, Hu P-S, Bai L, Jia J-Y, Rigler R, Pramanik A, Xu T, Hökfelt T, Xu Z-QD. Visualization of a functionally enhanced GFP-tagged galanin R2 receptor in PC12 cells: constitutive and ligand-induced internalization. PNAS. 2004;101:15207–15212. doi: 10.1073/pnas.0406571101. doi:10.1073/pnas.0406571101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Savage S, Elvander-Tottie E, Ogren SO, Kehr J. Galanin differentially regulates acetylcholine release in ventral and dorsal hippocampus: a microdialysis study in awake rat. Neuroscience. 2011;197:172–180. doi: 10.1016/j.neuroscience.2011.09.035. doi:10.1016/j.neuroscience.2011.09.035. [DOI] [PubMed] [Google Scholar]
- Zini S, Roisin MP, Langel U, Bartfai T, Ben-Ari Y. Galanin reduces release of endogenous excitatory amino acids in the rat hippocampus. European Journal of Pharmacology. 1993;245:1–7. doi: 10.1016/0922-4106(93)90162-3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Brennan M, Sabino V, Lu X, Bartfai T. Galanin type 1 receptor knockout mice show altered responses to high-fat diet and glucose challenge. Physiol. Behav. 2007;91:479–485. doi: 10.1016/j.physbeh.2006.11.011. doi:10.1016/j.physbeh.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]