Abstract
Context
Perfluorinated compounds (PFCs) have emerged as important food contaminants. They cause immune suppression in a rodent model at serum concentrations similar to those occurring in the US population, but adverse health effects of PFC exposure are poorly understood.
Objective
To determine whether PFC exposure is associated with antibody response to childhood vaccinations.
Design, Setting, and Participants
Prospective study of a birth cohort from the National Hospital in the Faroe Islands. A total of 656 consecutive singleton births were recruited during 1997-2000, and 587 participated in follow-up through 2008.
Main Outcome Measures
Serum antibody concentrations against tetanus and diphtheria toxoids at ages 5 and 7 years.
Results
Similar to results of prior studies in the United States, the PFCs with the highest serum concentrations were perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA). Among PFCs in maternal pregnancy serum, PFOS showed the strongest negative correlations with antibody concentrations at age 5 years, for which a 2-fold greater concentration of exposure was associated with a difference of −39% (95% CI, −55% to −17%) in the diphtheria antibody concentration. PFCs in the child’s serum at age 5 years showed uniformly negative associations with antibody levels, especially at age 7 years, except that the tetanus antibody level following PFOS exposure was not statistically significant. In a structural equation model, a 2-fold greater concentration of major PFCs in child serum was associated with a difference of −49% (95% CI, −67% to −23%) in the overall antibody concentration. A 2-fold increase in PFOS and PFOA concentrations at age 5 years was associated with odds ratios between 2.38 (95% CI, 0.89 to 6.35) and 4.20 (95% CI, 1.54 to 11.44) for falling below a clinically protective level of 0.1 IU/mL for tetanus and diphtheria antibodies at age 7 years.
Conclusion
Elevated exposures to PFCs were associated with reduced humoral immune response to routine childhood immunizations in children aged 5 and 7 years.
FLUORINE -SUBSTITUTED ORganic compounds have thousands of important industrial and manufacturing applications and occur widely in surfactants and repellants in food packaging and textile impregnation.1 The perfluorinated compounds (PFCs) are highly persistent and cause contamination of drinking water, food, and food chains.1 The most common PFCs, perfluorooctanoic acid (PFOA, sometimes called C8), perfluorooctane sulfonic acid (PFOS), and perfluorohexane sulfonic acid (PFHxS), have elimination half-lives in humans of at least 4 years2 and are commonly detected in human serum.3
Perfluorinated compounds are transferred through the placenta,4 and postnatal exposure sources include human milk and house dust.1 Because few prospective data are available on health risks to the general population, current risk assessment has relied on liver toxicity and peroxisome proliferation in animal models.5 The immune system in mice has recently been shown to be highly sensitive to PFOS, with adverse effects on humoral immunity detected at blood concentrations similar to those occurring in humans.6 In vitro studies support that immunotoxicity is plausible,7 also in regard to other PFCs.8
As a feasible parameter in population studies, the antibody response to childhood immunizations is clinically relevant and reflects major immune system functions.9 We therefore initiated an investigation of antibody responses to diphtheria and tetanus toxoids as indicators of immunotoxicity in children.10 Our study focused on the fishing community of the Faroe Islands, where frequent intake of marine food is associated with increased exposures to PFCs.11
METHODS
Cohort Formation and Clinical Examinations
The birth cohort was formed from 656 consecutive singleton births at the National Hospital in Tórshavn, Faroe Islands, during 1997-2000. Although cesarean deliveries and obstetric complications were usually not included, the cohort can be considered reasonably representative of Faroese births. Health care is free of charge in the Faroes, and childhood immunizations begin with vaccinations at age 3 months against diphtheria and tetanus, along with pertussis, polio, and Haemophilus influenzae type B. Repeat inoculations are given at ages 5 and 12 months, with a booster vaccination against diphtheria and tetanus at age 5 years.
To examine the long-term antibody responses to the immunizations, the birth cohort underwent prospective follow-up until age 7 years. A total of 587 children (89% of the cohort) participated in 1 or more of the examinations, which took place at age 5 years prebooster, approximately 4 weeks after the booster, and at age 7 years.12 Results from 6 children at the most recent examination were excluded, because an additional booster had been given at some point after the routine booster given at age 5 years. The 464 children (71%) participating at age 7 years did not significantly differ from nonparticipants in terms of sex, maternal exposure to PFCs, or antibody concentration at age 5 years.
The study protocol was approved by the ethical review committee serving the Faroe Islands and by the institutional review board at the Harvard School of Public Health, and written informed consent was obtained from all mothers.
Exposure Assessment
Prenatal exposures to PFCs were assessed from analyses of serum obtained from the mother at the last antenatal examination at week 32 of pregnancy; postnatal exposure was assessed from serum from the child at age 5 (prebooster). Analysis of coded samples for PFC concentrations was carried out by online solid-phase extraction and analysis, using high-pressure liquid chromatography with tandem mass spectrometry.13 The accuracy and reliability of the data was ensured by including, in each analytical series, quality control serum samples, calibration standards, and reagent and serum blanks.
PFOS was quantified by integration of 2 adjacent peaks, which represent the branched isomers and the linear isomer. Within-batch and between-batch imprecision (assessed by coefficient of variation) were better than 3.0% and 5.2% for all analytes. Results with excellent accuracy were obtained in the regular comparisons organized by the German Society of Occupational Medicine. Polychlorinated biphenyls (PCBs) were measured in maternal serum, milk, and the child’s serum as previously described.12 We used a simplified lipid-based lPCB concentration calculated as the sum of congeners CB-138, CB-153, and CB-180 multiplied by 2.14
Vaccines and Antibody Measurements
All children in the study were vaccinated according to the official Danish/Faroese vaccination program. Tetanus toxoid and diphtheria toxoid are both classic protein antigens that depend on T helper cells for primary as well as recall antibody responses. Toxoids for the routine childhood vaccinations and boosters were from Statens Serum Institut, Copenhagen, Denmark. All children received the same amount of tetanus and diphtheria toxoids and associated alum adjuvant. However, from September 1, 2003, the booster vaccine also contained pertussis antigen, and from July 1, 2004, it also contained polio antigen. Mercury-based preservatives were not used.
Serum concentrations of antibodies against the tetanus toxoid were measured in coded samples by the Statens Serum Institut using enzyme-linked immunosorbent assay,15 whereas antibodies against the diphtheria toxoid were measured using a standard Vero cell– based neutralization assay using 2-fold dilutions of serum samples.16 For both assays, calibration was performed using international and local standard antitoxins.
Data Analysis
Concentrations of PFCs and PCBs showed skewed distributions and were log transformed.12 Correlations were determined by pairwise Pearson correlation coefficients. Associations of PFC exposure with antibody concentrations were first determined using standard regression techniques. Antibody concentrations were also log transformed to obtain normally distributed residuals with a homogeneous variance. As obligatory covariates, we included sex and age.
For the 5-year postbooster data, we adjusted for the time since vaccination, using a restricted cubic spline.12 We also considered the possible effect of PCB exposure, birth weight, maternal smoking during pregnancy, and duration of breastfeeding, in regard to their possible influence on the PFC regression coefficients. Booster type was considered for the 2 most recent examinations.
Individual PFC exposure parameters were entered into the regression models one at a time. Because of the log transformation, the outcome measures could be expressed as the relative change (in %) of the antibody concentrations for each 2-fold increase of the exposure. The appropriateness of linear dose-response functions was explored in generalized additive models that produce flexible nonparametric relationships.17
Structural equation models18,19 were generated to determine the joint associations of PFCs with the overall antibody concentrations. This class of multivariate models typically consists of a measurement part in which the observed variables are linked to a limited number of latent variables and a structural part describing the relationship between the latent variables, with possible adjustment for the effects of covariates. In the measurement part, at least 3 independent exposure variables are desirable, and we therefore included the PFCs with the highest concentrations (PFOS, PFOA, PFHxS) as reflections of the latent variable representing the overall, true PFC exposure. In the structural part, the latent exposure variable is considered a predictor of the individual or joint antibody concentrations. Because information from multiple exposures and multiple outcomes can be included simultaneously, these models afford a greater statistical power, while avoiding the need for adjustment for multiple comparisons.20,21 The appropriateness of the model was determined by comparing the expected and the observed covariance in a χ2 test, with a P value less than .05 taken to indicate that the model did not properly represent the data.
Because of the clinical importance of protective antibody concentrations greater than 0.1 IU/mL, odds ratios were calculated to assess the effect of PFC exposure on the risk of having an antibody concentration below this level.
After log transformation, the PFC concentrations showed standard deviations similar to those previously observed for PCBs.10,12 Thus, a 90% power to detect a difference of 18% in antibody concentrations also applies for each 2-fold increase in the PFC concentrations. All tests were 2-sided, and we used a 5% significance level. The statistical analyses were performed using Stata version 11 and Mplus version 5.2.
RESULTS
The major characteristics of cohort members contributing serum samples at ages 5 and 7 years are presented in TABLE 1. Serum antibody concentrations were higher for tetanus than for diphtheria, and many diphtheria results at 5 years prebooster were close to the clinically protective level of 0.1 IU/L. The PFCs within each sample set were interrelated but showed weak associations between prenatal and post-natal exposures (TABLE 2). Most of the PFCs correlated only weakly with PCBs in maternal serum. Tertile exposure groups showed lower average antibody concentrations, especially at age 7 years in regard to higher serum PFC concentrations at age 5 years (eTable 1, available at http://www.jama.com). A lower antibody response was observed in 2 groups of 173 and 168 children, who had been inoculated with combination booster vaccines containing pertussis, polio, or both, as compared with the 151 who received diphtheria and tetanus toxoids only. Use of a combination booster was therefore included as a covariate for the post-booster data.
Table 1.
Characteristics of Faroese Birth Cohort Members (N = 587) Examined During 7 Years of Follow-up
| Variable | Result |
|---|---|
| Birth and infancy (n = 587) | |
| Maternal smoking during pregnancy, No. (%) | 171 (29.1) |
|
| |
| Girls, No. (%) | 278 (47.4) |
|
| |
| Birth weight, mean (SD), g | 3725.5 (505.9) |
|
| |
| Birth weight ≤2500 g, No. (%) | 5 (0.9) |
|
| |
| Total duration of breastfeeding, mean (SD), mo | 9.8 (6.7) |
|
| |
| Prebooster examination (n = 532) | |
| Age, mean (SD), y | 5.0 (0.1) |
|
| |
| Antibody concentration, median (IQR), IU/mL | |
| Tetanus | 0.22 (0.10-0.51) |
|
| |
| Diphtheria | 0.12 (0.05-0.40) |
|
| |
| Postbooster examination (n = 456) | |
| Age, mean (SD), y | 5.2 (0.1) |
|
| |
| Antibody concentration, median (IQR), IU/mL | |
| Tetanus | 35 (16-96) |
|
| |
| Diphtheria | 13 (6.4-26) |
|
| |
| Age 7 years examination (n = 464) | |
| Age, mean (SD), y | 7.5 (0.1) |
|
| |
| Antibody concentration, median (IQR), IU/mL | |
| Tetanus | 1.59 (0.65-4.60) |
|
| |
| Diphtheria | 0.68 (0.40-1.60) |
Abbreviation: IQR, interquartile range.
Table 2.
Concentrations of PFCs in Serum From Birth Cohort Mothers and Their Children at Age 5 Years and Correlations Pairwise and With the PCB Concentration in Maternal Serum
| PFC Concentration, Geometric Mean (IQR), ng/mL |
Pairwise Pearson Correlations at Age 5 y |
||||||
|---|---|---|---|---|---|---|---|
| Compound | PCB | PFOS | PFOA | PFHxS | PFNA | PFDA | |
| Maternal PFCs | |||||||
| PFOS | 27.3 (23.2 to 33.1) | 0.24 | 0.27 | 0.05 | 0.07 | 0.14 | 0.07 |
|
| |||||||
| PFOA | 3.20 (2.56 to 4.01) | −0.03 | 0.06 | 0.19 | 0.03 | −0.02 | −0.06 |
|
| |||||||
| PFHxS | 4.41 (2.24 to 8.43) | 0.05 | 0.28 | 0.01 | 0.11 | −0.01 | 0.03 |
|
| |||||||
| PFNA | 0.60 (0.46 to 0.79) | 0.55 | 0.23 | 0.03 | 0.14 | 0.32 | 0.20 |
|
| |||||||
| PFDA | 0.28 (0.22 to 0.38) | 0.48 | 0.28 | 0.01 | 0.13 | 0.30 | 0.20 |
|
| |||||||
| PFCs at age 5 y | |||||||
| PFOS | 16.7 (13.5 to 21.1) | 0.08 | 1 | ||||
|
| |||||||
| PFOA | 4.06 (3.33 to 4.96) | 0.00 | 0.50 | 1 | |||
|
| |||||||
| PFHxS | 0.63 (0.45 to 0.88) | 0.07 | 0.57 | 0.53 | 1 | ||
|
| |||||||
| PFNA | 1.00 (0.76 to 1.24) | 0.29 | 0.48 | 0.54 | 0.34 | 1 | |
|
| |||||||
| PFDA | 0.28 (0.21 to 0.38) | 0.14 | 0.39 | 0.35 | 0.22 | 0.78 | 1 |
Abbreviations: IQR, interquartile range; PCB, polychlorinated biphenyl; PFC, perfluorinated compound; PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoate; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Multiple regression analyses with covariate adjustment showed that prenatal exposures to both PFOS and PFOA, as indicated by the maternal serum concentrations, were negatively associated with antidiphtheria antibody concentrations (TABLE 3). Most clearly, a 2-fold increase in PFOS exposure was associated with a difference in antibody concentration of −39% (95% CI, −55% to −17%) at age 5 years before the booster. All but 1 of the PFC concentrations measured in the child’s serum at age 5 years showed negative associations with the antibody concentrations measured in serum both before and after the booster. For antibody concentrations at age 7 years, all PFC exposures measured at age 5 years showed negative associations, most strongly for PFOA and PFOS. Thus, a 2-fold increase in PFOA exposure was associated with differences of −36% (95% CI, −52% to −14%) and −25% (95% CI, −43% to −2%) for tetanus and diphtheria, respectively. The PFOS exposure was associated with a difference in diphtheria antibody of −28% (95% CI, −46% to −3%).
Table 3.
Estimated Percentage Difference in Specific Antibody Concentrations at Age 5 Years Prebooster, 5 Years Postbooster, and 7 Years, per 2-Fold Increase in Concentrations of PFCs in Maternal or 5-Year Serum
| Change, % (95% CI) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Maternal PFC |
PFC at Age 5 y |
|||||||
| Year 5 |
Year 7c, Adjusted for Age 5 Resultsd |
Year 5 |
Year 7 Adjusted for Age 5 Resultsd |
|||||
| Antibody | Preboostera | Postboosterb | Year 7c | Preboostera | Postboosterb | Year 7c | ||
| Tetanus | n = 509 | n = 419 | n = 424 | n = 380 | n = 537 | n = 440 | n = 408 | n = 401 |
|
| ||||||||
| PFOS | −10.1 (−31.9 to 18.7) |
−2.3 (−28.6 to 33.6) |
35.3 (−3.9 to 90.6) |
33.1 (1.5 to 74.6) |
−11.9 (−30.0 to 10.9) |
−28.5 (−45.5 to −6.1) |
−23.8 (−44.3 to 4.2) |
−11.4 (−30.5 to 12.8) |
|
| ||||||||
| PFOA | −10.5 (−28.2 to 11.7) |
14.5 (−10.4 to 46.4) |
7.4 (−17.1 to 39.0) |
12.3 (−8.6 to 38.1) |
−13.3 (−31.6 to 9.9) |
−9.7 (−30.7 to 17.7) |
−35.8 (−51.9 to −14.2) |
−28.2 (−42.7 to −10.1) |
|
| ||||||||
| PFHxS | −6.3 (−15.1 to 3.4) |
6.3 (−8.4 to 23.2) |
4.5 (−9.6 to 20.6) |
12.6 (0.6 to 25.9) |
−6.3 (−17.6 to 6.5) |
−19.0 (−29.8 to −6.6) |
−19.7 (−31.6 to −5.7) |
−14.0 (−24.0 to −2.6) |
|
| ||||||||
| PFNA | 11.2 (−8.6 to 35.1) |
−3.7 (−23.1 to 20.7) |
22.1 (−4.2 to 55.5) |
4.5 (−13.9 to 26.7) |
−5.9 (−21.8 to 13.4) |
−18.2 (−34.0 to 1.4) |
−17.4 (−34.1 to 3.6) |
−13.6 (−27.3 to 2.8) |
|
| ||||||||
| PFDA | −2.5 (−18.5 to 16.8) |
−6.1 (−23.5 to 15.3) |
16.4 (−6.7 to 45.2) |
4.9 (−11.8 to 24.7) |
−13.6 (−26.3 to 1.4) |
−19.9 (−33.1 to −3.9) |
−22.3 (−35.8 to −5.8) |
−12.5 (−24.6 to 1.5) |
|
| ||||||||
| Diphtheria | n = 510 | n = 419 | n = 424 | n = 382 | n = 537 | n = 440 | n = 408 | n = 403 |
|
| ||||||||
| PFOS | −38.6 (−54.7 to −16.9) |
−20.6 (−37.5 to 0.9) |
−19.7 (−41.8 to 10.7) |
−10.0 (−32.6 to 20.0) |
−16.0 (−34.9 to 8.3) |
−15.5 (−31.5 to 4.3) |
−27.6 (−45.8 to −3.3) |
−20.6 (−38.2 to 2.1) |
|
| ||||||||
| PFOA | −16.2 (−34.2 to 6.7) |
−6.2 (−22.4 to 13.3) |
−22.8 (−39.4 to −1.7) |
−16.8 (−32.9 to 3.3) |
−6.8 (−28.3 to 21.0) |
−6.1 (−23.6 to 15.5) |
−25.2 (−42.9 to −2.0) |
−23.4 (−39.3 to −3.4) |
|
| ||||||||
| PFHxS | −6.4 (−16.0 to 4.3) |
−3.7 (−14.1 to 7.9) |
−0.5 (−13.1 to 14.0) |
1.8 (−9.5 to 14.6) |
5.0 (−8.9 to 21.0) |
−9.1 (−18.7 to 1.7) |
−9.8 (−22.3 to 4.9) |
−12.5 (−23.2 to −0.4) |
|
| ||||||||
| PFNA | −14.8 (−31.2 to 5.5) |
−12.9 (−26.7 to 3.5) |
−5.1 (−24.4 to 19.2) |
−6.5 (−23.7 to 14.4) |
−17.7 (−33.0 to 1.1) |
−16.1 (−28.8 to −1.0) |
−17.1 (−32.8 to 2.2) |
−14.5 (−28.6 to 2.4) |
|
| ||||||||
| PFDA | −21.7 (−35.7 to −4.8) |
−18.8 (−30.5 to −5.0) |
0.7 (−18.2 to 24.0) |
2.3 (−14.8 to 22.8) |
−16.0 (−29.6 to 0.3) |
−8.7 (−20.6 to 5.0) |
−14.4 (−28.4 to 2.4) |
−10.3 (−23.1 to 4.6) |
Abbreviations: PFDA, perfluorodecanoate; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoate; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid.
Adjusted for age and sex.
Adjusted for age, sex, time since vaccination (as restricted cubic spline), and booster type.
Adjusted for age, sex, and booster type.
Adjusted for age, sex, booster type, and the child’s specific antibody concentration at age 5 years.
In an additional regression model, the antibody concentrations at age 7 years were further adjusted for each child’s antibody concentration at age 5 years (Table 3), thus focusing on the association between PFC exposure and the relative change in antibody concentrations between ages 5 and 7 years. After this adjustment, the associations for PFC exposure at age 5 years remained in the same direction, although weaker than without the adjustment. Serum concentrations of the linear isomer showed associations similar to those reported here for total PFOS, whereas the branched isomers generally showed weaker associations with the outcome variables (eTable 2). Results adjusted for PCBs in milk and 5-year serum as predictors of PCB immunotoxicity12 were not materially different (eTable 3).
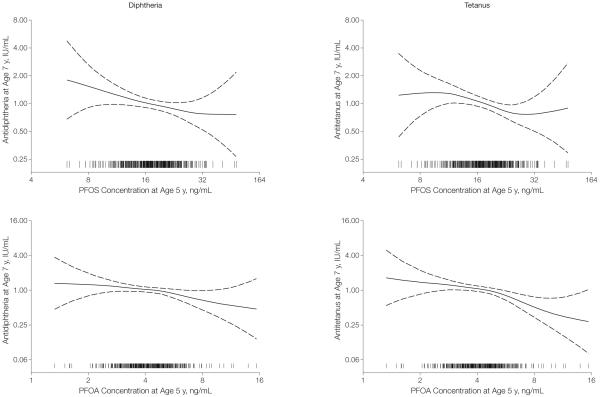
The 3 major PFC concentrations were combined into 2 latent variables representing prenatal and postnatal exposures, respectively, which were then included as predictors of the antibody concentration at ages 5 (prebooster) and 7 years (TABLE 4). Perfluorononanoate and perfluorodecanoate were not included because the structural equation model failed to converge, perhaps because these PFCs correlated less strongly with the major 3 compounds (Table 2). When the associations of PFC exposure with the 2 antibody concentrations were similar, both outcomes were included in a joint structural equation model. At a doubled postnatal PFC exposure, the antibody concentration at age 7 years was approximately halved, with a point estimate of −49% (95% CI, −67% to −23%) for the difference (Table 4). This highly significant difference remained after adjustment for prenatal PFC exposure. The dose-response relationships for PFOS and PFOA (FIGURE) indicate that the PFC-related differences in antibody concentrations are monotonic, with a curve shape that is approximately linear.
Table 4.
Differences in Tetanus and Diphtheria Antibody Concentrations at Age 5 Years Prebooster and Age 7 Associated With a Doubled Concentration of PFCs for Maternal Pregnancy Serum and Age-5 Serum in a Structural Equation Model
| Tetanus, % Change (95% CI) |
P
Value |
Diphtheria, % Change (95% CI) |
P
Value |
P Value for Same Effecta |
Joint Change, % (95% CI) |
P
Value |
|
|---|---|---|---|---|---|---|---|
| Age 5 prebooster | |||||||
| Maternal PFC | −20.2 (−49.2 to 25.2) | .33 | −47.9 (−67.7 to −15.9) | .008 | .17 | −31.1 (−56.8 to 9.8) | .12 |
|
| |||||||
| PFC at age 5 y | −20.5 (−44.4 to 13.6) | .21 | −7.9 (−38.0 to 37.0) | .69 | .47 | −15.6 (−38.5 to 15.8) | .29 |
|
| |||||||
| PFC at age 5 yb | −17.2 (−42.1 to 18.5) | .30 | −1.2 (−33.6 to 46.8) | .95 | .39 | −11.0 (−35.2 to 22.3) | .47 |
|
| |||||||
| Age 7 | |||||||
| Maternal PFC | 35.1 (−25.4 to 144.6) | .32 | −42.0 (−66.1 to −0.8) | .047 | .007 | ||
|
| |||||||
| PFC at age 5 y | −55.2 (−73.3 to −25.0) | .002 | −44.4 (−65.5 to −10.5) | .02 | .42 | −49.4 (−66.7 to −23.0) | .001 |
|
| |||||||
| PFC at age 5 yb | −58.8 (−76.0 to −29.3) | .001 | −45.5 (−66.9 to −10.3) | .02 | .31 | −51.8 (−68.9 to −25.1) | .001 |
Abbreviation: PFC, perfluorinated compound.
Determined by likelihood ratio test for the same effect of PFC on the 2 types of antibodies.
Adjusted for the PFC concentration in maternal pregnancy serum.
Figure.
Association Between Serum Concentrations of Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA) at Age 5 Years and Serum Antibody Concentrations Against Diphtheria and Tetanus at Age 7 Years
The generalized additive models have 3 df and were adjusted for age, sex, and vaccine booster type. Dashed lines indicate 95% confidence intervals; vertical bars on the horizontal scale indicate individual observations.
At the prebooster examination, 141 (26%) and 202 (37%) of the cohort members had antibody concentrations below a clinically protective level of 0.1 IU/mL for tetanus and diphtheria, respectively, while the same was observed for 18 and 32 children at age 7 years. The odds ratios (ORs) of antibody concentrations falling below the protective level for diphtheria at age 5 years were the strongest for the PFOS concentrations, both in maternal serum (OR, 2.48 [95% CI, 1.55 to 3.97]) and child serum (OR, 1.60 [95% CI, 1.10 to 2.34]). At age 7 years, the age 5 serum concentrations of PFOS (OR, 2.38 [95% CI, 0.89 to 6.35]) and PFOA (OR, 3.27 [95% CI, 1.43 to 7.51]) were the strongest predictors. Odds ratios for tetanus at age 7 years were 2.61 (95% CI, 0.77 to 8.92) for PFOS and 4.20 (95% CI, 1.54 to 11.44) for PFOA, both measured in child serum (eTable 4).
COMMENT
These results indicate that PFC exposures at commonly prevalent serum concentrations are associated with lower antibody responses to childhood immunizations and an increased risk of antibody concentrations below the level needed to provide long-term protection. In the prospective design of this study, PFCs were measured both in maternal pregnancy serum and in samples from the child at age 5 years, thus allowing assessment of prenatal and postnatal exposure, with antibody concentrations as outcomes at ages 5 and 7 years. Negative associations of serum PFC concentrations with antibody concentrations were particularly prominent for exposures measured at age 5 years. The prenatal exposure level, as indicated by the mother’s serum concentration during pregnancy, was less clearly associated with the antibody outcomes. Although all of the 5 PFCs measured showed negative associations with antibody levels, the overlapping confidence intervals and the lack of comparative toxicology studies prevent inference in regard to causal attribution. The structural equation models combined the information on the 3 major PFCs and suggested that a 2-fold greater concentration of the exposure was associated with a halving of the antibody concentration. The model showed an excellent fit, adjusted for imprecision, and avoided the need for adjustment for multiple comparisons, although it could not represent the full effects of all PFCs measured.
In agreement with PFC analyses previously carried out by the Centers for Disease Control and Prevention,11 the serum PFC concentrations from Faroese women are similar to or slightly less than those reported for adult US women in 1999-2000, except for PFHxS.3 Limited data suggest that US children may have higher serum concentrations than adults, and most PFC results for the Faroese children at age 5 years in 2004-2006 are lower than the concentrations in serum pools from US children aged 3 to 5 years in 2001-2002.22 In contrast, PCB exposures in the Faroes are higher than in the United States,23 but associations with PFC concentrations were weak, and adjustment for PCB exposure10,12 did not appreciably change the results.
The concentrations of specific antibodies in serum represent a useful and clinically relevant indicator of immune functions in children.9,24 All children in the study received the same dose of toxoids at about the same age and were examined on 3 occasions at similar intervals after the most recent vaccination. The addition of polio and pertussis antigens may have attenuated the antibody responses and thus was taken into account in the data analysis. Other covariates had only a negligible effect on the regression coefficients. The negative association of PFC exposure with diphtheria and tetanus antibody concentrations can be compared with our previous finding that a 2-fold greater concentration of PCB exposure was associated with a difference in antibody concentrations of up to about −20%.10,12 The PFC-associated effects appear to be substantially greater, with differences up to −49% for a 2-fold increase in exposure.
This study is apparently the first to link PFC exposure in children to deficits in immune system functions. PFOS (most likely the linear isomer) and PFOA appear to be the main culprits. From ongoing studies of a general population group exposed to PFOA through contaminated drinking water, an interim report recently stated that this exposure was associated with lower serum concentrations of IgA and IgE (in females only) but not IgG.25 Because these cross-sectional results refer to total immunoglobulin concentrations, they may not necessarily reflect the generation and maintenance of specific antibody levels. We are not aware of any other human studies in this area. Our findings are supported by several,6,26,27 though not all,28 experimental studies in rodents, in which adverse effects of PFOS on humoral immune function were observed at serum concentrations similar to those reported in the present study and at levels prevalent in the United States.22 The similarity in effect levels is noteworthy, given the interspecies differences in immune system development.
An antibody concentration greater than 0.1 IU/mL is considered an important indicator of protection in accordance with the public health rationale for routine vaccinations. Prenatal and postnatal PFOS exposures, as well as postnatal PFOA exposure, were associated with increased odds of antibody concentrations below the protective level. If the associations are causal, the clinical importance of our findings is therefore that PFC exposure may increase a child’s risk for not being protected against diphtheria and tetanus, despite a full schedule of vaccinations. Adequate formation of specific antibodies relies on several important immune functions,9,24 and serum antibody concentrations triggered by standardized antigen stimulations may therefore reflect the more general efficacy of the immune system in relation to infection. For this reason, PFC-associated decreases in antibody concentrations may indicate the potential existence of immune system deficits beyond the protection against the 2 specific bacteria examined in this study.
In summary, elevated exposures to PFCs in Faroese children aged 5 and 7 years were associated with reduced humoral immune response to routine childhood immunizations. These findings suggest a decreased effect of childhood vaccines and may reflect a more general immune system deficit. Assessment of risk related to exposure to these contaminants therefore needs to consider the immunotoxic potential of the PFCs.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by the National Institute of Environmental Health Sciences, NIH (ES012199); the US Environmental Protection Agency (R830758); the Danish Council for Strategic Research (09-063094); and the Danish Environmental Protection Agency as part of the environmental support program DANCEA (Danish Cooperation for Environment in the Arctic).
Role of the Sponsors: The study sponsors had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Additional Contributions: We thank the cohort families for participating in this project; laboratory technician Vibeke K. Pedersen of the University of Southern Denmark for PFC analyses (no extra compensation received); and the staff at the Quality Control Department of Statens Serum Institut, Copenhagen, for performing the antibody analyses.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Mølbak reported that he is employed as a civil servant at the government-owned Statens Serum Institut, which manufactures and markets vaccines in Denmark and other countries. No other authors reported disclosures.
Disclaimer: The authors are solely responsible for all results and conclusions reported herein, which do not necessarily reflect the position of any of the funding agencies.
Online-Only Material: eTables 1-4 are available at http://www.jama.com.
REFERENCES
- 1.Vestergren R, Cousins IT. Tracking the pathways of human exposure to perfluorocarboxylates. Environ Sci Technol. 2009;43(15):5565–5575. doi: 10.1021/es900228k. [DOI] [PubMed] [Google Scholar]
- 2.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) Environ Sci Technol. 2007;41(7):2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 4.Needham LL, Grandjean P, Heinzow B, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45(3):1121–1126. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Food Safety Authority Opinion of the Scientific Panel on Contaminants in the Food Chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008;653:1–131. doi: 10.2903/j.efsa.2008.653. http://www.efsa.europa.eu/de/scdocs/doc/653.pdf. Accessed December 29, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fair PA, Driscoll E, Mollenhauer MA, et al. Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J Immunotoxicol. 2011;8(1):17–29. doi: 10.3109/1547691X.2010.527868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsini E, Avogadro A, Galbiati V, et al. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs) Toxicol Appl Pharmacol. 2011;250(2):108–116. doi: 10.1016/j.taap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Fang X, Feng Y, Shi Z, Dai J. Alterations of cytokines and MAPK signaling pathways are related to the immunotoxic effect of perfluorononanoic acid. Toxicol Sci. 2009;108(2):367–376. doi: 10.1093/toxsci/kfp019. [DOI] [PubMed] [Google Scholar]
- 9.Dietert RR. Developmental immunotoxicology (DIT): windows of vulnerability, immune dysfunction and safety assessment. J Immunotoxicol. 2008;5(4):401–412. doi: 10.1080/15476910802483324. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med. 2006;3(8):e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weihe P, Kato K, Calafat AM, et al. Serum concentrations of polyfluoroalkyl compounds in Faroese whale meat consumers. Environ Sci Technol. 2008;42(16):6291–6295. doi: 10.1021/es800695m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilmann C, Budtz-Jørgensen E, Nielsen F, Heinzow B, Weihe P, Grandjean P. Serum concentrations of antibodies against vaccine toxoids in children exposed perinatally to immunotoxicants. Environ Health Perspect. 2010;118(10):1434–1438. doi: 10.1289/ehp.1001975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. J Chromatogr A. 2009;1216(3):385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 14.Grandjean P, Weihe P, Needham LL, et al. Relation of a seafood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res. 1995;71(1):29–38. doi: 10.1006/enrs.1995.1064. [DOI] [PubMed] [Google Scholar]
- 15.Hendriksen CF, vd Gun JW, Nagel J, Kreeftenberg JG. The toxin binding inhibition test as a reliable in vitro alternative to the toxin neutralization test in mice for the estimation of tetanus antitoxin in human sera. J Biol Stand. 1988;16(4):287–297. doi: 10.1016/0092-1157(88)90017-0. [DOI] [PubMed] [Google Scholar]
- 16.Miyamura K, Nishio S, Ito A, Murata R, Kono R. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using VERO cells, I: studies on factors affecting the toxin and antitoxin titration. J Biol Stand. 1974;2(3):189–201. doi: 10.1016/0092-1157(74)90015-8. [DOI] [PubMed] [Google Scholar]
- 17.Hastie TJ, Tibshirani RJ. Generalized Additive Models. Chapman & Hall/CRC Press; Boca Raton, FL: 1990. Monographs on Statistics and Applied Probability 43. [Google Scholar]
- 18.Bollen KA. Structural Equations With Latent Variables. John Wiley; New York, NY: 1989. [Google Scholar]
- 19.Skrondal A, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Chapman & Hall/CRC Press; Boca Raton, FL: 2004. [Google Scholar]
- 20.Budtz-Jørgensen E, Keiding N, Grandjean P, Weihe P. Estimation of health effects of prenatal methylmercury exposure using structural equation models. Environ Health. 2002;1(1):2. doi: 10.1186/1476-069X-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez BN, Budtz-Jørgensen E, Ryan L, Hu H. Structural equation models: a review with applications to environmental epidemiology. J Am Stat Assoc. 2005;100(472):1443–1455. http://pubs.amstat.org/doi/abs/10.1198/016214505000001005. Accessed December 29, 2011. [Google Scholar]
- 22.Kato K, Calafat AM, Wong LY, Wanigatunga AA, Caudill SP, Needham LL. Polyfluoroalkyl compounds in pooled sera from children participating in the National Health and Nutrition Examination Survey 2001-2002. Environ Sci Technol. 2009;43(7):2641–2647. doi: 10.1021/es803156p. [DOI] [PubMed] [Google Scholar]
- 23.Grandjean P, Weihe P, Burse VW, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23(4):305–317. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 24.van Loveren H, Germolec D, Koren HS, et al. Report of the Bilthoven Symposium: advancement of epidemiological studies in assessing the human health effects of immunotoxic agents in the environment and the workplace. Biomarkers. 1999;4(2):135–157. doi: 10.1080/135475099230949. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher T, Steenland K, Savitz D. C8 Science Panel. Status Report: PFOA and immune biomarkers in adults exposed to PFOA in drinking water in the mid Ohio valley. C8 Science Panel Web site. http://www.c8sciencepanel.org/pdfs/Status_Report _C8_and_Immune_markers_March2009.pdf. March 16, 2009. Accessed June 13, 2011.
- 26.Keil DE, Mehlmann T, Butterworth L, Peden-Adams MM. Gestational exposure to perfluorooctane sulfonate suppresses immune function in B6C3F1 mice. Toxicol Sci. 2008;103(1):77–85. doi: 10.1093/toxsci/kfn015. [DOI] [PubMed] [Google Scholar]
- 27.Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol Sci. 2008;104(1):144–154. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- 28.Qazi MR, Abedi MR, Nelson BD, DePierre JW, Abedi-Valugerdi M. Dietary exposure to perfluorooctanoate or perfluorooctane sulfonate induces hypertrophy in centrilobular hepatocytes and alters the hepatic immune status in mice. Int Immunopharmacol. 2010;10(11):1420–1427. doi: 10.1016/j.intimp.2010.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.