Abstract
Study Objectives:
When sounds associated with learning are presented again during slow-wave sleep, targeted memory reactivation (TMR) can produce improvements in subsequent location recall. Here we used TMR to investigate memory consolidation during an afternoon nap as a function of prior learning.
Participants:
Twenty healthy individuals (8 male, 19–23 y old).
Measurements and Results:
Participants learned to associate each of 50 common objects with a unique screen location. When each object appeared, its characteristic sound was played. After electroencephalography (EEG) electrodes were applied, location recall was assessed for each object, followed by a 90-min interval for sleep. During EEG-verified slow-wave sleep, half of the sounds were quietly presented over white noise. Recall was assessed 3 h after initial learning. A beneficial effect of TMR was found in the form of higher recall accuracy for cued objects compared to uncued objects when pre-sleep accuracy was used as an explanatory variable. An analysis of individual differences revealed that this benefit was greater for participants with higher pre-sleep recall accuracy. In an analysis for individual objects, cueing benefits were apparent as long as initial recall was not highly accurate. Sleep physiology analyses revealed that the cueing benefit correlated with delta power and fast spindle density.
Conclusions:
These findings substantiate the use of targeted memory reactivation (TMR) methods for manipulating consolidation during sleep. TMR can selectively strengthen memory storage for object-location associations learned prior to sleep, except for those near-perfectly memorized. Neural measures found in conjunction with TMR-induced strengthening provide additional evidence about mechanisms of sleep consolidation.
Citation:
Creery JD, Oudiette D, Antony JW, Paller KA. Targeted memory reactivation during sleep depends on prior learning. SLEEP 2015;38(5):755–763.
Keywords: memory reactivation, consolidation, reactivation, slow-wave sleep, sleep spindles
INTRODUCTION
Consolidation refers to a gradual process whereby new knowledge is integrated with prior knowledge such that memory storage can be stabilized and strengthened, probably through reactivation during both sleep and waking.1,2 Sleep may be an optimal time for consolidation, because it is relatively free from competing sensory information and because the distinctive neurophysiology of slow-wave sleep (SWS) may be particularly conducive to consolidation. If so, multiple factors may determine which memories are prone to reactivation during sleep, possibly including emotional salience, motivation to remember, and pre-sleep memory strength.3 Here, we use the technique of targeted memory reactivation (TMR) to investigate the influence of memory strength prior to sleep.
Studies of human memory consolidation during sleep have produced behavioral evidence of superior retrieval after a period of sleep compared to an equal period of wake.4,5 Specific benefits from memory processing during sleep are also suggested by evidence from sleep physiology. For example, measures of SWS correlate with memory improvement from before to after a period of sleep.6,7 Furthermore, slow waves have greater amplitudes and lower frequencies after a period of intentional learning than after a period without intentional learning.8 Also, electrically inducing SWS starting in stage-2 sleep can boost overall slow-wave amplitude and post-sleep memory performance.9,10 Accordingly, a current hypothesis is that slow-wave oscillations temporally frame the communication between relevant areas of the neocortex, where memories are integrated into existing schema, and the hippocampus, where links among cortical regions are first formed.11
Compelling evidence of neural reactivation of memories in the rodent hippocampus provides clues about the mechanisms of sleep-dependent memory benefits.12,13 Hippocampal place-cells fired in the same temporal order during sleep as during prior learning, which was interpreted as memory replay. In a human neuroimaging study, hippocampal activity during SWS was similar to that previously observed during a spatial navigation task.6 However, we have very little information about which measures of neural activity in humans index specific instances of memory reactivation during sleep.
Directly influencing which memories are reactivated using targeted memory reactivation (TMR) may provide a new, noninvasive strategy to shed light on memory processing during sleep. The literature on TMR was recently reviewed by Oudiette and Paller.14 In a landmark study, Rasch and colleagues15 used odors to cue learning during sleep. Before sleep, participants learned object-location associations in a rose-odor context. Presenting the odor again during SWS led to an increase in location recall accuracy, and a control condition was used to show that the benefit required odor presentation both during learning and during SWS.
Rudoy and colleagues16 also demonstrated TMR following object-location learning. Whereas Rasch and colleagues15 used odor cues associated with the context of learning, Rudoy and colleagues16 used sound cues that were each linked with a specific object. Results showed less forgetting for objects that were cued by a sound during sleep compared to objects that were not cued. This TMR effect showed that sounds during sleep can cue specific memories, which in this case were unique object-location associations. In rodents, TMR was used to directly link external reactivation with hippocampal replay; tones associated with spatial learning were played during SWS and corresponding hippocampal place cells were preferentially reactivated.17
On the whole, these methods for reactivating recently learned information during sleep have allowed researchers to manipulate which items are reactivated during sleep, thereby providing a way to obtain new evidence concerning how reactivation influences memory.14 Whereas memory comparisons following a period of sleep versus wake are usually confounded by indirect effects on performance due to differential alertness or interference, TMR studies are advantageous because these confounds are avoided in within-subject contrasts of post-sleep memory performance for cued versus uncued material.
To firmly establish TMR as an effective tool for manipulating consolidation, it is essential to determine what types of learned material can be externally reactivated and which brain regions and mechanisms are associated with reactivation during sleep. The aforementioned studies showed that TMR can influence memory for spatial locations. TMR has also been used to influence skill learning,18 fear extinction19 and word recall.20 In the latter study, which was conducted in patients with epilepsy, results showed that one requirement for TMR is a relatively intact medial temporal region. Using a variant of the spatial location test with words, word recall benefits were found for healthy controls and patients with unilateral damage, but not patients with bilateral damage. Furthermore, the degree of word recall benefit after TMR was correlated with the degree of medial temporal damage across subjects.
Additional information is still needed about the boundary conditions for TMR benefits in post-sleep memory testing. The efficacy of TMR cues may depend on sufficiently strong associations between the cues and learning, as well as on whether other memory processing is competing with cued memory processing. Given findings that sleep consolidation is contingent on the perceived need to remember the information in question,21–24 motivation to remember may also play a role. Pragmatic aspects of memory testing may also come into play; at the extremes, TMR benefits would not be observed if memory was tested after insufficient learning or too much forgetting, or if memory performance was at ceiling levels. Some basic sensory processing of cues during sleep is probably also essential. In a study with functional magnetic resonance imaging during TMR, sounds related to learning were played during sleep, but these sounds did not produce a reliable memory benefit overall.25 Most likely, subjects in this study tended to suppress auditory processing due to the exceedingly loud environment in the scanner in which they were trying to sleep. Supporting the idea of sensory gating operative at the level of the thalamus, the degree of memory benefit was correlated with brain activation in the thalamus across subjects. The degree of memory benefit was also correlated with activity in the medial temporal lobe and the cerebellum, as well as degree of parahippocampal-precuneus connectivity, thus identifying several candidate measures of brain activity associated with sound-cued memory reactivation.
In this study we set out to replicate and extend the TMR results of Rudoy et al.16 As in the prior study, the object-location recall test afforded fine-grained measures of recall accuracy. After learning, participants moved each object to the location where they remembered it. Using this method, we analyzed TMR results as a function of memory accuracy prior to sleep. So that pre-nap accuracy measures would reflect the conspicuous retrieval of declarative memories—not contamination from information presented moments earlier and kept in mind—we included a longer delay after learning than had been used by Rudoy and colleagues. We thus tested whether memory benefits of cueing recently learned spatial locations during sleep varied as a function of level of initial learning, while also investigating whether measures of sleep physiology were related to TMR benefits.
METHODS
Participants
Healthy individuals (n = 20, 8 male, 19–23 y old) who anticipated that they would be able to sleep in the afternoon were recruited from the university community. They were instructed to wake up by 8:00 a.m. and avoid caffeine on the morning of the experiment. Participants arrived at the laboratory at approximately 1:00 p.m. Data from an additional five participants were not included because they never entered SWS, such that no cues were presented.
Procedure
Before beginning the study, participants gave informed consent and completed a set of questionnaires. One questionnaire concerned their sleep the night before, and two standard sleepiness scales were used to gauge alertness: the Stanford Sleepiness Scale26 and the Epworth Sleepiness Scale.27 Each participant was tested individually in an electrically shielded room. A 1,000 × 800 pixel (35.7 × 28.6 cm) grid on a video screen was viewed from a distance of 100 cm. Figure 1 shows the timeline of the experimental procedure.
Figure 1.
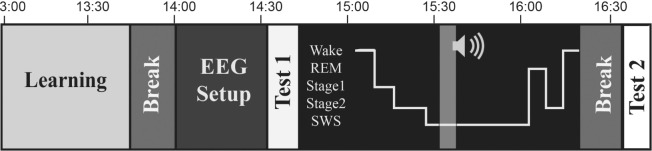
Study timeline. EEG, electroencephalography.
At the start of the learning phase, participants encoded the locations of 50 objects by viewing each object once in its correct location. The center of each object was indicated by a red dot. The 50 locations were randomly selected for each participant by computer. Objects (5.3 × 5.3 cm) appeared for 3,000 ms, with a 1,000-ms interstimulus interval. At stimulus onset, a corresponding sound was presented (e.g., the cat appeared with a “meow” sound). The duration of each sound was 500 ms.
In the remaining portion of the learning phase, objects appeared in the middle of the screen, sequentially, each accompanied by its corresponding sound. On each trial, the participant used a computer mouse to attempt to move the object to the correct location. The participant registered each answer with a mouse-click, at which point the object was displayed at the correct location. This instantaneous jump constituted feedback. An error of less than 150 pixels (5.3 cm, which corresponds to the size of each object) was considered a correct response. After each object location was tested in this manner, the set of 50 objects appeared in a different random order for a second run of testing. Any object for which the response was correct on both runs did not appear again in the learning phase. The remaining objects were shown again in the third run. Each subsequent run included only objects that had not yet elicited two correct responses in a row. The learning phase varied from 20–45 min depending on the number of runs required for participants to learn the object locations to criterion (i.e., object placed within 150 pixels of the original location twice in a row). Overall, the mean number of runs required was 3.5 (± 0.2 standard error); learning to criterion occurred in just two runs for 49% of the objects.
Following the learning phase, a 15-min break was allowed before the EEG set-up phase, which lasted approximately 30 min. Electrodes in an elastic cap were placed at 21 scalp locations from the 10–20 system (Cz, C3, C4, Fpz, Fp1, Fp2, Fz, F3, F4, F7, F8, Pz, P3, P4, T3, T4, T5, T6, Oz, O1, O2), and both mastoids. Additional electrodes were placed on the face for recording the vertical and horizontal electro-oculogram (EOG) and chin electromyogram (EMG). Electrode impedance was reduced to 5 kΩ.
Test-1 was an assessment of location recall before sleep. Beginning approximately 45 min after the end of learning, location recall was tested for all objects presented in a random order. Test format was the same as in the learning phase, except no feedback was given. Each object appeared in the middle of the screen, and the participant tried to move it to the correct location.
Next, the futon chair in the testing room was folded down to make a small bed, with sheets, a blanket and a pillow, and the participant was asked to rest quietly and attempt to take a nap. At approximately 3:00 p.m., lights were turned off and white noise was presented. Sound intensity, measured from where participants' heads were located while they slept, was approximately 37 dB(A) sound pressure level (note that this sound level was approximately the same as that used by Rudoy et al., 2009, although an incorrect dB value was reported in that paper16).
Sleep physiology was scored online using standard criteria, within the constraints of making decisions in real time. When the participant was deemed to be in SWS for two 30-sec epochs, sound cues were presented for 25 of the objects. Using performance data from Test-1, a computer algorithm ranked all 50 objects by recall error and then bifurcated the list to equate performance for cued and uncued objects. The 25 selected cue sounds were intermixed with 25 guitar strums. One sound was played approximately every 5 sec, yielding a stimulation period slightly over 4 min. Each cue sound was played only once. All participants stayed in SWS during sound presentation and none of the sounds provoked an arousal response or signs of waking in EEG recordings.
When 90 min had elapsed, the participant was awakened if not already awake. Electrodes were removed and the participant had time to freshen up. The participant then filled out the Karolinska Sleep Diary28 for the nap, as well as the Stanford Sleepiness Scale25 and the Epworth Sleepiness Scale26 to gauge alertness. Test-2, which was identical to Test-1, began at least 15 min after awakening (mean delay 17.4 ± 1.6 standard error/min).
Participants were debriefed after Test-2 about whether they had noticed sounds during the nap. They were then given an additional test in which each object was presented with the corresponding sound, and for each they indicated whether they thought they heard the cue while asleep.
EEG Data
Data were amplified with a bandpass of 0.1–100 Hz and sampling rate of 250 Hz. EEG channels were referenced offline to averaged mastoids. Data from noisy electrodes were interpolated using the spherical interpolation method in EEGlab (http://sccn.ucsd.edu/eeglab/). Offline sleep scoring was completed by a rater (who was blind to when sounds were presented) using the sleepSMG package (http://sleepsmg.sourceforge.net) for Matlab (The MathWorks Inc, Natick, MA).
EEG spectral power was calculated for each sleep stage, as well as for before, during, and after the 4-min period of sound presentation. Five EEG bands were designated slow (0.6–1 Hz), delta (0.6–4 Hz), theta (4–8 Hz), alpha (8–12 Hz) and sigma (12–15 Hz). Fast Fourier transform was applied using a Hanning function and 5-sec intervals, yielding a frequency resolution of 0.2 Hz. Mean power was computed for six clusters of electrodes: frontopolar (Fpz, Fp1, Fp2), frontal (F3, F4, Fz, F7, F8), central (C3, Cz, C4), parietal (P3, Pz, P4), temporal (T3, T4, T5, T6) and occipital (O1, Oz, O2).
Spindles were calculated in each channel separately using an established algorithm that was verified by an expert sleep scorer.29 After the EEG signal was filtered between 11–16 Hz, the root mean square (RMS) was calculated at every point with a moving window of 0.2 sec. A spindle was counted if the RMS crossed and remained above a threshold of 1.5 standard deviations of the signal in that channel for 0.5–3.0 sec. Spindle onset was taken as the moment the RMS signal crossed the threshold and offset when it fell back below threshold. Mean spindle frequency was taken as the number of peaks during the spindle divided by spindle duration. Slow spindles were those with a mean frequency of 11–13.5 Hz, and fast spindles those with a mean frequency of 13.5–16 Hz. Spindle density, the number of spindles per minute, was calculated separately for slow and fast spindles. Calculations of slow and fast spindle density were averaged for each electrode cluster.
Statistical Analysis
The chief behavioral measure was location recall accuracy after sleep, computed separately for objects cued by the corresponding sound during sleep versus for those not cued. Recall accuracy measures were computed for each object as the distance between the object placement and the study location for that object (i.e., error in pixels). Thus, lower values for the Test-1 error and the Test-2 error indicated better memory. To control for level of initial learning, a forgetting score was computed for each participant as the mean Test-2 error minus the mean Test-1 error (a larger forgetting score indicates more forgetting).
Behavioral analyses were conducted using paired-sample t-tests, analysis of covariance (ANCOVA), analysis of variance (ANOVA), and Pearson correlations. Further analyses, some of which included measures from EEG recordings, were conducted using paired-sample t-tests and Pearson correlations. All tests were two-tailed (alpha = 0.05).
RESULTS
Recall Accuracy
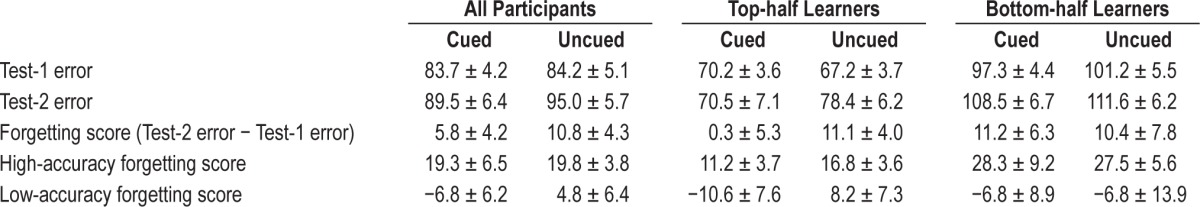
At Test-1, participants placed objects on average 84.0 ± 4.4 pixels away from corresponding study locations. This mean recall error was equivalent to 3.1 cm, or 8% of the horizontal screen length, and represents highly specific knowledge of the arbitrary locations of the 50 objects. At Test-2, participants placed objects on average 92.2 ± 5.4 pixels away from corresponding study locations, representing a small decline in recall accuracy from that in Test-1. The average amount of forgetting from Test-1 to Test-2, termed the forgetting score, was thus 8.3 pixels (reflecting the greater mean recall error at Test-2 than at Test-1; t19 = 2.74, P = 0.01). Note that for recall error values and forgetting scores, higher values imply poorer recall accuracy.
Table 1 shows mean values for Test-1 error, Test-2 error, and forgetting score separately for cued and uncued objects. There was a negligible difference between Test-1 accuracy for cued objects versus those not cued (t19 = 0.18, P = 0.80, not signifi-cant). This close match was expected, because objects were assigned to cued and uncued conditions to match the Test-1 recall error between these two conditions, although in some individuals the means were not matched due to large variability in error on the least accurate trials. Indeed, the occasionally large errors highlight the fact that object locations were not all learned to the same level. Because a central aim is to investigate the relevance of the level of initial learning for sleep reactivation, we conducted analyses both as a function of each participant's overall accuracy at Test-1 and as a function of accuracy for each individual object at Test-1.
Table 1.
Recall performance (mean errors in pixels ± SEM).
Recall Conditionalized on Each Participant's Test-1 Accuracy
Analyses focused on the cueing benefit, computed as the forgetting score for uncued objects minus the forgetting score for cued objects. Accordingly, a positive cueing benefit would imply that there was less forgetting after the nap for cued objects than for uncued objects (as found in the prior study by Rudoy et al.16). We analyzed the cueing benefit in several different ways. On one hand, when the cueing benefit was calculated by including all objects for all individuals, and not taking level of initial learning into account, the cueing benefit was nonsignificant (F1,19 = 0.70, P = 0.41). On the other hand, as shown in Figure 2, the cueing benefit was significantly influenced by each participant's Test-1 accuracy (r = 0.44, P = 0.05), such that higher accuracy at Test-1 was associated with a larger cueing benefit. In other words, retention was superior for cued objects compared to uncued objects to the extent that participants' mean initial recall accuracy was high. This result was further substantiated through another analysis in which we calculated each participant's Test-1 performance as the percent of objects remembered to the initial learning criterion (i.e., within 150 pixels of the correct location, which yielded a range from 70% to 98%). With this method, a significant correlation between Test-1 performance accuracy and cueing benefit was also demonstrated (r = 0.60, P < 0.01). A full assessment of the cueing benefit thus requires a consideration of the influence of Test-1 accuracy.
Figure 2.
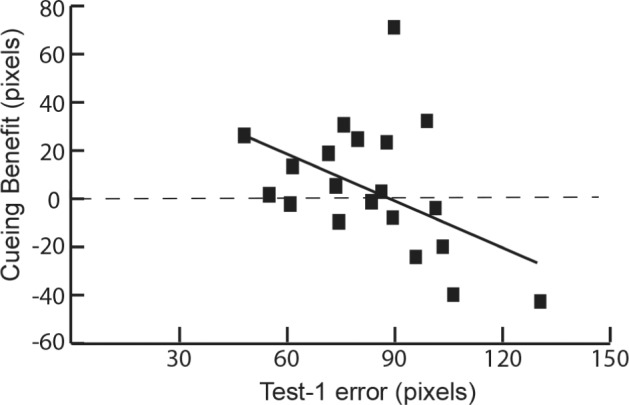
Variability of Test 1 recall error across participants was systematically related to the cueing benefit, computed as the forgetting score for cued objects minus the forgetting score for uncued objects.
To facilitate a simple demonstration of the influence of participants' Test-1 recall accuracy, we divided the sample into two groups using a median split on Test-1 recall accuracy, and then analyzed forgetting scores in each group. We refer to the group with higher accuracy as top-half learners (mean Test-1 error = 68.7 pixels) and the group with lower accuracy as bottom-half learners (mean Test-1 error = 99.3 pixels).
As shown in Figure 3, forgetting scores for top-half learners were significantly lower for cued trials than for uncued trials (t9 = 2.45, P = 0.04), as predicted. For bottom-half learners, forgetting scores for cued and uncued trials were not significantly different (t9 = 0.01, P = 0.94). The 2-way interaction of cueing condition (cued/uncued) × learner type (top-half/bottom-half) was not significant (F1,18 = 0.95, P = 0.34). A potentially relevant observation is that bottom-half learners varied widely in their performance—Test-1 error ranged from 86.6 to 130.8 pixels and the cueing benefit ranged from +71.6 to −42.6 pixels (see Figure 2).
Figure 3.
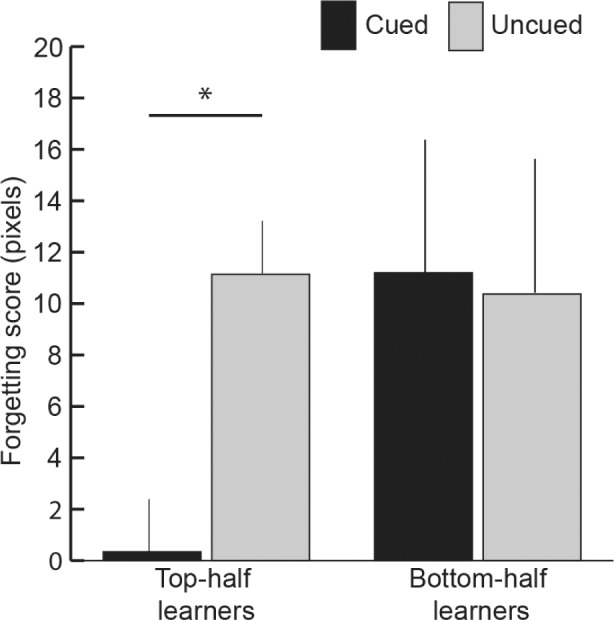
Forgetting score (Test 2 error minus Test 1 error) for cued and uncued conditions, computed separately for top-half learners and bottom-half learners (mean ± standard error of the mean, error bars adjusted for within-subject comparisons, *P < 0.05).
To take into account the effect of Test-1 accuracy on cueing benefit within the entire sample, we used a one-way repeated-measures ANCOVA between cued and uncued error with Test-1 recall accuracy as a covariate, which yielded a significant effect of cueing (F1,18 = 5.05, P = 0.04). This finding implies that the sound cues produced a significant benefit when cross-participant variability related to Test-1 accuracy was removed.
Recall Conditionalized on Test-1 Accuracy Trial-by-Trial
Given that the cueing benefit varied systematically as a function of participants' average Test-1 accuracy, we sought to determine whether individual Test-1 trial accuracy was similarly relevant. As an initial step, we combined trials from all participants and excluded outlier trials. Outlier trials amounted to 4.8% of the trials and were defined as those trials with a Test-1 error exceeding the group mean by more than two standard deviations (252 pixels). Next, cut-offs for Test-1 error values were selected to divide the remaining trials into three equal categories. The categories were: low Test-1 error (2.2–40.8 pixels), intermediate Test-1 error (41–79.8 pixels), and high Test-1 error (80.1–251.7 pixels). The cueing benefit was greatest for the high-error category, as shown in Figure 4. Supporting this impression, Test-2 error scores were submitted to a two-way repeated-measures ANCOVA, with within-subject factors cueing (cued/uncued) and Test-1 error category (low/intermediate/high), and with average Test-1 accuracy for each participant as a covariate. There was a significant interaction (F2,17 = 4.03, P = 0.03) and a significant quadratic trend (F1,17 = 7.01, P = 0.01), indicating that the cueing benefit increased from low to intermediate to high Test-1 error category. Follow-up ANCOVAs assessing the cueing effect for each category, controlling for learner Test-1 error, revealed nonsignificant differences for the low-error category (F1,18 = 0.67, P = 0.42), a marginal difference for the intermediate-error category (F1,18 = 2.83, P = 0.11), and a significant difference between cued and uncued trials for the high-error category (F1,18 = 6.59, P = 0.02). The same analysis was also computed including outlier trials such that the three equal categories were: low Test-1 error (2.2–42.3 pixels), intermediate Test-1 error (42.4–84.3 pixels), and high Test-1 error (84.6–596.8 pixels). When all items were included in the same two-way ANCOVA previously mentioned, the significant interaction (F2,17 = 11.03, P < 0.01) and a significant quadratic trend (F1,17 = 20.31, P < 0.01) remained.
Figure 4.
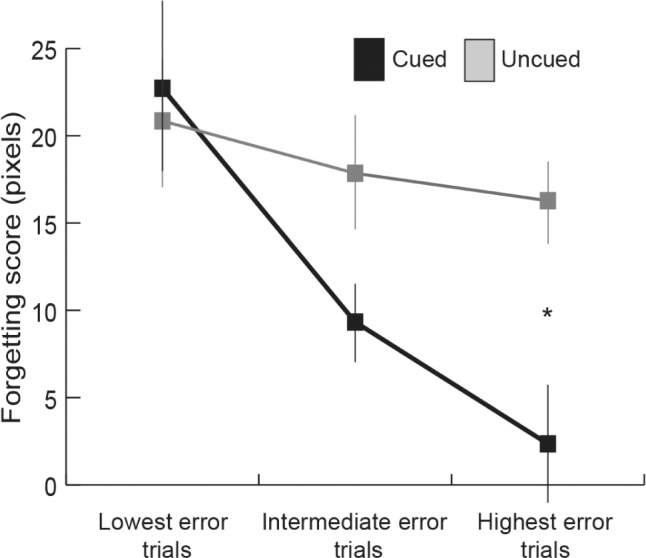
Forgetting score for cued and uncued conditions as a function of trial-by-trial Test 1 accuracy, computed for all participants. Low-error trials have the smallest Test 1 error, intermediate trials an intermediate Test 1 error, and high error trials the largest Test 1 error (mean ± standard error of the mean, error bars adjusted for within-subject comparisons, *P < 0.05).
Optimal Conditions for a Cueing Benefit in the Present Study
The cueing benefit appeared to require a certain level of learning at the individual level. Participants were grouped together as top-half learners if their average Test-1 error was below 85.2 pixels. However, this criterion was selected because it happened to be the median, and it may not optimally define the level of initial learning necessary for producing a cueing benefit. Indeed, average Test-1 accuracy was only slightly lower in many of the bottom-half learners (Figure 2). Accordingly, in an exploratory analysis a criterion of 100 pixels (average individual Test-1 error) allowed 16 participants to be included, and a significant cueing benefit was observed (forgetting score of 3.1 ± 4.6 pixels for cued and 16.0 ± 3.7 pixels for uncued; t15 = 2.30, P = 0.04).
We also asked how the conditionalized trial-by-trial results could be taken into account most effectively. When all participants were included and the high-error plus intermediate-error categories combined, there was a significant cueing benefit, even without using average Test-1 error as a covariate (t19 = 2.22, P = 0.04). Although it may generally be preferable to exclude participants on the basis of poor initial learning (e.g., 20% of the participants in the aforementioned analysis above), it is intriguing that a cueing benefit was found with all participants when trials were selected such that Test-1 recall accuracy for each trial was neither too good nor too poor.
Knowledge of Sleep Cues
At debriefing, all participants said they did not hear any sounds during the nap. Participants were then asked to guess which sounds were played, with each sound-object pair presented individually in a yes/no task. Participants indicated virtually no explicit knowledge of which sounds were presented during sleep, as they endorsed cued sounds 38.2% of the time and uncued sounds 39.2% of the time (mean values, t19 = 0.26, P = 0.80). Likewise, in an analysis restricted to participants who showed the cueing benefit, endorsement rates again differed by about 1% for cued and uncued sounds across the group, indicated no knowledge of which sounds were presented during sleep.
Relationships between Sleep Physiology and Cueing Benefit
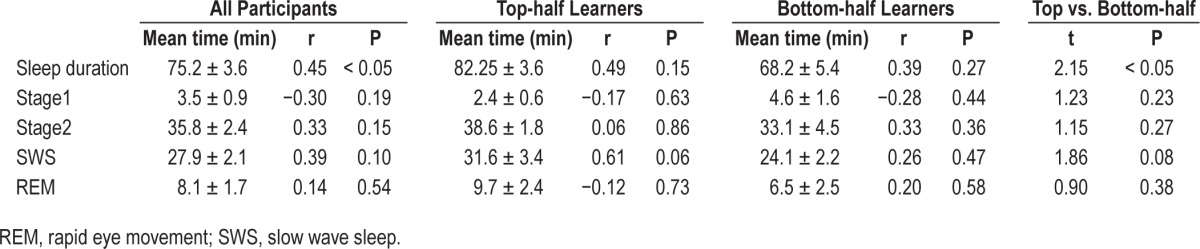
Table 2 shows sleep staging times for all participants, along with correlation coefficients between time in each sleep stage and the cueing benefit. Of all these measures, only sleep duration was associated with cueing benefit. Sleep duration was also greater for top-half learners than for bottom-half learners. Although we did not postulate a correlation between sleep duration and the cueing effect, this correlation fits with the idea that the cueing benefit depends on memory processing during sleep. However, it does not provide specific information about which aspect of sleep might be important.
Table 2.
Sleep-stage data: correlation between time in each stage and cueing benefit, with t-tests between top-half and bottom-half learners for time in each stage.
Because delta power and spindle density during SWS have been found to correlate with memory performance after sleep,8,9,30 we analyzed these measures in relation to the cueing benefit in cross-subject correlational analyses. Delta was computed as the percentage of power from 0.6–4 Hz when compared with other frequencies, and averaged over all SWS periods. We computed values for all electrode clusters (averaging values for electrodes within each cluster). Because the frontal electrode cluster yielded the largest values for relative delta power, correlational analyses were computed for that cluster. In contrast, spindle density values were greatest for the parietal cluster, so that cluster was used for spindle correlational analyses. Other clusters were not examined, based on the assumption that weaker measures of the same brain activity were shown at other scalp locations.
The cueing benefit was correlated with relative delta power (r = 0.48, P = 0.03). Similar correlation results were also found when analyses were restricted to either the SWS interval before sound presentation began, the sound presentation interval, or the SWS interval following sound presentation. We also found that top-half learners had greater relative delta power than bottom-half learners (t18 = 2.54, P = 0.02). There were no significant correlations between the cueing benefit and relative theta, alpha, or sigma, measured at the electrode clusters where they were maximal (all P > 0.20).
The cueing benefit was also correlated with fast spindle density, but in a way that varied depending on time interval. Greater fast spindle density during the sound presentation interval was associated with a greater cueing effect (r = 0.45, P < 0.05; Figure 5). In contrast, fast spindle density in the period before or the period after sound presentation bore no significant association with the cueing effect (r = 0.13, P = 0.58, and r = 0.24, P = 0.31, respectively). Top-half learners and bottom-half learners did not differ significantly on fast spindles during the sound presentation interval (t18 = 0.96, P = 0.35). There was no significant correlation between slow spindle density, measured at the frontopolar cluster before, during, or after sounds, and the cueing benefit (all P > 0.18).
Figure 5.
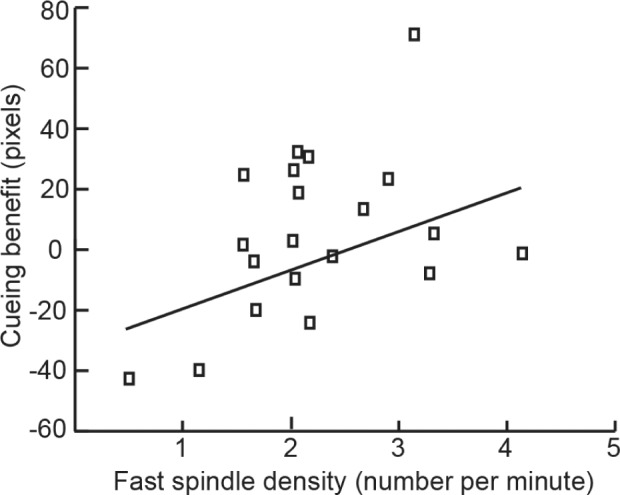
Variability of sleep physiology across subjects was systematically related to the cueing benefit. Greater fast spindle density, averaged over parietal locations in the interval while cues were presented, correlated with a larger cueing benefit.
DISCUSSION
Substantiation of TMR
In the current study, we revisited the discovery made by Rudoy and colleagues16 that cueing learned objects during sleep with a related sound reactivates and strengthens corresponding spatial memories. Our findings confirmed previous findings that TMR can enhance sleep-dependent consolidation,14 while providing additional information about the boundary conditions for this phenomenon. In short, TMR produced benefits provided that spatial recall measured prior to sleep was neither too strong nor too weak.
Spatial Recall and Timing Considerations
The spatial memory test that we used provided fine-grained information on location recall for each of 50 objects. If a participant placed an object close to the original location, we infer that memory storage for that individual object was accurate. As time passes, location recall gradually becomes less accurate.31 An important advantage of this test is that it can be used to monitor forgetting with high resolution.
Pre-sleep errors in the current study were larger than those from the study by Rudoy et al.16 (where the mean Test-1 error was 63.9 pixels). This divergence is understandable, given that testing procedures differed; the test was given directly after learning in the study by Rudoy et al. and after a 45-min delay in the current study. Mean recall performance on the initial test was highly accurate in nearly all participants in the study by Rudoy et al., thus limiting the chances of identifying poor learners. With a test immediately after learning, much of the spatial information was recently activated, and some type of short-term memory may have also supported recall performance. We designed the current study specifically to include a significant delay before the pre-sleep memory measure was obtained. The current test thus provided a more trustworthy assessment of memory storage prior to sleep.
Optimal Conditions for TMR
Participants with reasonably high Test-1 accuracy showed the predicted cueing benefit. For subjects with relatively poor Test-1 recall, cueing results were more variable. Yet, the cueing benefit was significant when results from the best 80% of the sample were included, as well as when 100% of the sample was included with Test-1 accuracy as a covariant. If cues promote reactivation, it makes sense that memory cannot be improved using cues that reactivate memories for grossly incorrect locations. Cueing might even make such memories worse, as the wrong location is stamped in more strongly. Thus, it might be sensible to terminate a TMR experiment if a certain level of learning is not achieved prior to sleep, or to prolong training until a high-enough level is achieved. Yet, we also found that individual objects recalled with extremely high accuracy at Test-1 yielded no cueing benefit. There are several ways in which this combination of results is consistent with the literature on encoding and sleep consolidation.
On one hand, prior studies found that the best learners gained a larger benefit from sleep.5,32,33 For example, a nap produced a benefit on three declarative memory tasks only for the top-half performers in one study.5 Preferential sleep-related benefits for top-half learners could result from the influence of factors such as motivation, reward, emotional salience, and relevance.22–24,34,35 Additionally, reactivation studies using a variant of the current task during waking produced informative results based on retrieval errors measured at the time of reactivation, as shown by Bridge and Paller31 and Bridge and Voss.36 When incorrect locations were retrieved for objects at Test-1 (as was often the case for bottom-half learners here), cues for those objects could reactivate incorrect locations, leading to poor recall at a later time. If learning is extremely poor, cue presentation might fail to produce memory retrieval at all. In general, when memory for object locations is erratic, TMR may be beneficial only for objects with strong sound-object-location associations. By this account, in participants who exhibited poor Test-1 recall, there are good reasons for why cueing retrieval during sleep might not benefit subsequent recall. That is, a key requirement for successful TMR during sleep is adequate learning prior to sleep.
On the other hand, other studies showed that well-learned objects did not benefit from sleep-related consolidation.37,38 For example, Drosopoulos et al.37 taught participants word pairs with intense encoding or weak encoding, and subsequently observed a sleep-related benefit only for weakly encoded word-pairs. The investigators suggested that sleep does not influence memories that are already strong. A similar result was found in a study of sleep-dependent learning of a motor skill; the largest sleep-dependent gains were for objects with lower performance before sleep.38 Memory enhancement from sleep likely follows an inverted U-shaped function in relationship to pre-sleep memory strength, as proposed by Stickgold, where very weak and very strong memories do not tend to benefit from sleep.3,39 The current results likewise suggest that TMR benefits are not found for either the most accurate spatial associations or for individuals with overall weak learning.
Perspectives based on an extension of the Complementary Learning Systems model provide a possible explanation for why consolidation of weaker memories might be preferentially favored during sleep. In considering possibilities for avoiding catastrophic interference, Norman and colleagues40 argued that facilitating weak memories during sleep would be preferable to facilitating strong memories. To the extent that strong memories are reinforced, a small number of memories could become too strong and then inhibit all other memories.
The idea of endogenous reactivation can be used to address why strong associations did not benefit from TMR. Perhaps the strongest memories are likely to be reactivated during sleep whether or not corresponding cues are presented. If so, then memories that were strong at Test-1 might not benefit from TMR. Additional reactivation might be redundant for those items. In support of this idea, Oudiette and colleagues found that high-value memories were facilitated by sleep more than low-value memories.22 In addition, the sleep-related benefit for high-value memories remained the same when cues for low-value memories were presented during sleep, suggesting that high-value memories were reactivated spontaneously. Furthermore, the benefits of TMR may be most apparent for weaker memories that have more room for gains associated with reactivation.
In the current study, the magnitude of the cuing benefit correlated with sleep duration, along with measures of sleep physiology in the form of relative delta power over the course of the nap and fast spindle density in the interval while cues were played. These results lend additional support to the hypothesis that spindle activity is associated with enhanced memory during sleep.32,33 In rodents, neuronal bursts in deep cortical layers are believed to trigger synaptic plasticity correlated with spindle activity detected from the scalp.41 The relevant spindle activity here may arise from cortical regions involved in the spatial and motor associations relevant for learning in the current task. Scalp recordings may be sensitive only to a subset of that activity. The memory reactivation associated with spindle activity was presumably facilitated by low-frequency oscillations prevalent during SWS.2
TMR methods provide novel perspectives on sleep consolidation, but there are limitations. One question is whether external cues influence later memory through the same or different mechanisms from sleep consolidation that occurs without external cues. Currently, this question is difficult to answer with confidence, but as additional neural evidence accrues we can ask whether the same neural mechanisms seem to be operative in the two cases. There will thus be a way to compare the neural basis of TMR effects to the typical neural mechanisms of memory change during sleep. Although the advantage of memory reactivation has been found in both sleep and waking,1,22,31,36 the goal of the current paper was to understand the best conditions for TMR during sleep. There is still more to learn to be able to define optimal conditions for TMR benefits. Future studies may usefully explore other factors such as test timing, cognitive load during learning, reward value of remembering, and strategic factors.
CONCLUSIONS
Our results confirm previous research that showed that external reactivation of learned information during sleep can strengthen memory storage.15–20,22,25,42 Segregating participants or trials as a function of recall achieved prior to sleep further showed that cueing benefits depend on learning status in relation to either extreme. When overall object-location learning is too weak, sleep cues are unlikely to reactivate valid information, so no memory benefit is accrued. When individual-item object-location learning is too strong, a benefit from TMR is also unlikely, perhaps because sleep reactivation for well-learned associations is likely whether or not cues are presented, or because there is little room for further improvement. One implication of these findings is that paradigms that produce learning that is too weak or individual memories that are too strong can obscure behavioral effects of TMR. Nonetheless, when the extremes are avoided, TMR can be used to preferentially reactivate specific information learned prior to sleep.
TMR is a noninvasive tool that can enhance investigations into the behavioral and physiological aspects of memory consolidation during sleep. A long-standing research strategy in this area has been to compare memory performance following a period of sleep versus a period of wake (or sleep deprivation), but confounding variables such as interference and fatigue limit the conclusions that can be drawn from such results. These problems can be avoided by using TMR to selectively influence memory performance, taking advantage of powerful within-subject comparisons.
TMR has thus provided direct evidence for the relevance of memory processing during sleep. We conclude that sleep is beneficial in the selective consolidation of memories, that this consolidation is associated with the reactivation of previously learned information, and that this reactivation can be triggered by external cues.
DISCLOSURE STATEMENT
This was not an industry supported study. This material is based upon work supported by the National Science Foundation under grant number BCS-1025697. The authors have indicated no financial conflicts of interest. All work was performed at the Department of Psychology at Northwestern University.
ACKNOWLEDGMENTS
The authors thank S. M. Florczak, D. J. Bridge, J. L. Voss, G. Larson, G. Santostasi, and J. A. Gottfried for helpful discussion.
REFERENCES
- 1.Paller KA. Memory consolidation: systems. In: Squire LR, editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009. pp. 741–9. [Google Scholar]
- 2.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 3.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 5.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, Fishbein W. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiol Learn Mem. 2006;86:241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Takashima A, Petersson KM, Rutters F, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci U S A. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;101:13963–8. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–3. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 10.Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–92. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sirota A, Buzsáki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3:245–59. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–18. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–9. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 14.Oudiette D, Paller KA. Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci. 2013;17:142–9. doi: 10.1016/j.tics.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- 16.Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. doi: 10.1126/science.1179013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012;15:1439–44. doi: 10.1038/nn.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antony JW, Gobel EW, O'Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012;15:1114–6. doi: 10.1038/nn.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat Neurosci. 2013;16:1553–5. doi: 10.1038/nn.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuentemilla L, Miró J, Ripollés P, Vilà-Balló A, Juncadella M, Castañer S, et al. Hippocampus-Dependent Strengthening of Targeted Memories via Reactivation during Sleep in Humans. Curr Biol. 2013;23:1769–75. doi: 10.1016/j.cub.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Van Dongen EV, Thielen J-W, Takashima A, Barth M, Fernández G. Sleep supports selective retention of associative memories based on relevance for future utilization. PloS One. 2012;7:e43426. doi: 10.1371/journal.pone.0043426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oudiette D, Antony JW, Creery JD, Paller KA. The role of memory reactivation during wakefulness and sleep in determining which memories endure. J Neurosci. 2013;33:6672–8. doi: 10.1523/JNEUROSCI.5497-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Mölle M, Born J. Sleep selectively enhances memory expected to be of future relevance. J Neurosci. 2011;31:1563–9. doi: 10.1523/JNEUROSCI.3575-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer S, Born J. Anticipated reward enhances offline learning during sleep. J Exp Psychol Learn Mem Cogn. 2009;35:1586. doi: 10.1037/a0017256. [DOI] [PubMed] [Google Scholar]
- 25.Van Dongen EV, Takashima A, Barth M, Zapp J, Schad LR, Paller KA, et al. Memory stabilization with targeted reactivation during human slow-wave sleep. Proc Natl Acad Sci. 2012;109:10575–80. doi: 10.1073/pnas.1201072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. Quantification of sleepiness: a new approach. Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Akerstedt T, Hume KEN, Minors D, Waterhouse JIM. The subjective meaning of good sleep, an intraindividual approach using the Karolinska Sleep Diary. Percept Mot Skills. 1994;79:287–96. doi: 10.2466/pms.1994.79.1.287. [DOI] [PubMed] [Google Scholar]
- 29.Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011;34:1411–21. doi: 10.5665/SLEEP.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schabus M, Gruber G, Parapatics S, Sauter C, Klosch G, Anderer P, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27:1479–85. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 31.Bridge DJ, Paller KA. Neural correlates of reactivation and retrieval-induced distortion. J Neurosci. 2012;32:12144–51. doi: 10.1523/JNEUROSCI.1378-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, et al. Interindividual sleep spindle differences and their relation to learning-related enhancements. Brain Res. 2008;1191:127–35. doi: 10.1016/j.brainres.2007.10.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schabus M, Hödlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23:1738–46. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 34.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17:891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 35.Sterpenich V, Albouy G, Darsaud A, et al. Sleep promotes the neural reorganization of remote emotional memory. J Neurosci. 2009;29:5143–52. doi: 10.1523/JNEUROSCI.0561-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bridge DJ, Voss JL. Hippocampal binding of novel information with dominant memory traces can support both memory stability and change. J Neurosci. 2014;34:2203–13. doi: 10.1523/JNEUROSCI.3819-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drosopoulos S, Schulze C, Fischer S, Born J. Sleep's function in the spontaneous recovery and consolidation of memories. J Exp Psychol Gen. 2007;136:169. doi: 10.1037/0096-3445.136.2.169. [DOI] [PubMed] [Google Scholar]
- 38.Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–13. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stickgold R. How do I remember? Let me count the ways. Sleep Med Rev. 2009;13:305. doi: 10.1016/j.smrv.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norman KA, Newman EL, Perotte AJ. Methods for reducing interference in the Complementary Learning Systems model: oscillating inhibition and autonomous memory rehearsal. Neural Netw. 2005;18:1212–28. doi: 10.1016/j.neunet.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Sirota A, Csicsvari J, Buhl D, Buzsáki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci. 2003;100:2065–9. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diekelmann S, Büchel C, Born J, Rasch B. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci. 2011;14:381–6. doi: 10.1038/nn.2744. [DOI] [PubMed] [Google Scholar]


