Abstract
Study Objectives:
Alcohol and sleep loss are leading causes of motor vehicle crashes, whereby attention failure is a core causal factor. Despite a plethora of data describing the effect of alcohol and sleep loss on vigilant attention, little is known about their effect on voluntary and involuntary visual attention processes.
Design:
Repeated-measures, counterbalanced design.
Setting:
Controlled laboratory setting.
Participants:
Sixteen young (18–27 y; M = 21.90 ± 0.60 y) healthy males.
Interventions:
Participants completed an attention test battery during the afternoon (13:00–14:00) under four counterbalanced conditions: (1) baseline; (2) alcohol (0.05% breath alcohol concentration); (3) sleep restriction (02:00–07:00); and (4) alcohol/sleep restriction combined. This test battery included a Psychomotor Vigilance Task (PVT) as a measure of vigilant attention, and two ocular motor tasks—visually guided and antisaccade—to measure the involuntary and voluntary allocation of visual attention.
Measurements and Results:
Only the combined condition led to reductions in vigilant attention characterized by slower mean reaction time, fastest 10% responses, and increased number of lapses (P < 0.05) on the PVT. In addition, the combined condition led to a slowing in the voluntary allocation of attention as reflected by increased antisaccade latencies (P < 0.05). Sleep restriction alone however increased both antisaccade inhibitory errors [45.8% errors versus < 28.4% all others; P < 0.001] and the involuntary allocation of attention, as reflected by faster visually guided latencies (177.7 msec versus > 185.0 msec all others) to a peripheral target (P < 0.05).
Conclusions:
Our data reveal specific signatures for sleep related attention failure: the voluntary allocation of attention is impaired, whereas the involuntary allocation of attention is enhanced. This provides key evidence for the role of distraction in attention failure during sleep loss.
Citation:
Lee J, Manousakis J, Fielding J, Anderson C. Alcohol and sleep restriction combined reduces vigilant attention, whereas sleep restriction alone enhances distractibility. SLEEP 2015;38(5):765–775.
Keywords: alcohol, attention, sleep restriction, vigilance
INTRODUCTION
Alcohol intoxication and drowsiness are leading causes of motor vehicle crashes,1–5 which are likely to result in serious injury or fatality to the driver or other road users.5–7 Legal limits of blood alcohol levels while driving exist to endorse safer driving practices, and are based on performance impairment following alcohol intake in alert, non-drowsy individuals.8 It is well documented however that legal levels of blood alcohol concentration (BAC) and prior insufficient sleep (5 h) combine to form a synergistic deterioration on driving performance.9,10 Although driving is a complex behavior involving a combination of motor control, cognitive processing, and visual attention,11 the primary manifestation of alcohol and drowsiness-related motor vehicle crashes is attention failure, typified by a lack of, or delayed response to visual stimuli in the environment.12,13 Despite a plethora of research examining the independent effect of alcohol and sleep loss on attention, little is known about the combination of these factors on different types of attentional failure.
Attention is not a unitary construct but a combination of voluntary and involuntary processes. The frontoparietal network model of attention is the most widely accepted neural model and describes dynamic interactions between dorsal (“top-down”) and ventral (“bottom-up”) subnetworks, allowing individuals to focus attention in a goal-oriented manner (“top down” voluntary attention) while inhibiting more automatic responses to irrelevant stimuli (“bottom-up” involuntary attention).14 Selective attention, or the ability to attend to one information source while excluding all others, is dependent on an adequate state of vigilance, which is often characterized and measured by changes in sustained attention.15 Vigilance, or sustained attention, is highly dependent on the sleep-wake state,16 whereby sleep loss results in “state-instability”— rapid and uncontrolled sleep initiation with simultaneous fluctuations in sustained attention.17 Within the field of sleep and circadian science, this is typically captured by the Psychomotor Vigilance Task (PVT).19,20
The effect of sleep deprivation on selective attention was first documented over four decades ago. Norton demonstrated that sleep deprived individuals were inefficient at sorting cards containing irrelevant information,22 suggesting a deficit in selective attention processes following sleep loss. Since then, studies examining the effect of sleep loss on selective attention have been comparatively scarce compared to those focusing on sustained attention. Because focusing attention in the presence of irrelevant distractors is essential in many occupational settings, there exists renewed interest in examining the effect of sleep loss on selective attention; more specifically the ability to inhibit the involuntary capturing of attention while maintaining voluntary control of attention allocation.23–25 Our recent work suggests sleep deprivation affects the ability to inhibit a response to a peripheral, irrelevant stimulus thus enhancing “distractibility.” Sleep deprivation led to slower responses, more head turns, and increased number of attention lapses on the PVT when performed in the presence of a peripheral dis-tractor.23,24 This may be due to a different effect of sleep deprivation on voluntary and involuntary attention mechanisms. For instance, in separate studies, insufficient sleep has been associated with increased responsiveness of involuntary attention26 yet reduced capability in the voluntary allocation of attention, as reflected by slower responses when a voluntary inhibitive action was required (i.e., when responding to a target when preceded by an invalid cued target on a cued reaction time task).27 Notwithstanding different experimental approaches, collectively these findings suggest that when sleep is deficient the voluntary “top down” control of attention worsens27 while the involuntary “bottom up” allocation of attention becomes sensitized.26
The ocular motor system and the attention system share neuroanatomical networks.28,29 The allocation of visual attention, whether voluntary (“top-down”) or involuntary (“bottom-up”), consequently depends on the interaction of these networks.30–36 Examination of voluntary and involuntary aspects of attention therefore can be examined using ocular motor paradigms,37,38 whereby participants initiate or inhibit a response to visual targets via an ocular saccade. Bocca and colleagues25 examined inhibitory control using an antisaccade task, where the eye movement is generated in the opposite direction of a suddenly appearing target. Here, sleep loss had a detrimental effect on the voluntary allocation of attention, as reflected by slower initiation of correct inhibitory antisaccades and increased number of inhibition errors. Similar to sleep loss, the effect of alcohol on attention is well documented,40 especially with respect to deficits in sustained attention.41–45 Evidence suggests clear impairment in the voluntary control of attention following alcohol intake. Utilising an antisaccade task, alcohol (BAC 0.07%) has consistently led to slower latencies for correctly directed antisaccades.46–48
Although much data exist on the effect of sleep loss and alcohol on sustained and selective attention separately, studies examining the combined effects of sleep loss and alcohol remain largely focussed on sustained attention50,51 or global aspects of driving impairment, such as lane departures.9,50,52–56 Clear increases in lane drifting (typical of sleep related crashes) and steering deviation have been reported during a 2-h simulated driving task in the afternoon9,10,54 and evening55 following a night of partial sleep restriction (between 4–5 h) when combined with alcohol within legal limits (0.05% BAC). These adverse driving outcomes have been largely attributed to changes in vigilance, drowsiness, and sleep onset/microsleep.9,10 However, we have previously described that poor driving outcomes such as lane drifting following restricted sleep may not be due to falling asleep or vigilant decrements, but instead may be due to the driver being distracted.57 The extent to which the poor driving outcomes in previous driving studies combining sleep loss and alcohol are due to reductions in vigilance and/or deficits in the voluntary and involuntary allocation of attention remains unknown.
To understand further the cause of sleep related motor vehicle crashes, it is important to elucidate the facets of attention most vulnerable to sleep loss. These include sustaining attention, the voluntary allocation of attention to important safety critical stimuli, and the inhibition of attention to irrelevant stimuli. In addition, it is important to examine these (1) with and without alcohol due to the synergistic effect of alcohol on performance impairment following restricted sleep and (2) following more typical sleep restriction regimens that are known to elevate crash risk; i.e., 5 h.58 To our knowledge no study has examined changes in voluntary and involuntary attentional processes in individuals following restricted sleep combined with moderate amounts of alcohol intake. This forms the basis of our study.
METHODS
Participants
Sixteen healthy male participants (age range: 18–27 y; M = 21.90 ± 0.60 y) were recruited to take part in the study. All participants had a body mass index within the range 18–33 (24.05 ± 0.81), were nonsmokers, did not consume more than 300 mg of caffeine per day, and reported being mild-moderate alcohol drinkers (between 2 and 14 standard drinks per week).59 Participants were screened to ensure all had habitual sleep times between 22:00–01:00 and wake times between 06:00–09:00; reported no history of psychiatric or sleep disorders, epilepsy or migraines; were not taking medication affecting the central nervous system; did not have nystagmus or corrected vision via optical frames/lenses; or had not worked shifts in the past 3 months or traveled across two time zones in the past month. Daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS) and sleep quality via the Pittsburgh Sleep Quality Index (PSQI). Those who were positive for daytime sleepiness (ESS > 10)60 or poor sleep quality (PSQI > 5)61 were also excluded from the study.
All participants were reimbursed for their time and provided full, written informed consent. The study was approved by the Monash University Human Ethics Research Committee.
Design
The study comprised a repeated-measures design whereby participants undertook all four conditions of the study. These were: 1. Baseline – where no alcohol consumption nor sleep restriction occurred; 2. Alcohol – where participants consumed alcohol to reach a breath alcohol concentration (BrAC) of 0.05% after a normal night's sleep; 3. Sleep restriction – where participants had their sleep restricted to 5 h from 02:00 until 07:00 prior to the experiment; and 4. Combined – where participants had their sleep restricted to 5 h (02:00 – 07:00) and consumed alcohol to reach a BrAC of 0.05%. Conditions were counterbalanced across participants and each condition separated by at least a 5-day washout period.
The study was powered on a similar study design using the proxy measure of braking reaction time.52 With a group size of 16, for four conditions, we had 95.2% power to detect a signifi-cant main effect of condition (Δ = 529.4 ms, σ = 355.7, α = 0.05).
Procedure
Eligible participants were required to attend the laboratory prior to the study to complete the PVT and the ocular motor tasks to ensure familiarity and/or eliminate known practice effects.62 During both the practice trial and the main study, participants were asked to maintain their normal sleep habits except when otherwise instructed. Participants abstained from alcohol and caffeine for 24 h prior to all testing. Compliance with sleep requirements was monitored using Actiwatch Spectrums (Philips Respironics, BMedical, Australia) and sleep diaries, and compliance with alcohol intake were monitored via BRaC using an AlcoLimit Enforcer 2 (AlcoLimit Breath-alysers Pty Ltd, Manly, Australia).
For the main study, and for each condition, participants were asked to eat breakfast at habitual times followed by a snack at 11:00. Following this they were required to abstain from eating before arriving at the laboratory to facilitate accurate alcohol calculations. Participants arrived at the laboratory at 12:00 where actigraphy and BrAC were examined for compliance. Participants were provided with a 480-mL drink at 12:30, from which they took a 30-mL sip every minute for 16 min. In the nonalcohol conditions (Baseline and Sleep Restriction), the 480 mL contained orange juice with the rim dipped in vodka.54,63 In the alcohol conditions (Alcohol and Combined), the 480-mL drink contained a calculated amount of 37.5% proof vodka (Smirnov Triple Distilled) to achieve a BrAC of 0.05%, with orange juice added to make the drink content up to 480 mL. Alcohol volume was calculated based on previous work10 using the following formula:
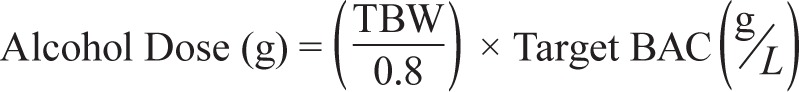 |
where TBW = 2.447 − (0.09516 × age[yrs]) + (0.1074 × height[cm]) + (0.3362 × weight[kg]).
Alcohol was administered single-blind. Breathalyzer tests were taken at 13:00 and 13:30. Because alcohol is well known for its biphasic effects (stimulating arousal on the ascending arm of the BrAC curve and sedation on the descending arm of the BrAC curve64–66), our study was designed such that the attention tasks were administered during the soporific descending arm of the BrAC curve.
The attention test battery began at 13:00 and consisted of (1) ocular motor tasks to examine voluntary and involuntary control of selective attention (13:00–13:15) and (2) the PVT to examine sustained attention (13:30–13:45). The Karolinska Sleepiness Scale (KSS) was used to examine subjective sleepiness every 15 min during testing. Total test time was 45 min. The study protocol can be seen in Figure 1.
Figure 1.
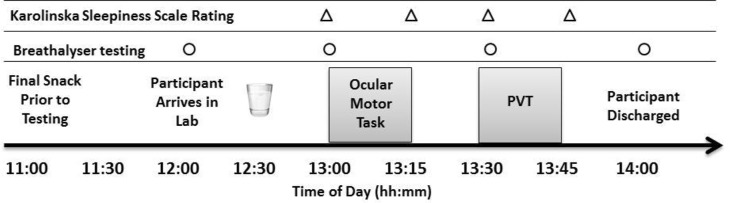
Study protocol indicating timing of drink administration (12:30), ocular motor tasks (between 13:00–13:15) and PVT (between 13:30–13:45). Timing of drink administration, testing, breathalyzer tests (○) and Karolinska Sleepiness Scale ratings (Δ) are shown. Participants were discharged from the laboratory when breath alcohol concentration had returned to < 0.02%. PVT, Psychomotor Vigilance Test.
Subjective Sleepiness
Subjective sleepiness was measured with the KSS.67 This is a nine-point verbally anchored scale whereby participants rate their subjective level of sleepiness, ranging from (1) Extremely alert to (9) Very sleepy, great effort to keep alert, fighting sleep. Individuals rated their sleepiness every 15 min from 13:00 until 13:45, before and after each attention test.
Voluntary and Involuntary Selective Attention: Ocular Motor Test Battery
Participants completed two ocular motor paradigms (see next paragraphs). These tasks require participants to initiate or inhibit a saccade (eye movement) in response to visual target stimuli displayed on a computer screen. All instructions were scripted to ensure consistency between participants and conditions: “In this task, you are required to look at the target cross which will appear in the centre of the screen. At random intervals, a second cross will appear either to the left or the right of that central cross. As soon as you see these left or right crosses appear, you must look at (a) that cross as quickly and as accurately as possible [visually guided] or (b) the opposite location to that cross as quickly and as accurately as possible [antisaccade]. The central cross will then reappear, you should look again at the central cross and the task will repeat. Do you understand?”.
Binocular eye movements were recorded using an Eyelink 1000 eye tracker (SR Research Ltd, Ontario, Canada). The Eyelink 1000 consisted of a desktop-mounted camera with a sampling rate of 500 Hz, and a stimulus display monitor with a resolution of 1068 × 1050 and a refresh rate of 60 Hz. Participants were seated 51 cm from the camera and 82 cm from the stimulus display monitor in a darkened, silent room. The order of presentation of the ocular motor tasks was counterbalanced.
Visually Guided Task
A visually guided task may be used to measure involuntary, reflexive attention.68 Participants were asked to fixate on a central fixation stimulus before generating a saccade to a peripheral target cross when it appeared. The central fixation stimulus was presented between 750 and 1,500 ms (subtending 0.05° × 0.05° of visual angle), before a peripheral target cross (subtending 1.6° × 1.6° of visual angle) with a black crosshair at the center (subtending 0.6° × 0.6° of visual angle) appeared to the right or left of the fixation stimulus, at either 5° or 10° from center. Only one cross would be presented at a time (no gap). After presentation of the peripheral target cross, the central fixation stimulus was re-presented and a new trial began. Forty-eight trials were presented in two separate blocks of 24. Total task duration: 2 min 10 sec (time between blocks 5–10 sec).
Target crosses were presented equally to the right and left side and equally to the 5° and 10° positions to eliminate preemptive responses.
Antisaccade Task
An antisaccade task requires individuals to inhibit a reflexive saccade to a target stimulus and instead generate a saccade to its mirror location.69 This task was used to measure the voluntary control of attention.
Stimuli consisted of crosses presented in the horizontal plane on the stimulus display monitor. A central fixation stimulus was presented (subtending 1.6° × 1.6° of visual angle), with a central black crosshair (subtending 0.6° × 0.6° of visual angle) to serve as a fixation stimulus. With a random interstimulus interval of 1,200–1,600 ms, a target stimulus cross was presented to the right or left of the fixation stimulus, at either 5° or 10° from center in the horizontal plane. Upon presentation, participants were required to inhibit a reflexive saccade toward the target stimulus and instead generate a saccade to its mirror location. Simultaneously with the presentation of the target stimulus, the central fixation stimulus disappeared such that only one stimulus cross appeared on the screen at one time (no gap). After presentation of a peripheral target stimulus (1,500 ms), the central fixation stimulus was re-presented and a new trial started. Forty-eight trials were presented in two sequential blocks of 24. Total task duration: 2 minutes 19 seconds (time between blocks 5–10 sec).
Sustained Attention: The PVT
Sustained attention was assessed using a 10-min visual PVT.19 Participants sat with their preferred finger from their dominant hand on a response button, and responded as quickly as possible when a stimulus appeared on the screen in the form of a millisecond counter. The interstimulus interval ranged from 2–10 sec. Response times for each trial were displayed on the screen prior to the next trial. If no response occurred within 10,000 ms, an on/off audio tone rang. Data were cleaned to remove any response < 100 ms including errors of commission. Mean response time, 10% fastest and slowest response times, and number of lapses (responses times > 500 ms) were calculated.
Data Analysis
Paired-samples t-tests were conducted to ensure no difference in BrAC in the Alcohol and Combined conditions at both 13:00 and 13:30.
For the ocular motor tasks, data were analyzed using a customized program written in MATLAB (MATLAB, The Math-Works Inc., Natick, MA). Response latencies were calculated for each correct saccade, characterised as the temporal difference between fixation offset and saccade onset. Saccade onset was determined using a velocity criterion of 30° per second. In addition, according to previous studies,69 for the antisaccade task the first two trials were discarded if the initial saccade was classified as a direction error.
In the antisaccade task, directional error frequency (a saccade to the cue, not its mirror location) was calculated. All trials were examined for quality to ensure a valid trial. Trials were deemed invalid and eliminated from analysis due to blinking within 200 ms either side of cue presentation or when interfering directly with a saccade; small saccades < 3° may be indicative of saccadic intrusions such as square wave jerks; anticipatory errors defined as a saccade occurring before target presentation or < 100 ms after target presentation; or an unstable baseline defined as a movement greater in magnitude than ± 3° directly before or during the target onset. Each saccadic response latency was transformed using its reciprocal [1/(×/1000)] to normalize the data. Latencies that were ± 2.3 standard deviations greater than the transformed mean were removed. All ocular motor measures were averaged, within participant. Participants with an average latency ± 2.3 standard deviations greater than the group mean were also removed from analysis.
As reaction time data typically are not normally distributed,70 all PVT data were normalized. The reciprocal reaction time [1/ (×/1000)] was calculated for mean reaction time, slowest 10% of reaction times, and fastest 10% of reaction times. Lapses were transformed using [(√n)+(√n+1)].71 Cumulative distribution plots were created as previously described.72 Area under the curve (AUC) was calculated for both the normal response range (100 ms–500 ms) and the “lapse range” (responses > 500 ms).
Linear mixed-model analysis was performed on all transformed ocular motor (response latency; % directional errors) and PVT outcomes (mean reaction time [RT], fastest 10%, slowest 10%, and number of lapses). Condition was modeled as a fixed factor and Participant as a random factor. For KSS only, a two-way (Condition*Time) linear mixed model using Condition (4 levels) and Time (4 levels) as fixed factors and Participant as a random factor was used. For all linear mixed models, a compound symmetry covariance type was used as this provided the lowest Schwarz Bayesian Criterion (BIC).73 Post hoc pairwise comparisons were conducted using a false discovery rate (FDR) comparison to control for familywise error.74,75 For all post hoc tests, adjusted P values (Padj) are provided using the FDR “q” adjusted significance value.
All statistical analyses were performed using SPSS 20.0 (IBM Corp., Armonk, NY). Means and standard error of the mean (SEM) are reported unless otherwise stated.
RESULTS
Data were obtained from 16 participants. One participant was excluded from all analyses due to lack of compliance to procedures, and one participant was excluded from ocular motor analysis due to excessive blinking. The final analysis was performed on n = 14 for all ocular motor analyses, and n = 15 for PVT and KSS data. Due to technical difficulties, two participants did not provide a BrAC at 13.00 (n = 14) and one participant at 13.30 (n = 15). Finally, of a possible total of 5,264 ocular motor trials (or saccades) 5,002 were classified as valid, representing a 5% loss of data. These include 56 trials removed on the antisaccade task (2.2% loss) and 206 trials removed from the visually guided task (7.7% loss).
Prior Sleep: Adherence to Sleep Restriction Protocol
Sleep duration was significantly different due to condition (F(3,42) = 125.48, P < 0.001), such that individuals slept longer prior to Baseline (424.3 ± 15.4 min) and Alcohol (430.9 ± 14.4 min) conditions compared to both Sleep Restriction (271.2 ± 7.0 min) and Combined (269.9 ± 8.6 min) conditions (P < 0.001). Consistent with protocol adherence, there was no difference in sleep duration between Baseline and Alcohol conditions (P = 0.567), and Sleep Restriction and Combined conditions (P = 0.912).
Breath Alcohol Concentration
All participants across all conditions had a BrAC of 0.00% upon arrival to the laboratory. There was no difference in BrAC, at either time point (P > 0.5), between the Alcohol (M1300h = 0.045 ± 0.004%; M1330h = 0.04 ± 0.004%) and Combined condition (M1300h = 0.048 ± 0.004; M1330h = 0.041 ± 0.003%).
Subjective Sleepiness
Subjective sleepiness significantly differed according to condition (F(3,210) = 23.44, P < 0.001), such that participants reported higher sleepiness in all conditions compared to Baseline (Padj < 0.001). In addition, they also rated themselves sleepier in the Combined condition compared to Sleep Restriction (Padj = 0.0012) and Alcohol conditions (Padj < 0.001).
There was a significant main effect of time (F(3,210) = 49.02, P < 0.001), such that pretest sleepiness ratings at 13:00 were significantly lower than all other time points (Padj < 0.001). There was also a significant increase in sleepiness from 13:30 to 13:45 (Padj = 0.011). There was no interaction between condition and time (P = 0.59). See Figure 2.
Figure 2.
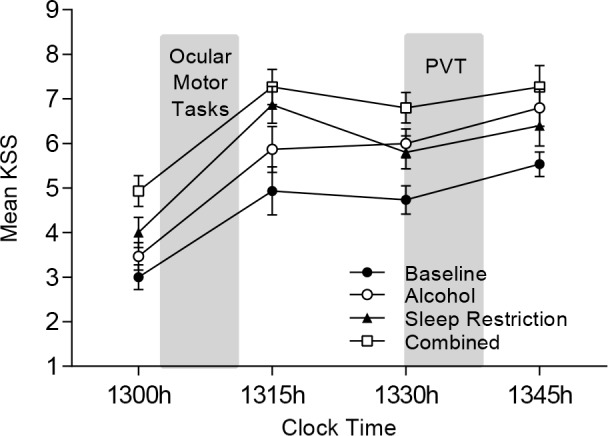
Mean KSS Score (± standard error of the mean) for each condition. Shaded areas represent the ocular motor test paradigm (13.00–13.15) and the PVT (13.30–13.40). KSS, Karolinska Sleepiness Scale; PVT, Psychomotor Vigilance Test.
Vigilant Attention: PVT
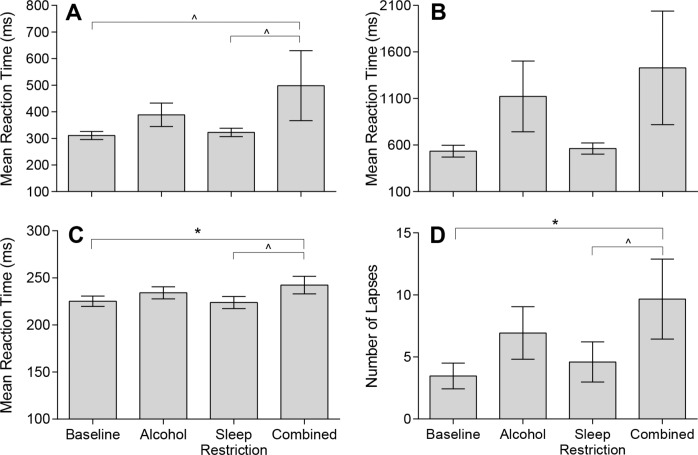
Changes in PVT performance due to condition can be seen in Figure 3. As seen in Figure 3A, there was a main effect of condition on mean RT (F(3,42) = 3.09, P = 0.037). Although mean RT was slower in the Combined condition compared to both Baseline (P = 0.015; Padj = 0.09) and Sleep Restriction conditions (P = 0.031; Padj = 0.093) these were above the level of significance when adjusted. Fastest 10% of reaction times (see Figure 3C) also increased due to condition (F(3,42) = 3.49, P = 0.024), such that the fastest responses slowed under the Combined condition compared to Sleep Restriction (Padj = 0.042) and Baseline (Padj = 0.054). Lapses increased due to condition (F(3, 42) = 3.15, P = 0.035), such that more lapses occurred in the Combined condition compared to Baseline (Padj = 0.048) and Sleep Restriction (Padj = 0.099); see Figure 3D. There was no significant change in slowest 10% of reaction times (Figure 3B) due to condition (F(3,42) = 2.54, P = 0.069).
Figure 3.
Mean and standard error of the means for Psychomotor Vigilance Test performance under Baseline (BL), Alcohol (AL), Sleep Restriction (SR), and Combined (C) conditions. These include (A) Mean Reaction Time (BL: 311.0 ± 15.2ms; AL: 388.7 ± 44.1 ms; SR: 322.5 ± 15.8 ms; C: 498.3 ± 131.4 ms); (B) Slowest 10% of reaction times (BL: 533.5 ± 36.3 ms; AL: 1122.1 ± 380.2ms; SR: 562.8 ± 60.4 ms; C: 1429.3 ± 610.1 ms); (C) Fastest 10% of reaction times (BL: 225.2 ± 5.5 ms; AL: 232.2 ± 6.4ms; SR: 223.9 ± 6.4 ms; C: 242.4 ± 9.3ms); and (D) Number of Lapses (BL: 3.5 ± 1.0; AL: 6.9 ± 2.1ms; SR: 4.6 ± 1.6 ms; C: 9.7 ± 3.2 ms). ∧Padj < 0.10; *Padj < 0.05. Untransformed data are plotted.
To examine change in RT distribution, we examined the cumulative distribution of PVT responses. The distribution of responses for each condition is comparable with a normal distribution followed by a long tail (Figure 4A). Area under the curve (AUC) analysis revealed no differences between condition (F(3,42) = 1.11, P = 0.356). However, on specific analysis of the normal response range (100–500 ms) (Figure 4B) and lapse range (> 500 ms) (Figure 4C) subtle changes were observed. For the normal response range, there was a signifi-cant difference between condition for AUC (F(3,42) = 3.33, P = 0.029). Here, the Combined condition had significantly fewer responses than the Baseline condition (Padj = 0.036). Likewise, analysis of the lapse range revealed a significant difference between condition (F(3,42) = 3.69, P = 0.019), with the Combined condition having significantly more responses than the Baseline condition (Padj = 0.024).
Figure 4.
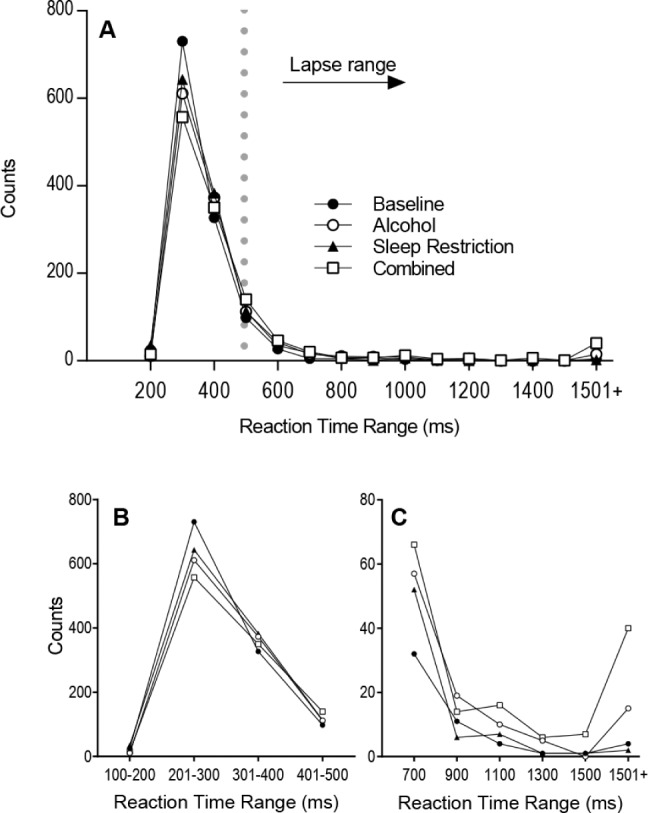
Comparison of the total sum of responses occurring: (A) across the entire reaction time range for each condition; (B) in the normal response range (100–500 ms); and (C) in the lapse range (> 500 ms).
Ocular Motor Paradigms
Antisaccade Task
There was a significant increase in response latency on the antisaccade task due to condition (F(3,39) = 3.65, P = 0.021), such that the Combined condition resulted in a slower generation of the antisaccade compared to Baseline (Padj = 0.036). In addition, there was an increase in the percentage of directional errors (F(3,39) = 17.18, P < 0.001) in the Sleep Restriction condition compared to all other conditions (Padj < 0.001). Data are shown in Figure 5 for mean response latency (left panel) and percentage of directional errors (right panel).
Figure 5.
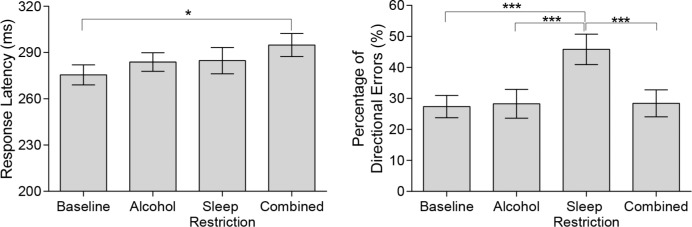
Mean and SEMs for response latency (left panel) and percentage of directional errors (right panel) on the antisaccade task under Baseline (BL), Alcohol (AL), Sleep Restriction (SR), and Combined (C) conditions. (Left panel) Mean Response Latency (BL: 275.6 ± 6.5 ms; AL: 283.8 ± 6.0 ms; SR: 284.7 ± 8.5 ms; C: 294.8 ± 7.5ms). (Right panel) Percentage of Directional Errors (BL: 27.4 ± 3.6%; AL: 28.3 ± 4.7%; SR: 45.8 ± 4.9%; C: 28.4 ± 4.3%). ***Padj < 0.0001, *Padj < 0.05. Untransformed data are plotted.
Visually Guided Task
Response latencies differed significantly between conditions (F(3,39) = 6.19, P = 0.002) such that the Sleep Restriction condition resulted in faster latencies compared to Baseline (Padj = 0.044), Alcohol (Padj = 0.003) and Combined conditions (Padj < 0.001). See Figure 6.
Figure 6.
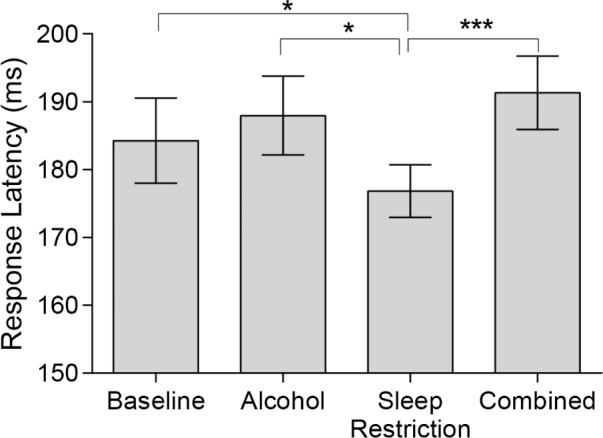
Mean ± standard error of the mean for response latency on the visually guided task for all conditions. (Baseline: 185.4 ± 6.2 ms; Alcohol: 189.0 ± 5.7 ms; Sleep Restriction: 177.7 ± 3.9 ms; Combined: 191.7 ± 5.7ms). *Padj < 0.05; ***Padj < 0.001. Untransformed data are plotted.
DISCUSSION
Our data indicate alcohol intake within legal limits (BrAC 0.05%) following 5 h of restricted sleep creates a synergistic detrimental effect on vigilant/sustained attention. We observed more lapses in attention and a slower mean response time on the PVT when alcohol and restricted sleep were combined, compared to when each existed alone. Although these data confirm previous findings that sleep loss and alcohol combined affects the capacity to continually attend to external stimuli, they provide little insight into the attentional mechanisms involved in this process. To address this, we also examined the effects of alcohol and sleep restriction, alone and combined, on the voluntary and involuntary allocation of attention using well-established ocular motor paradigms. Although a combination of sleep restriction and alcohol slowed down the voluntary allocation of attention (reflected by slower latencies on the antisaccade task), sleep restriction alone increased inhibitory errors in the voluntary allocation of attention while accelerating the involuntary allocation of attention (reflected by faster latencies on the visually guided task). These data suggest sleep restriction alone enhances distractibility.
Although deficits in sustained attention have been well described in the sleep literature,13,17,76 attention is not a unitary construct.15,77 Because sleep loss is unlikely to exert only a nonspecific de-arousing effect, understanding which aspects of attention are detrimentally affected by sleep loss is vital to overcoming attention failure in safety critical environments. Previously, we have alluded to enhanced distractibility during sleep loss as reflected by an increased number of attention lapses when sleep restricted participants performed the PVT in the presence of distracting, task-irrelevant peripheral stimuli (TV programme).23,24 In addition, the collective findings of Salmi et al.26 and Versace et al.27 provide evidence for enhanced distractibility following sleep loss. Although these studies report on different types of sleep disruption and tasks to separately examine voluntary and involuntary allocation of attention, their observations mirror ours in that the voluntary allocation of attention is impaired,27 whereas the involuntary allocation of attention to irrelevant stimuli is heightened26 following restricted sleep. This enhanced distractibility during sleep loss may have a neurophysiological basis. The ability to attend to external stimuli in the environment involves filtering out distracting stimuli.14,78 Although the prefrontal cortex (PFC) is not critical for performing simple, automatic behaviors,79 it is part of a network that is crucial for continually focusing attention by filtering out distracting stimuli.14 Because the PFC is preferentially susceptible to sleep deprivation,80–82 the reduced capacity to filter incoming information and become more responsive to irrelevant stimuli is expected following sleep loss. Because our study did not capture neural correlates of attention allocation however, this explanation remains speculative, and further work utilizing imaging techniques is required to elucidate the underlying neural basis for enhanced distractibility following sleep loss.
Our data correspond with Bocca et al.25 in that sleep loss decreases the voluntary control of attention on an antisaccade ocular motor task. Despite this, previous studies have reported no deficit on the antisaccade task for either latency or inhibitory errors following 20 h awake.83,84 These conflicting findings may be due to (1) a lack of control over laboratory procedures including caffeine access83; (2) differences in the ocular motor task including number of trials and the use of a “gap” paradigm84; or (3) differences in the time of day for testing under baseline and sleep loss conditions.83,84 We can address each of these in turn. First, as environment24 and caffeine85 affect attention failure, a lack of control over these factors may affect study outcomes. Second, Zils et al.84 used different parameters on the antisaccade task compared to ours: they evaluated a relatively small number of trials (20 versus 48 in our study) and incorporated a gap paradigm (where a 200ms temporal gap occurred between the fixation stimulus disappearing and the target stimulus appearing compared to our study with no gap where the fixation stimulus disappeared at the same time the target stimulus appeared). As Bocca et al. also reported increased latencies and directional errors (comparable to our findings) using a 200-ms gap paradigm on the antisaccade task, this does not explain the conflicting findings reported in the study by Zils et al. In addition, Bocca and colleagues also reported significant effects when examining only the first 20 trials (we also found the same in our study; analysis not shown, Padj < 0.001). As such, we argue that the conflicting findings between our study and Zils et al.84 was not due to differences in the antisaccade task. Third, we suspect a major contributory factor to the conflicting findings on the antisaccade task was the lack of control over time of day in previous studies. Similar to Bocca et al.,25 we examined ocular motor performance during the midafternoon circadian nadir (13:00–14:00) controlling for time of day, i.e., baseline and experimental conditions occurred at the same clock time. In contrast, those studies reporting nil effects of 24 h of sleep loss83,84 had no time of day control as ocular motor testing occurred in the morning hours compared to a baseline collected the previous evening. In addition, in those studies sleep deprivation testing occurred from 07:30 to 08:00, which may have corresponded with a wake-promoting alerting signal from the circadian clock.86,87 As performance varies throughout the 24-h day,87,88 a lack of control for time of day likely exerts a large effect on attention outcomes. Because no data currently exist describing changes in voluntary and involuntary control of attention due to circa-dian phase, this interpretation remains speculative.
Although sleep restriction alone led to increased directional errors on the antisaccade task, the combination of alcohol and sleep restriction resulted in decreased directional errors with slower antisaccadic response latencies. This result may be due to: (1) a speed-accuracy tradeoff, where participants sacrificed the speed of the response in favor of improved accuracy; (2) a speed-interference effect, where slowing of attention processing caused by alcohol resulted in more time for inhibitory systems to engage89; or (3) a differential effect of alcohol on neural function at different sites (i.e., those involved in latency versus those involved in generating the saccade).46 Awareness of alcohol intake by study participants may exacerbate a speed-accuracy tradeoff for the combined condition. As our study was designed in a single blind manner comparable to previous studies,53–55,63 we did not evaluate whether participants were aware of alcohol intake and were unable to elucidate whether this had a true effect in our study. We suspect this had minimal effect on our data outcomes however because we did not find uniformity of impairment across alcohol conditions, plus increased awareness and performance compensation is typically associated with higher doses of alcohol intake. (e.g., 0.8–0.9 g/kg).90,91 As suggested by the speed-interference theory, the delayed response might allow for a longer period of time for inhibition systems to engage thus reducing directional errors. Although our data are consistent with previous studies reporting an alcohol-induced slowing of voluntary allocation of attention, with a corresponding decrease or no change in directional errors,46–48 no study has yet confirmed the mechanism by which alcohol may exert this behavioral effect.
Our data may provide some insight into current theories of sleep loss on cognitive outcomes.94 The Controlled Hypothesis theory posits that long, monotonous tasks are affected by sleep loss due to a greater top-down control required to sustain performance,95 whereas the Neuropsychological Hypothesis theory describes how sleep loss preferentially impacts the pre-frontal cortex.81,96,97 as evidenced by hypoactivation of lateral and medial PFC following sleep deprivation.98,99 Although our data demonstrate impaired top-down control of attention (increased errors on the antisaccade task) during sleep loss, as the tasks we employed were short in duration, we argue that this is driven by a physiological consequence of sleep loss (neuropsychological hypothesis), rather than the task itself (controlled hypothesis). Our data also concur, in part, with the Vigilance Hypothesis theory, which argues that arousal/vigilance is a common, underlying factor responsible for the wide array of cognitive deficits observed.100,101 Because the involuntary capturing of attention on the visually guided task was significantly faster, this cannot be explained by the vigilance hypothesis. Taking this into consideration, our study outcomes do not support one single theory of sleep loss on cognition, but instead suggest a combined, integrative theory is required.
Our study is novel in two respects. First, it examines changes in voluntary and involuntary control of attention following sleep restriction, with and without alcohol intake. Previous studies have examined the combined effect of sleep restriction and alcohol using driving simulation and/or PVT measures.10,52–56,90 These tasks provide little insight into specific attentional mechanisms affected by the combination of these factors. For instance, driving taps into more global aspects of performance impairment, whereas the PVT examines vigilant attention.18 Although we have previously shown the PVT can be used to disentangle causes of attentional failure, in particular distraction,23,24 when combined with synchronized video/electroencephalography/eye tracking data, this is time-consuming and requires a large amount of offline processing. In comparison, the ocular motor tasks we employed are shorter (∼2 min), require less offline processing, and target the voluntary and involuntary attention networks within the same task, while yielding the same research outcomes as the combined PVT/eye tracking approach. As such, future work might consider incorporating both measures of sustained vigilant attention (i.e., the PVT) and selective attention (i.e., ocular motor paradigms) to enhance the understanding of attentional failure during sleep loss. Second, our study protocol included more typical levels of sleep restriction (5 h versus 24 h used previously), with and without the addition of legal levels of alcohol. Our research findings can therefore be translated to real-world outcomes, and may promote future ecologically based research. Our data suggest drivers or other personnel involved in safety critical monitoring (i.e., air traffic control, intensive care units, security monitoring) may be prone to enhanced distractibility following reduced sleep. We therefore encourage future work to evaluate changes in both sustained attention, and voluntary and involuntary allocation of attention, using more ecologically valid tasks and environments.
To ensure adherence to the protocol, we ensured BrAC readings were comparable between conditions employing alcohol (Alcohol versus Combined) and that prior sleep was comparable between conditions involving restricted sleep (Baseline versus Alcohol; Sleep Restriction versus Combined). As such, our findings were not due to any uncontrolled changes in these factors. In addition, although the tasks themselves induced sleepiness, as reflected by significant changes in pre- and post-KSS test scores, this increase was observed for both ocular motor and PVT test sessions (Figure 2). Therefore, any interpretation of the differential effect of condition on sustained attention (PVT) and selective attention (ocular motor) is not wholly due to an increase in task-induced sleepiness, but likely to altered effects on attention mechanisms combined with reduced vigilance and arousal.
Finally, we address our study limitations. First, this study may be limited by its sample size. Although we powered our study a priori based on data examining braking reactions time,52 our study lost power in post hoc comparisons due to correcting for multiple comparisons. To address this we employed a less conservative correction method (i.e., FDR) and provide unadjusted and adjusted values for data outcomes, where applicable. Second, alcohol exerts a biphasic effect, stimulating arousal on the ascending arm of the BrAC curve and sedating on the descending arm of the BrAC curve.64–66 The timing of our study protocol was designed to ensure testing took place during the soporific descending arm of the BrAC curve. Although this was true for all participants in the combined condition, for the alcohol condition four participants were at peak alcohol rather than on the descending arm when testing commenced. However, because our results indicate a general slowing of attention due to alcohol, rather than arousing, we do not believe this altered the direction of our findings.
In summary, we examined the effect of 5 h of sleep restriction and alcohol intake within legal limits (BrAC 0.05%), alone and combined, on sustained attention and voluntary/ involuntary allocation of attention. Although the combination of sleep restriction and alcohol led to a general slowing of all attentional processes, sleep restriction alone led to enhanced distractibility. This was indicated by reduced ability to ignore a peripheral stimulus on the antisaccade task, and a faster involuntary response to a peripheral target on the visually guided task. As alcohol and drowsiness are leading causes of motor vehicle crashes in Australia and worldwide, these data suggest (1) future work evaluating the effect of sleep loss and legal alcohol intake in ecologically valid, safety critical environments requiring the voluntary control of attention and inhibition of distractors; (2) targeted interventions aimed at reducing distractibility in sleep restricted individuals working in safety-critical occupational settings; and (3) modification of legal limits of alcohol consumption during periods of extended wake or restricted sleep. Although many safety critical tasks including driving involve a combination of complex behaviors, the primary manifestation of a sleep related accident is attentional failure. By further understanding the effect of sleep loss on vigilance/sustained attention, and the voluntary and involuntary allocation of attention, our study provides key evidence for specific signatures of sleep-related attention failure to better inform interventions targeting sleep-related accident risk.
DISCLOSURE STATEMENT
This was not an industry supported study. This study was funded by an investigator-initiated grant from VicRoads. The content is solely the responsibility of the authors and does not necessarily represent the official views of VicRoads. All authors report no conflicts of interest with data as described in this manuscript. Dr. Anderson has received a research award/ prize from Sanofi-Aventis; additional research support from VicRoads; lecturing fees from Brown Medical School/Rhode Island Hospital and Ausmed. She has also received contract research funding from Pacific Brands through an agreement between Monash University and Pacific brands. In addition, she has served as consultant to the Rail, Bus and Tram Union, the National Transport Commission, and the Transport Accident Commission on issues related to fatigue in transportation, and has served as an expert witness on sleep-related motor vehicle crashes to VicPolice. Dr Anderson leads a theme within the Cooperative Research Centre for Alertness, Safety and Productivity which involves working with a consortium of industry, academic and government partners. Dr Fielding has received grant funding from BioGen Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the study participants, the Monash Biomedical Imaging centre for testing facilities, and the Ocular Motor Research Laboratory for equipment and assistance. We are particularly thankful to Dr. Beth Johnson for assistance with the ocular motor tasks, and Ms. Ahuva Segal for aiding with neurobehavioral task programming.
REFERENCES
- 1.Austroads. Road facts 2005: an overview of the Australian and New Zealand road system. Australia: Austroads; 2005. [Google Scholar]
- 2.Garbarino S, Nobili L, Beelke M, De Carli F, Ferrillo F. The contributing role of sleepiness in highway vehicle accidents. Sleep. 2001;24:203–6. [PubMed] [Google Scholar]
- 3.Horne JA, Reyner L. Vehicle accidents related to sleep: a review. Occup Environ Med. 1999;56:289–94. doi: 10.1136/oem.56.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Highway Traffic Safety Administration; 2013. Traffic safety facts 2012 data: alcohol impaired driving. (Accessed at http://wwwnrd.nhtsa.dot.gov/Pubs/811870.pdf.) [Google Scholar]
- 5.Zeckey C, Dannecker S, Hildebrand F, et al. Alcohol and multiple trauma—is there an influence on the outcome? Alcohol. 2011;45:245–51. doi: 10.1016/j.alcohol.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Brady JE, Li G. Prevalence of alcohol and other drugs in fatally injured drivers. Addiction. 2013;108:104–14. doi: 10.1111/j.1360-0443.2012.03993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Knipling RR, Goodman MJ. The role of driver inattention in crashes: new statistics from the 1995 Crashworthiness Data System. 40th Annual Proceedings of the Association for the Advancement of Automotive Medicine; 1996. pp. 377–92. [Google Scholar]
- 8.Martin TL, Solbeck PAM, Mayers DJ, Langille RM, Buczek Y, Pelletier MR. A review of alcohol-impaired driving: the tole of blood alcohol concentration and complexity of the driving task. J Forensic Sci. 2013;58:1238–50. doi: 10.1111/1556-4029.12227. [DOI] [PubMed] [Google Scholar]
- 9.Horne JA, Reyner LA, Barrett PR. Driving impairment due to sleepiness is exacerbated by low alcohol intake. Occup Envir Med. 2003;60:689–92. doi: 10.1136/oem.60.9.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vakulin A, Baulk SD, Catcheside PG, et al. Effects of moderate sleep deprivation and low-dose alcohol on driving simulator performance and perception in young men. Sleep. 2007;30:1327–33. doi: 10.1093/sleep/30.10.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstey KJ, Wood J, Lord S, Walker JG. Cognitive, sensory and physical factors enabling driving safety in older adults. Clin Psychol Rev. 2005;25:45–65. doi: 10.1016/j.cpr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y-C, Ho CH. Effects of different blood alcohol concentrations and post-alcohol impairment on driving behavior and task performance. Traffic Inj Prev. 2010;11:334–41. doi: 10.1080/15389581003747522. [DOI] [PubMed] [Google Scholar]
- 13.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 14.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 15.Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol. 2006;117:1885–901. doi: 10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dongen H, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep–wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res. 2003;12:181–7. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- 17.Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001;139:253–67. [PubMed] [Google Scholar]
- 18.Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: a neurocognitive assay sensitive to sleep loss. In: Kushida C, editor. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. New York: Marcel Dekker; 2005. pp. 39–70. [Google Scholar]
- 19.Dinges DF, Powell J. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods. 1985;17:652–5. [Google Scholar]
- 20.Dinges DF, Pack F, Gillen K, et al. Cumulative effects of acute partial sleep deprivation on PVT Lapses and SSS ratings. Sleep Res. 1994;23:408. [Google Scholar]
- 21.Robertson IH, Garavan H. Vigilant attention. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: The MIT Press; 2004. [Google Scholar]
- 22.Norton R. The effects of acute sleep deprivation on selective attention. Br J Psychol. 1970;61:157–61. doi: 10.1111/j.2044-8295.1970.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson C, Horne JA. Sleepiness enhances distraction during a monotonous task. Sleep. 2006;29:573–6. doi: 10.1093/sleep/29.4.573. [DOI] [PubMed] [Google Scholar]
- 24.Anderson C, Wales AW, Horne JA. PVT lapses differ according to eyes open, closed, or looking away. Sleep. 2010;33:197–204. doi: 10.1093/sleep/33.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bocca M, Marie S, Chavoix C. Impaired inhibition after total sleep deprivation using an antisaccade task when controlling for circadian modulation of performance. Physiol Behav. 2014;124:123–8. doi: 10.1016/j.physbeh.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Salmi J, Huotilainen M, Pakarinen S, Siren T, Alho K, Aronen ET. Does sleep quality affect involuntary attention switching system? Neurosci Lett. 2005;390:150–5. doi: 10.1016/j.neulet.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Versace F, Cavallero C, De Min Tona G, Mozzato M, Stegagno L. Effects of sleep reduction on spatial attention. Biol Psychol. 2006;71:248–55. doi: 10.1016/j.biopsycho.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Casarotti M, Lisi M, Umiltà C, Zorzi M. Paying attention through eye movements: a computational investigation of the premotor theory of spatial attention. J Cogn Neurosci. 2012;24:1519–31. doi: 10.1162/jocn_a_00231. [DOI] [PubMed] [Google Scholar]
- 29.Corbetta M, Akbudak E, Conturo TE, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–73. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 30.Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–6. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- 31.Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22:5081–90. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottleib JP, Kusonoki M, Goldberg ME. The represenation of visual salience in monkey parietal cortex. Nature. 1998;391:481–4. doi: 10.1038/35135. [DOI] [PubMed] [Google Scholar]
- 33.Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J Neurophysiol. 2001;86:2634–7. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- 34.Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78:1574–89. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- 35.Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–50. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- 36.Wardak C, Olivier E, Duhamel J-R. Saccadic target selection deficits after lateral intraparietal area inactivation in monkeys. J Neurosci. 2002;22:9877–84. doi: 10.1523/JNEUROSCI.22-22-09877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–95. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- 38.DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–23. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- 39.Trujillo LT, Kornguth S, Schnyer DM. An ERP examination of the different effects of sleep deprivation on exogenously cued and endogenously cued attention. Sleep. 2009;32:1285–97. doi: 10.1093/sleep/32.10.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koelega HS. Alcohol and vigilance performance: a review. Psychopharmacology. 1995;118:233–49. doi: 10.1007/BF02245951. [DOI] [PubMed] [Google Scholar]
- 41.Fuster JM, Willey TJ, Riley DM, Ashford JW. Effects of ethanol on visual evoked responses in monkeys performing a memory task. Electroencephalogr Clin Neurophysiol. 1982;53:621–33. doi: 10.1016/0013-4694(82)90138-9. [DOI] [PubMed] [Google Scholar]
- 42.Rohrbaugh J, Stapleton J, Parasuraman R, et al. Alcohol intoxication reduces visual sustained attention. Psychopharmacology. 1988;96:442–6. doi: 10.1007/BF02180021. [DOI] [PubMed] [Google Scholar]
- 43.Lamb MR, Robertson LC. Effect of acute alcohol on attention and the processing of hierarchical patterns. Alcohol Clin Exp Res. 1987;11:243–8. doi: 10.1111/j.1530-0277.1987.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 44.Zoethout RWM, Delgado WL, Ippel AE, Dahan A, van Gerven JMA. Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. Br J Clin Pharmacol. 2011;71:331–50. doi: 10.1111/j.1365-2125.2010.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayette MA, Reichle ED, Schooler JW. Lost in the sauce: the effects of alcohol on mind wandering. Psychol Sci. 2009;20:747–52. doi: 10.1111/j.1467-9280.2009.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blekher T, Beard J, O'Connor S, et al. Response of saccadic eye movements to alcohol in African American and non-Hispanic white college students. Alcohol Clin Exp Res. 2002;26:232–8. [PubMed] [Google Scholar]
- 47.Khan SA, Ford K, Timney B, Everling S. Effects of ethanol on anti-saccade task performance. Exp Brain Res. 2003;150:68–74. doi: 10.1007/s00221-003-1400-1. [DOI] [PubMed] [Google Scholar]
- 48.Vorstius C, Radach R, Lang AR, Riccardi CJ. Specific visuomotor deficits due to alcohol intoxication: evidence from the pro-and antisaccade paradigms. Psychopharmacology. 2008;196:201–10. doi: 10.1007/s00213-007-0954-1. [DOI] [PubMed] [Google Scholar]
- 49.Abroms BD, Fillmore MT, Marczinski CA. Alcohol-induced impairment of behavioral control: effects on the alteration and suppression of prepotent responses. J Stud Alcohol. 2003;64:687–96. doi: 10.15288/jsa.2003.64.687. [DOI] [PubMed] [Google Scholar]
- 50.Howard ME, Jackson ML, Kennedy GA, Swann P, Barnes M, Pierce RJ. The interactive effects of extended wakefulness and low-dose alcohol on simulated driving and vigilance. Sleep. 2007;30:1334–40. doi: 10.1093/sleep/30.10.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krull KR, Smith LT, Kalbfleisch LD, Parsons OA. The influence of alcohol and sleep deprivation on stimulus evaluation. Alcohol. 1992;9:445–50. doi: 10.1016/0741-8329(92)90046-d. [DOI] [PubMed] [Google Scholar]
- 52.Banks S, Catcheside P, Lack L, Grunstein RR, McEvoy RD. Low levels of alcohol impair driving simulator performance and reduce perception of crash risk in partially sleep deprived subjects. Sleep. 2004;27:1063–7. doi: 10.1093/sleep/27.6.1063. [DOI] [PubMed] [Google Scholar]
- 53.Vakulin A, Baulk SD, Catcheside PG, et al. Effects of alcohol and sleep restriction on simulated driving performance in untreated patients with obstructive sleep apnea. Ann Intern Med. 2009;151:447–55. doi: 10.7326/0003-4819-151-7-200910060-00005. [DOI] [PubMed] [Google Scholar]
- 54.Barrett PR, Horne JA, Reyner LA. Sleepiness combined with low alcohol intake in women drivers: greater impairment but better perception than men? Sleep. 2004;27:1057–62. doi: 10.1093/sleep/27.6.1057. [DOI] [PubMed] [Google Scholar]
- 55.Barrett PR, Horne JA, Reyner LA. Early evening low alcohol intake also worsens sleepiness-related driving impairment. HumPsychopharmacol. 2005;20:287–90. doi: 10.1002/hup.691. [DOI] [PubMed] [Google Scholar]
- 56.Roehrs T, Beare D, Zorick F, Roth T. Sleepiness and ethanol effects on simulated driving. Alcohol Clin Exp Res. 1994;18:154–8. doi: 10.1111/j.1530-0277.1994.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 57.Anderson C, Horne JA. Driving drowsy also worsens driver distraction. Sleep Med. 2013;14:466–8. doi: 10.1016/j.sleep.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Connor J, Norton R, Ameratunga S, et al. Driver sleepiness and risk of serious injury to car occupants: population based case control study. BMJ. 2002;324:1125. doi: 10.1136/bmj.324.7346.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.NHMRC. Australian alcohol guidelines: health risks and benefits: National Health and Medical Research Council, 2001. Report No: 1864961546. [Google Scholar]
- 60.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 61.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 62.Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology. 2006;43:302–13. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 63.Barrett PR, Horne JA, Reyner LA. Alcohol continues to affect sleepiness related driving impairment, when breath alcohol levels have fallen to near-zero. Hum Psychopharmacol. 2004;19:421–3. doi: 10.1002/hup.601. [DOI] [PubMed] [Google Scholar]
- 64.Earleywine M, Martin CS. Anticipated stimulant and sedative effects of alcohol vary with dosage and limb of the blood alcohol curve. Alcohol Clin Exp Res. 1993;17:135–9. doi: 10.1111/j.1530-0277.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 65.Papineau KL, Roehrs TA, Petrucelli N, Rosenthal LD, Roth T. Electrophysiological assessment (the multiple sleep latency test) of the biphasic effects of ethanol in humans. AlcoholClin Exp Res. 1998;22:231–5. [PubMed] [Google Scholar]
- 66.Pohorecky LA. Biphasic action of ethanol. Biobehav Rev. 1977;1:231–40. [Google Scholar]
- 67.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 68.Theeuwes J, Kramer AF, Hahn S, Irwin DE. Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol Sci. 1998;9:379–85. [Google Scholar]
- 69.Unsworth N, Spillers GJ, Brewer GA, McMillan B. Attention control and the antisaccade task: a response time distribution analysis. Acta Psychologica. 2011;137:90–100. doi: 10.1016/j.actpsy.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Ratcliff R. Group reaction time distributions and an analysis of distribution statistics. Psychol Bull. 1979;86:446–61. [PubMed] [Google Scholar]
- 71.Basner M, Dinges DF. Maximising sensitivity of the psychomotor vigilance test (PVT) to sleep loss. Sleep. 2011;34:581–91. doi: 10.1093/sleep/34.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinclair KL, Ponsford JL, Rajaratnam SM, Anderson C. Sustained attention following traumatic brain injury: use of the Psychomotor Vigilance Task. J Clin Exp Neurophysiol. 2013;35:210–24. doi: 10.1080/13803395.2012.762340. [DOI] [PubMed] [Google Scholar]
- 73.Schwarz G. Estimating the dimension of a model. Ann Stat. 1978;6:461–4. [Google Scholar]
- 74.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodology) 1995;57:289–300. [Google Scholar]
- 75.Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–7. [Google Scholar]
- 76.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 77.Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychol Rev. 1991;2:109–45. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- 78.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 80.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J of Psychiatry. 1993;162:413–9. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 81.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 82.Thomas ML, Sing HC, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- 83.Crevits L, Simons B, Wildenbeest J. Effect of sleep deprivation on saccades and eyelid blinking. Eur Neurol. 2003;50:176–80. doi: 10.1159/000073060. [DOI] [PubMed] [Google Scholar]
- 84.Zils E, Sprenger A, Heide W, Born J, Gais S. Differential effects of sleep deprivation on saccadic eye movements. Sleep. 2005;28:1109–15. doi: 10.1093/sleep/28.9.1109. [DOI] [PubMed] [Google Scholar]
- 85.Brunye TT, Mahoney CR, Lieberman HR, Taylor HA. Caffeine modulates attention network function. Brain Cogn. 2010;72:181–8. doi: 10.1016/j.bandc.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 86.Czeisler CA, Weitzman ED, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–7. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 87.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277:R1152–63. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 88.Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ. EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277:R640–9. doi: 10.1152/ajpregu.1999.277.3.r640. [DOI] [PubMed] [Google Scholar]
- 89.Ridderinkhof KR, van den Wildenberg WP, Wijnen J, Burle B. Response inhibition in conflict tasks is revealed in delta plots. Cogn Neurosci Attention. 2004:369–77. [Google Scholar]
- 90.Roehrs T, Burduvali E, Bonahoom A, Drake C, Roth T. Ethanol and sleep loss: a “dose” comparison of impairing effects. Sleep. 2003;26:981–5. doi: 10.1093/sleep/26.8.981. [DOI] [PubMed] [Google Scholar]
- 91.Quillian WC, Cox DJ, Kovatchev BP, Phillips C. The effects of age and alcohol on simulated driving performance, awareness, and self restraint. Age Ageing. 1999;28:59–66. doi: 10.1093/ageing/28.1.59. [DOI] [PubMed] [Google Scholar]
- 92.Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–74. [PMC free article] [PubMed] [Google Scholar]
- 93.Wang GJ, Volkow ND, Franceschi D, et al. Regional brain metabolism during alcohol intoxication. Alcohol Clin Exp Res. 2000;24:822–9. [PubMed] [Google Scholar]
- 94.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pilcher JJ, Band D, Odle-Dusseau HN, Muth ER. Human performance under sustained operations and acute sleep deprivation conditions: toward a model of controlled attention. Aviat Space Environ Med. 2007;78:B15–24. [PubMed] [Google Scholar]
- 96.Harrison Y, Horne JA, Rothwell A. Prefrontal neuropsychological effects of sleep deprivation in young adults--a model for healthy aging? Sleep. 2000;23:1067–73. [PubMed] [Google Scholar]
- 97.Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7:95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 98.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 99.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–29. doi: 10.1055/s-2005-867080. [DOI] [PubMed] [Google Scholar]
- 101.Balkin TJ, Rupp T, Picchioni D, Wesensten NJ. Sleep loss and sleepiness: current issues. Chest. 2008;134:653–60. doi: 10.1378/chest.08-1064. [DOI] [PubMed] [Google Scholar]