Abstract
Study Objectives:
A link between sleep loss and increased risk for the development of diabetes is now well recognized. The current study investigates whether sleep extension under real-life conditions is a feasible intervention with a beneficial impact on glucose metabolism in healthy adults who are chronically sleep restricted.
Design:
Intervention study.
Participants:
Sixteen healthy non-obese volunteers (25 [23, 27.8] years old, 3 men).
Intervention:
Two weeks of habitual time in bed followed by 6 weeks during which participants were instructed to increase their time in bed by one hour per day.
Measurements and Results:
Continuous actigraphy monitoring and daily sleep logs during the entire study. Glucose and insulin were assayed on a single morning blood sample at the end of habitual time in bed and at the end of sleep extension. Home polysomnography was performed during one weekday of habitual time in bed and after 40 days of sleep extension. Sleep time during weekdays increased (mean actigraphic data: +44 ± 34 minutes, P < 0.0001; polysomnographic data: +49 ± 68 minutes, P = 0.014), without any significant change during weekends. Changes from habitual time in bed to the end of the intervention in total sleep time correlated with changes in glucose (r = +0.53, P = 0.041) and insulin levels (r = −0.60, P = 0.025), as well as with indices of insulin sensitivity (r = +0.76, P = 0.002).
Conclusions:
In healthy adults who are chronically sleep restricted, a simple low cost intervention such as sleep extension is feasible and is associated with improvements in fasting insulin sensitivity.
Citation:
Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. SLEEP 2015;38(5):707–715.
Keywords: time in bed, sleep loss, sleep extension, type 2 diabetes, fasting glucose, fasting insulin, insulin resistance, insulin sensitivity, QUICKI
INTRODUCTION
For the past few decades, self-reported sleep duration has decreased markedly, especially during workdays.1,2 This decrease in sleep duration is paralleled by a higher prevalence of diagnosed diabetes, as reported by the Centers for Disease Control and Prevention (http://www.cdc.gov/). The International Diabetes Federation recently presented alarming data indicating that the number of people with diabetes is estimated at 382 million worldwide and that this number will reach 592 million in 2035.3 Type 2 diabetes represents the most common form of diabetes, leading to a vast number of comorbidities, such as cardiovascular disease, retinopathy, kidney failure, and poor limb circulation.4 In addition, healthcare costs represent a huge burden that result in a true public health concern.5
Education campaigns to prevent and manage type 2 diabetes recommend weight loss through nutrition hygiene and increased physical activity.6 However, accumulating evidence strongly suggests that habitual sleep curtailment and disturbed sleep are additional factors contributing to the development of type 2 diabetes. Several well-controlled laboratory studies designed to address the metabolic and hormonal consequences of recurrent partial sleep restriction have demonstrated that insulin sensitivity, assessed by a clamp procedure or by intravenous glucose tolerance testing, decreases in a state of sleep debt as compared to a fully rested condition,7–13 although the findings are not uniform.14,15 In a laboratory study that obtained one single fasting blood sample, five nights of sleep restriction to four hours lead to a marked increase in insulin levels, without significant change in glucose concentration, resulting in an elevated insulin-to-glucose ratio, a proposed marker of insulin resistance.16 Many epidemiological studies are consistent with these laboratory results.17 A meta-analysis of prospective studies showed that self-reported short sleep (< 5–6 h) is associated with a higher risk of developing type 2 diabetes.18 Whether interventions to extend the time spent asleep in short sleepers are feasible under real-life conditions and whether they can reduce diabetes risk remain open questions.
Studies investigating the impact of sleep extension in healthy individuals on cognitive, biological, and neurophysiological functions19–34 highlight the potential effects of sleep extension to recover from deleterious consequences of chronic sleep curtailment. Conversely, “banking sleep” (i.e., sleep extension before sleep restriction) for one week has been shown to improve recovery on alertness and on a math test after sleep restriction.26,35 Most of these findings are, however, based on protocols that include relatively short sleep extension periods (one to five nights19,23,25,28,30,31 or one week22,26,29,32,35), and/or often using a between-subject design.20,22,23,26,30,31,33–35 Only four studies recruited constrained short sleepers,28,29,32,34 and only five studies included electroencephalographic (EEG) data.20–22,26,29 In three of these latter studies, sleep recordings were conducted in a sleep laboratory,20,22,26 which involves a change in the usual sleeping environment and may lead to sleep disturbances. Lastly, none of these studies included measures of glucose metabolism.
The current study therefore investigates whether increasing time in bed in adults with habitual sleep restriction over a relatively long period of time (6 weeks) could have metabolic benefits that are commensurate with the increase in sleep duration.
METHODS
Participants and Protocol
Each participant signed a consent form prior to participating in the current study, approved by the Ethical Committee of ULB University - Erasme Hospital (Brussels, Belgium).
Healthy non-obese volunteers aged 20–50 years were recruited using advertisements inviting participation in a sleep extension study. Potential participants who contacted the investigator were asked to complete a short questionnaire on demographics, occupation, and sleep duration during both weekdays and weekends. Individuals who were night workers or reported sleeping > 7 h/night during the week or reported no difference between weekday and weekend sleep durations were excluded. Eligible volunteers had a face-to-face interview with the investigator and were asked whether they believed that they could extend their sleep time. Those who gave a negative response were excluded. The remaining participants were consented and completed validated questionnaires listed below.
Additional exclusion criteria included having a body mass index (BMI) > 30 kg/m2; having any known sleep disorder or poor sleep quality based on screening interview, Berlin questionnaire,36 or Pittsburgh Sleep Quality Inventory37; and being an extreme chronotype (either morning or evening types) based on a circadian typology questionnaire (Morningness-Eveningness Questionnaire38).
Participants wore a wrist activity monitor (Actiwatch-2, Medys, Kappellen, Belgium) and completed a daily sleep log39 during the entire time of the study (8 weeks), including 2 weeks while following their habitual time in bed and 6 weeks during the intervention of sleep extension. The intervention consisted of increasing time in bed by 1 h/night while following the instructions for sleep hygiene adapted from the recommendations of the American Academy of Sleep Medicine. Participants received information on the deleterious neurobehavioral and biological effects of insufficient sleep.
Sleep extension was scheduled individually, in accordance with each participant's lifestyle (habitual sleep/wake cycle, work schedules, and hobbies). The only advice concerning physical activity consisted of emphasizing to physically active participants that exercising < 2 h before bedtime could delay sleep onset. Participants were provided with a phone number and an email address to contact the investigator whenever they had a concern or a question regarding the study. In addition, a meeting was scheduled every 2 weeks to discuss the difficul-ties the participants might have encountered and how the new schedule could be improved. These meetings also served to encourage compliance with the protocol.
To evaluate sleep architecture, sleep was polygraphically recorded during a weekday at the participant's home toward the end of the 2 study periods, i.e., after 12 days of habitual time in bed and after 40 days of sleep extension.
At the end of the 2 weeks of habitual time in bed and at the end of the 6 weeks of sleep extension, a fasting blood sample was obtained at Erasme Hospital, where glucose and insulin levels were assayed. On the day that preceded the first fasting blood sample (under habitual time in bed), the participants were requested to complete a detailed questionnaire reporting on food intake, including types and quantities. They were asked to follow the exact same diet on the day preceding the second session of blood sampling, i.e., at the end of the sleep extension intervention.
Sleep Recording and Analysis
Participants pressed an event marker button on their wrist activity monitor to mark their bedtimes and their wake times without affecting the data collection. They also completed daily sleep logs that included timings of lights off, sleep onset, sleep offset, and sleep duration. The combination of these 2 collection methods provided accurate determinations of bedtimes and wake times for analysis of actigraphic recordings using the Respironics Actiware 5 software (Medys, Kappellen, Belgium). Sleep times and sleep efficiency were computed from these analyses.
Polysomnographic (PSG) recordings included EEG (2 central, 1 frontal and 1 occipital lead), electrooculography (EOG), and electromyography (EMG). Abdominal and thoracic belts, nasal pressure transducer, oronasal thermocouple, and sensor for oxygen saturation by finger pulse oximetry were placed to verify that respiratory events were minor (apnea-hypopnea index < 5/h). Each 30-s epoch of recording was scored as stage Wake, N1 (NREM stage 1), N2, N3, or REM sleep following standard criteria.40 Sleep onset corresponded to the time of the first 30-s epoch scored either N2, SWS, or REM. Final awakening was defined as the time corresponding to the last 30-s epoch scored N2, SWS, or REM. Sleep period time was defined as the time interval separating sleep onset from final awakening. Total sleep time was calculated as sleep period time minus the duration of intra-sleep awakenings, and sleep maintenance as total sleep time, expressed in percent of the sleep period time.
Assays
Glucose levels were assayed using an automate “Modular P800” (Roche Diagnostics). The intra-assay coefficient of variation was < 1.1%. Insulin levels were assayed using an automate “Modular E170” (Roche Diagnostics). The limit of sensitivity was 0.2 μU/mL and the intra-assay coefficient of variation was < 1.5%.
Statistical Analysis
Results are expressed as mean ± SD when normally distributed and as median (Q1, Q3) otherwise. Data were log-transformed where appropriate to perform the statistics.
Overnight sleep variables obtained during the habitual time in bed period were compared to those obtained during the sleep extension intervention using a repeated-measure analysis of variance (ANOVA).
The primary metabolic outcome measures were blood levels of glucose and insulin. Additionally, 3 validated indices combining the fasting glucose and insulin levels were calculated for each participant: the homeostatic model assessment (HOMA)41,42 and the insulin-to-glucose ratio,16,43 which are both markers of fasting insulin resistance, and the Quantitative Insulin Sensitivity Check Index (QUICKI),44 which is a marker of insulin sensitivity. Glucose, insulin, HOMA, insulin-to-glucose ratio, and QUICKI after sleep extension were expressed as percent changes from the habitual time in bed condition. Sleep variables at the end of the sleep extension were also expressed as percent change from the habitual time in bed condition. Correlations were calculated using Pearson coefficients of correlation (r) when data followed a normal distribution and Spearman coefficients of correlation (rSP) otherwise.
All statistical calculations were performed using JMP software (SAS Institute Inc., Cary, NC).
RESULTS
Participants
Seventeen healthy adults were included in the study. One dropped out for personal reasons. The remaining 16 participants (3 men, median [Q1, Q3]: 25 [23, 27.8] years old, 20.8 [19.2, 23.9] kg/m2) completed the entire study. Thirteen of 16 participants were of European descent, 2 were of North African ethnicity, and one was from Central Africa. Six participants were students with classes during the daytime, and 10 had daytime employment; all individuals were thus under constraining daily schedules. Participants' scores on the Pittsburgh Sleep Quality Inventory averaged 4.8 ± 2.5, indicating that they exhibited good sleep quality except for short sleep duration. Fourteen of the 16 volunteers were neutral chronotypes (neither morning nor evening types), and 2 were moderate morning chronotypes, based on the circadian typology questionnaire (Morningness-Eveningness Questionnaire). Self-reported time in bed during weekly nights averaged 6.5 [5.8, 6.8] h, and self-reported time in bed during weekend was increased in comparison to weekly nights (7.7 ± 1.1 h, P = 0.0002), indicating that our participants were sleep restricted during the week and tried to recover during weekends.
Sleep Variables
Sleep logs
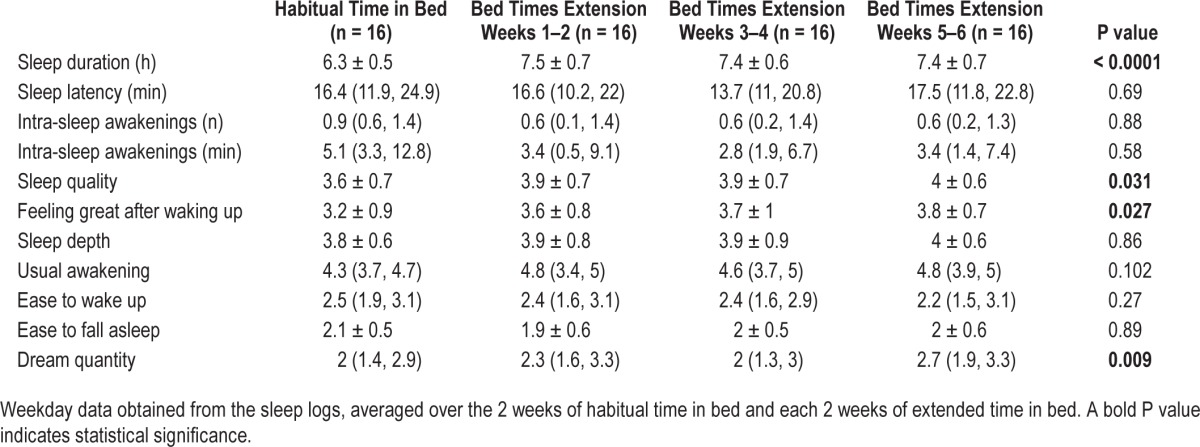
Table 1 reports the weekday data derived from sleep logs and averaged over the 2 weeks of habitual time in bed and each 2-week period during the intervention. Self-reported sleep duration increased by about 1 h during the intervention of extended time in bed (P < 0.0001) without increase in either sleep latency (P = 0.69) or intra-sleep awakenings (P = 0.88 for number; P = 0.58 for duration). Participants reported a better sleep quality (P = 0.031), feeling better after waking up (P = 0.027), and dreaming more (P = 0.009) after the intervention. No significant difference was reported for sleep depth (P = 0.86), ease of waking up (P = 0.27), or ease of falling asleep (P = 0.89).
Table 1.
Self-reported sleep variables during weekdays.
Wrist Activity Monitoring
Habitual bedtimes and wake times averaged 0h 37 ± 57 min and 7h 05 ± 52 min, respectively, whereas extended sleep bedtimes and wake times averaged 23h 49 ± 40 min and 7h 00 ± 47 min, respectively, based on actigraphic data averaged over the 2 weeks of habitual bed times and over the last 2 weeks of the intervention.
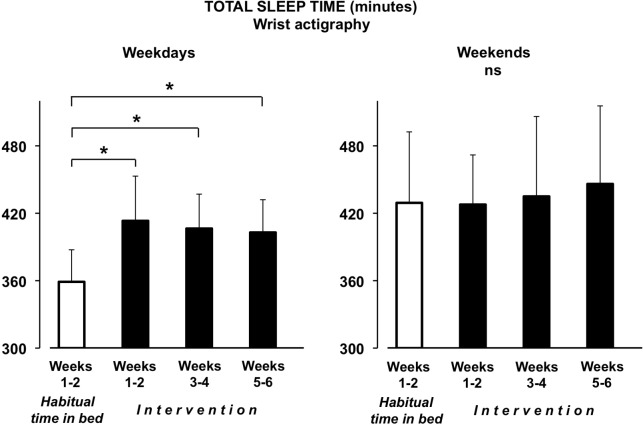
Figure 1 illustrates actigraphic data for the total sleep time during weekdays (left panel) and during weekends (right panel), averaged over the 2-week habitual time in bed and over the three 2-week periods during the intervention. Actigraphic data are missing for 2 participants in the block weeks 1–2 and for 1 participant in the block weeks 3–4 of the intervention.
Figure 1.
Total sleep time from actigraphic data. Minutes of total sleep time (+SD) derived from actigraphic data, averaged over the 2 weeks of habitual time in bed and by 3 blocks of 2 weeks during the intervention period. Data are presented for weekdays (left) and weekends (right). *P < 0.0001.
During the first 2 weeks of the pre-intervention period (habitual time in bed), participants slept about 6 h during week-days (5h 59 min ± 29 min) with an increase by more than one hour during weekends (7h 09 min ± 63 min, P = 0.0006). The intervention of sleep extension was largely successful, as a clear increase in total sleep time during weekdays was observed throughout the 6 weeks of the intervention (P < 0.0001). Indeed, total sleep time increased by 54 ± 33 minutes over the 2 first weeks of the intervention, by 48 ± 31 minutes over the 2 middle weeks of the intervention, and by 44 ± 34 minutes over the 2 last weeks of the intervention (P ≤ 0.0001 for all comparisons to habitual time in bed and P > 0.25 for the comparisons between any 2-week periods of the intervention) without any significant change in sleep efficiency (P > 0.35). A large inter-individual variability emerged as increased sleep time during weekdays from habitual time in bed to the three 2-week periods of the intervention ranged from 13 to 119 minutes, −4 to 118 minutes, and −15 to +106 minutes, respectively. Total sleep time and sleep efficiency during weekends remained unchanged throughout the entire protocol (P = 0.36 and P = 0.30, respectively).
Polysomnography
PSG data are missing for one participant due to technical failure. The following analyses therefore include 15 volunteers.
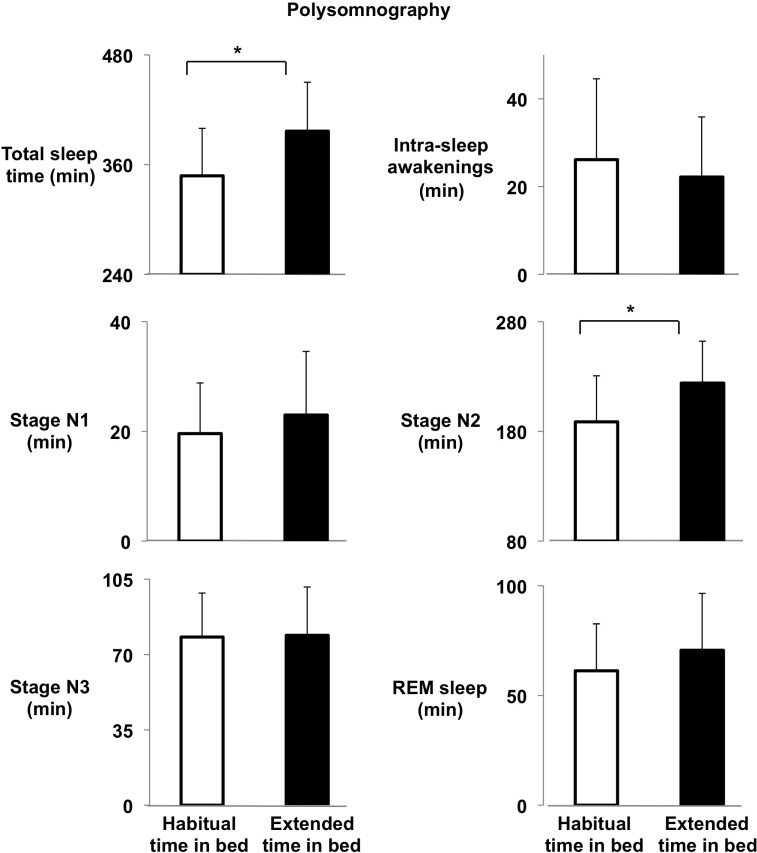
Sleep variables obtained via polysomnography are shown in Figure 2. Consistent with actigraphic data, total sleep time was increased by 49 ± 68 min (from 5h 48 ± 52 min to 6h 37 ± 54 min, P = 0.014) during the sleep extension intervention. The increase in total sleep time was essentially due to an increase in the duration of stage N2 (+35 ± 54 min, from 178 [161, 206] min to 224 [199, 260] min, P = 0.021) without a significant change in durations of stage N1 (+3 ± 11 min, from 20 ± 9 min to 23 ± 12 min, P = 0.26), slow wave sleep (+1 ± 19 min, from 78 ± 20 min to 79 ± 22 min, P = 0.87) or REM sleep (+9 ± 21 min, from 61 ± 21 min to 71 ± 26 min, P = 0.11). Importantly, the duration of the intra-sleep awakenings did not increase after the sleep extension (−4 ± 14 min, from 21 [12, 37] min to 21 [9, 36] min, P = 0.23). Despite extended time in bed, sleep maintenance tended to increase after the intervention (94.9% [90.7, 96.9] vs. 95.3% [92.4, 98.2], P = 0.098). A very large inter-individual variability in the increase of total sleep time after the intervention was observed compared to habitual time in bed, ranging from −86 to +142 minutes.
Figure 2.
Sleep variables from polysomnographic data. Mean (+SD) total sleep time, intra-sleep awakenings, stage N1, stage N2, stage N3, and REM sleep (in minutes) obtained during one unattended home polysomnography towards the end of the habitual time in bed condition, after 12 days of habitual time in bed (white bars), and one toward the end of the intervention period, after 40 days of sleep extension (black bars). *P < 0.04.
Correlation analyses revealed that percent changes from pre- to post-intervention in total sleep time were associated with the changes in stage N1 (r = +0.61, P = 0.016), stage N2 (r = +0.82, P = 0.0002), and REM sleep (rSp = +0.55, P = 0.035), but not with those of stage 3 (P = 0.37), indicating that the participants who were able to augment their sleep time increased the duration of stages N1, N2, and REM sleep.
Correlations between Actigraphic and Polysomnographic Data
Total sleep durations as assessed via PSG recordings were compared to sleep durations derived from actigraphic recordings obtained during the same nights. This analysis revealed a strong association between the 2 measurements on the first session, i.e., pre-intervention (n = 13, r = +0.95, P < 0.0001), and on the second session, after the intervention (n = 14, r = +0.82, P = 0.0003). Changes in sleep time from habitual time in bed to extended time in bed were also highly correlated between PSG-derived and actigraphic-derived measurements (n = 12, r = +0.85, P = 0.0004).
Weight Change
Body weight measurements were obtained at the end of both habitual bed times and the intervention of sleep extension. There were no significant changes in weight between pre (60 ± 9.6 kg) and post intervention (60.1 ± 9.7 kg, P = 0.81).
Fasting Glucose and Insulin Levels
Fasting insulin level after intervention is missing for one participant. No significant difference was detected between pre- and post-intervention in fasting glucose and insulin levels.
To examine the relationship between the increase in total sleep time and the blood levels of glucose and insulin, correlations between the percent changes in sleep and metabolic measures were calculated, using the data from 15 participants when actigraphy data were considered and from 14 participants when PSG data were considered.
Correlation analyses between changes in levels of glucose and insulin and changes in actigraphic measures obtained on the day of PSG revealed significant associations between changes in sleep time and changes in fasting glucose (r = +0.65, P = 0.017) and insulin levels (r = −0.57, P = 0.053). There was no other significant association between glucose-related measures and actigraphic sleep data.
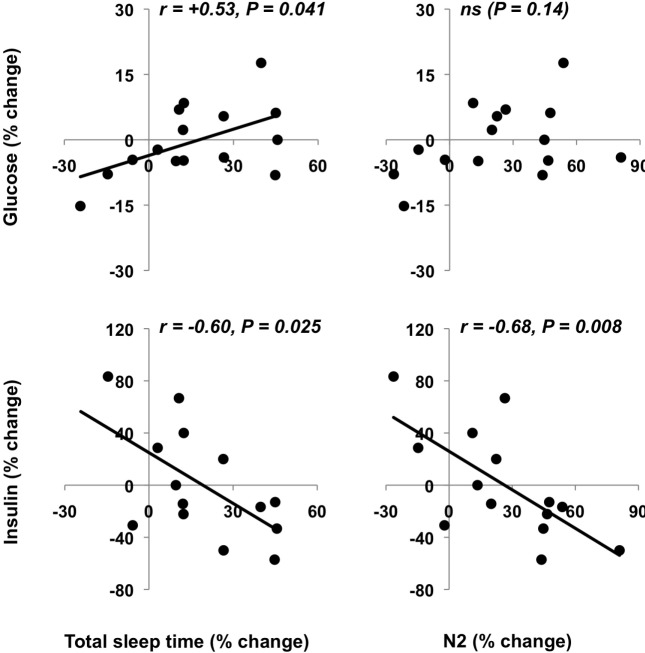
Percent changes from pre- to post-intervention in PSG total sleep time were positively associated with relative changes in fasting glucose levels (P = 0.041 Figure 3A, left upper panel) indicating that participants who were able to increase their sleep time the most had the highest increases in fasting glucose levels. However, sleep extension was beneficial in reducing fasting insulin levels as the changes in total sleep time correlated negatively with the changes in fasting insulin levels (Figure 3A, lower left panel, P = 0.025). As reflected by the different scales of the glucose and insulin changes in Figure 3A, the decreases in insulin were considerably larger than the increases in glucose.
Figure 3A.
Percent changes in sleep variables and in fasting glucose and insulin. Associations between the percent changes between pre- and post-intervention in total sleep time and stage N2, and the percent changes between pre- and post-intervention in fasting glucose and insulin.
Changes in stage N2 also correlated negatively with changes in fasting insulin levels (Figure 3A, lower right panel, P = 0.008).
Indices of Insulin Sensitivity
Significant associations were observed between changes in actigraphic sleep time and changes in insulin-to-glucose ratio (r = −0.66, P = 0.019) and QUICKI (r = +0.57, P = 0.053).
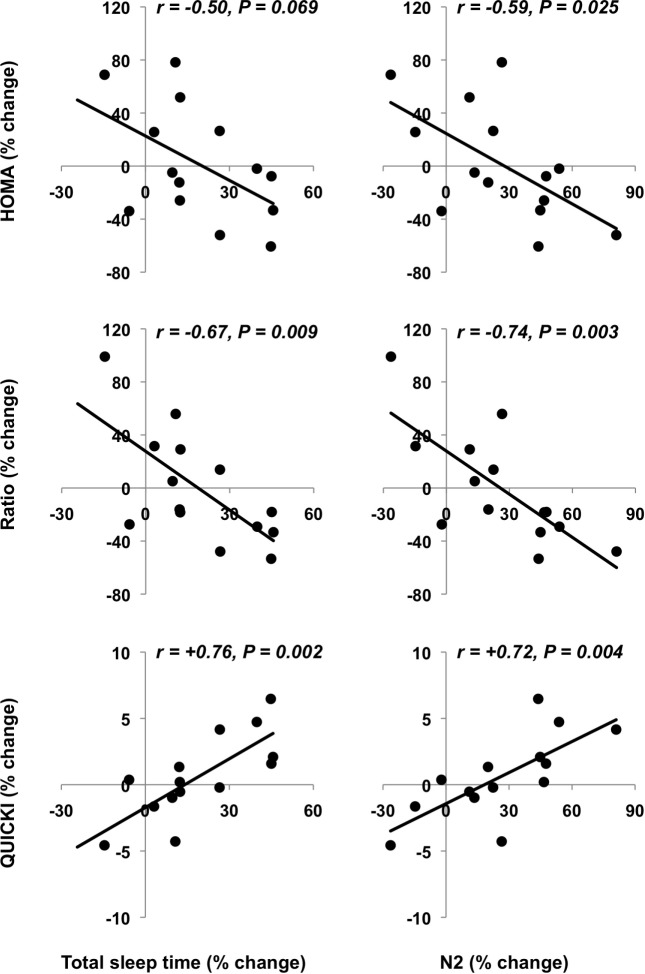
Changes in polysomnographic total sleep time tended to be associated with changes in HOMA (Figure 3B, upper left panel, P = 0.069) and correlated with changes in insulin-toglucose ratio (Figure 3B, middle left panel, P = 0.009) and QUICKI (Figure 3B, lower left panel, P = 0.002). Furthermore, changes in stage N2 were associated with changes in HOMA, insulin-to-glucose ratio, and QUICKI (Figure 3B, right panels, P = 0.025, P = 0.003, and P = 0.004, respectively). Other associations between sleep scores and glucose-related measurements did not reach statistical significance (P ≥ 0.15). Further, regression analyses with changes in insulin-to-glucose ratio or QUICKI as the dependent variable and changes in both total sleep time and body weight as independent variables revealed that only changes in total sleep time was significant (ratio: P = 0.042 for changes in total sleep time and P = 0.62 for weight changes and QUICKI: P = 0.016 for changes in total sleep time and P = 0.28 for weight changes).
Figure 3B.
Percent changes in sleep variables and in fasting glucose and insulin. Associations between pre- and post-intervention percent changes in total sleep time and stage N2, and those in HOMA, insulin-to-glucose ratio, and QUICKI.
DISCUSSION
The current study demonstrates that six weeks of sleep extension at home in adults who habitually restrict their sleep time during the week is a feasible intervention with metabolic benefits. There was a high inter-individual variability in the amount of sleep extension and increases in objectively assessed sleep times were strongly correlated with improvements in indices of insulin sensitivity.
In our study, the additional amount of sleep obtained during the intervention was mainly achieved by an augmentation of the time spent in NREM stage 2 as well as (although modestly) REM sleep, without any significant changes in either slow wave sleep or intra-sleep awakenings. Moreover, sleep efficiency as assessed via actigraphy was not modified, and sleep maintenance evaluated via polysomnography tended to be improved after sleep extension. These results indicate that sleep quality was not altered by extended sleep duration with the intervention of an extra hour of sleep, at variance with sleep extension studies that have imposed long hours in bed, i.e., 10 hours in bed20,21 and/or 2 additional hours compared to habitual bed times.20,28,29 In these latter studies, long hours in bed resulted in decreased sleep efficiency and/ or increased intra-sleep awakenings, even when participants were short sleepers and total sleep time was increased by 1.7 hours during one week of sleep extension.29 Unaltered sleep efficiency in the present study during weekdays and the fact that weekend sleep times were not modified by the intervention (participants preserved the extra amount of sleep during weekend) suggest that the requirement of one additional hour of sleep per day in habitually sleep-restricted adults is an adequate amount that preserves sleep quality.
Accumulating evidence indicates that reduced sleep duration in healthy7,8,10–12 and overweight adults9 induces deleterious effects on glucose metabolism resulting in a higher risk of diabetes. Our study shows, for the first time, that the reverse intervention can have beneficial metabolic effects (i.e., improvements in insulin sensitivity) in healthy adults who are engaged in habitual sleep restriction. In fact, our findings faithfully mirror the elevated insulin resistance observed in a recent study of 5 nights of sleep restriction to 4 hours16 that used the exact same index of insulin resistance, i.e., the insulin-to-glucose ratio. Since our study included a homogenous group of participants, i.e., relatively young and lean, these findings need to be replicated in older, and overweight and obese populations.
The duration of the sleep extension intervention could be a crucial factor in our positive findings. Indeed, most studies that have investigated the potential benefits of sleep extension were performed over one to seven nights.19,22,23,25,26,28–32,35 In the present study, sleep extension was required for 6 weeks to allow enough time for a potential physiological adaptation. It is noteworthy that a 6-week intervention designed to increase sleep duration in adults with prehypertension or type 1 hypertension was effective in decreasing blood pressure.33 The individual design of the daily schedule of sleep extension in accordance with the participant's preferences is an additional factor that may have contributed to our positive findings. Additionally, the participants were encouraged to follow the instructions of sleep hygiene and were educated in the benefits of adequate sleep duration for health. The investigator could be reached by phone or via email and individually bi-weekly meetings were programmed to encourage compliance. Additionally, inclusion criteria involved being a restricted sleeper (< 7 h) who believed that sleep extension was compatible with his/her current lifestyle. In sum, we believe that three factors played a role in the success of our experimental strategy: first, the fact that the participants were individuals who habitually curtailed their sleep but did not consider sleep extension as unfeasible; second, the relatively small amount of additional sleep requested (i.e., one hour) over a long period of time; and third, the flexibility provided to the participants to implement the extra amount of sleep without imposing a fixed schedule. Importantly, limiting the extra amount of sleep to one hour seems a reasonable goal, as excessive sleep duration is not recommended. Indeed, epidemiological studies have demonstrated a U-shape curve for the relationship between sleep duration and diabetes risk, indicating that long sleep is also a risk factor for diabetes.18 In addition, the mechanisms linking the amount of sleep and the risk of diabetes are still not fully understood. They most likely involve multiple pathways linked to each other and (partially) controlled by sleep, including counterregulatory hormones, sympatho-vagal balance, and the inflammatory system.45 Therefore, future sleep extension studies should assess the potential benefits of sleep extension using an appropriate amount of extra sleep on a relatively long period of time, while assessing potential mechanisms.
A potential limitation of the present study is the absence of a control group. For this first study of the potential metabolic benefits of sleep extension, based on our experience with studies manipulating sleep duration, we expected that there would be a wide inter-individual variation in the amount of extra sleep obtained and chose to complete the study in a sufficiently large number of participants to examine correlations between the success of the intervention and the metabolic changes. We indeed observed a high inter-individual variability with the sleep extension intervention, confirming that not all subjects are able to comply with the instructions. The highly significant correlations between changes in the amount of sleep and changes in metabolic measures are a powerful argument for a causal relationship. Nonetheless, future studies of sleep extension should include a control group.
A second potential limitation is the assessment of basal (i.e., fasting) glucose metabolism without evaluating the response to a challenge. The two well-established methods for the quantification of insulin sensitivity during a glucose challenge, the euglycemic-hyperinsulinemic clamp and the minimal model analysis of the frequently sampled intravenous glucose tolerance test, cannot easily be applied in a field study because of their complexity, cost, and invasiveness. We therefore focused on surrogate measures of fasting insulin resistance, HOMA,41,42 and the insulin-to-glucose ratio used in van Leeuwen et al.,16 as well as the surrogate measure of insulin sensitivity, QUICKI44—these can all be derived from glucose and insulin levels obtained in one fasting blood sample and are sufficient to show an improvement in basal insulin sensitivity after sleep extension, as observed in our study. Of note, these indices of fasting insulin resistance/sensitivity could be more easily applied in larger population studies.
The lack of control of food intake throughout the protocol could be another potential limitation to the study. However, body weight was not modified from pre to post intervention. In addition, the 16 participants reported no lifestyle modifications, in particular no changes in physical activities, during the bi-weekly meetings other than those related to sleep hours and sleep quality.
In conclusion, our study shows that sleep extension over 6 weeks in sleep-restricted adults has beneficial effects on insulin sensitivity. Further investigations using sleep extension paradigms in populations at risk such as pre-diabetic and diabetic patients are therefore needed, considering that diabetes is currently managed by recommendations of more physical activity and less caloric intake to lose weight without any systematic investigation of sleep habits.
DISCLOSURE STATEMENT
Dr. Rachel Leproult is a recipient of a grant “Brains Back to Brussels” from INNOVIRIS, the Brussels Institute for Research and Innovation, Région Bruxelles-Capitale, Belgium. This grant entirely supported the current research. Dr. Gaétane Deliens was during the study, and Médhi Gilson is, Research Fellow of the FRS-FNRS Fonds National de la Recherche Scientifique, Belgium. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Prof. Eve Van Cauter for useful comments and the volunteers for participating in the study.
Footnotes
A commentary on this article appears in this issue on page 663.
REFERENCES
- 1.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–16. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 2.National Sleep Foundation. 2013 International Bedroom Poll. 2013 [Google Scholar]
- 3.International Diabetes Federation. IDF Diabetes Atlas. 6th ed. Brussels, Belgium: International Diabetes Federation; 2013. [PubMed] [Google Scholar]
- 4.Harris MI. Diabetes in America: epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–4. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–46. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwarz PE, Lindstrom J, Kissimova-Scarbeck K, et al. The European perspective of type 2 diabetes prevention: diabetes in Europe--prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp Clin Endocrinol Diabetes. 2008;116:167–72. doi: 10.1055/s-2007-992115. [DOI] [PubMed] [Google Scholar]
- 7.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 8.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med. 2012;157:549–57. doi: 10.7326/0003-4819-157-8-201210160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism. 2013;62:204–11. doi: 10.1016/j.metabol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St-Onge MP, O'Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24:731–43. doi: 10.1016/j.beem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taub JM, Globus GG, Phoebus E, Drury R. Extended sleep and performance. Nature. 1971;233:142–3. doi: 10.1038/233142a0. [DOI] [PubMed] [Google Scholar]
- 20.Roehrs T, Shore E, Papineau K, Rosenthal L, Roth T. A two-week sleep extension in sleepy normals. Sleep. 1996;19:576–82. [PubMed] [Google Scholar]
- 21.Harrison Y, Horne JA. Long-term extension to sleep--are we really chronically sleep deprived? Psychophysiology. 1996;33:22–30. doi: 10.1111/j.1469-8986.1996.tb02105.x. [DOI] [PubMed] [Google Scholar]
- 22.Belenky G, Wesensten NJ, Thorne DR, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: what a difference an hour makes. Child Dev. 2003;74:444–55. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- 24.Kamdar BB, Kaplan KA, Kezirian EJ, Dement WC. The impact of extended sleep on daytime alertness, vigilance, and mood. Sleep Med. 2004;5:441–8. doi: 10.1016/j.sleep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Horne J, Anderson C, Platten C. Sleep extension versus nap or coffee, within the context of 'sleep debt'. J Sleep Res. 2008;17:432–6. doi: 10.1111/j.1365-2869.2008.00680.x. [DOI] [PubMed] [Google Scholar]
- 26.Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–21. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mah CD, Mah KE, Kezirian EJ, Dement WC. The effects of sleep extension on the athletic performance of collegiate basketball players. Sleep. 2011;34:943–50. doi: 10.5665/SLEEP.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubo T, Takahashi M, Sato T, Sasaki T, Oka T, Iwasaki K. Weekend sleep intervention for workers with habitually short sleep periods. Scand J Work Environ Health. 2011;37:418–26. doi: 10.5271/sjweh.3162. [DOI] [PubMed] [Google Scholar]
- 29.Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Bowyer S, Drake CL. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep. 2011;34:1659–70. doi: 10.5665/sleep.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1667–72. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children's emotional lability and impulsivity. Pediatrics. 2012;130:e1155–61. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 32.Gumenyuk V, Korzyukov O, Roth T, Bowyer SM, Drake CL. Sleep extension normalizes ERP of waking auditory sensory gating in healthy habitually short sleeping individuals. PLoS One. 2013;8:e59007. doi: 10.1371/journal.pone.0059007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haack M, Serrador J, Cohen D, Simpson N, Meier-Ewert H, Mullington JM. Increasing sleep duration to lower beat-to-beat blood pressure: a pilot study. J Sleep Res. 2013;22:295–304. doi: 10.1111/jsr.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: an experimental study. Sleep Med. 2013;14:510–7. doi: 10.1016/j.sleep.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Rupp TL, Wesensten NJ, Balkin TJ. Sleep history affects task acquisition during subsequent sleep restriction and recovery. J Sleep Res. 2010;19:289–97. doi: 10.1111/j.1365-2869.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 36.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 37.Buysse DJ, Reynolds CF, III, Monk TH, Berman SB, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 38.Horne JA, Ostberg O. The Owl and Lark Questionnaire. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 39.Ellis BW, Johns MW, Lancaster R, Raptopoulos P, Angelopoulos N, Priest RG. The St. Mary's Hospital sleep questionnaire: a study of reliability. Sleep. 1981;4:93–7. doi: 10.1093/sleep/4.1.93. [DOI] [PubMed] [Google Scholar]
- 40.Iber C, Ancoli-Israel S, Chesson AJ, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 41.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 42.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 43.Guerrero-Romero F, Rodriguez-Moran M. Glucose intolerance is predicted by the high fasting insulin-to-glucose ratio. Diabetes Metab. 2001;27(2 Pt 1):117–21. [PubMed] [Google Scholar]
- 44.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 45.Morselli L, Leproult R, Balbo M, Spiegel K. Role of sleep duration in the regulation of glucose metabolism and appetite. Best Pract Res Clin Endocrinol Metab. 2010;24:687–702. doi: 10.1016/j.beem.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]