Abstract
Study Objectives:
The aim of the study was to determine the association of objectively measured sleep disordered breathing (SDB) with incident coronary heart disease (CHD) or heart failure (HF) in a nonclinical population.
Design:
Longitudinal analysis of a community-dwelling cohort followed up to 24 y.
Setting:
Sleep laboratory at the Clinical Research Unit of the University of Wisconsin Hospital and Clinics.
Participants:
There were 1,131 adults who completed one or more overnight polysomnography studies, were free of CHD or HF at baseline, were not treated by continuous positive airway pressure (CPAP), and followed over 24 y.
Interventions:
None.
Measurements and Results:
In-laboratory overnight polysomnography was used to assess SDB, defined by the apnea-hypopnea index (AHI) using apnea and hypopnea events per hour of sleep. Incident CHD or HF was defined by new reports of myocardial infarction, coronary revascularization procedures, congestive heart failure, and cardiovascular deaths. We used baseline AHI as the predictor variable in survival analysis models predicting CHD or HF incidence adjusted for traditional confounders. The incidence of CHD or HF was 10.9/1,000 person-years. The mean time to event was 11.2 ± 5.8 y. After adjusting for age, sex, body mass index, and smoking, estimated hazard ratios (95% confidence interval) of incident CHD or HF were 1.5 (0.9–2.6) for AHI > 0–5, 1.9 (1.05–3.5) for AHI 5 ≤ 15, 1.8 (0.85–4.0) for AHI 15 ≤ 30, and 2.6 (1.1–6.1) for AHI > 30 compared to AHI = 0 (P trend = 0.02).
Conclusions:
Participants with untreated severe sleep disordered breathing (AHI > 30) were 2.6 times more likely to have an incident coronary heart disease or heart failure compared to those without sleep disordered breathing. Our findings support the postulated adverse effects of sleep disordered breathing on coronary heart disease and heart failure.
Citation:
Hla KM, Young T, Hagen EW, Stein JH, Finn LA, Nieto FJ, Peppard PE. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. SLEEP 2015;38(5):677–684.
Keywords: coronary heart disease, heart failure, sleep disordered breathing
INTRODUCTION
Sleep disordered breathing (SDB), characterized by repeated episodes of airway obstruction during sleep, is reportedly increasing in prevalence commensurate with the obesity epidemic.1,2 SDB is associated with independent risk factors for coronary heart disease (CHD) including prevalent and incident hypertension, nocturnal nondipping of blood pressure, and diabetes mellitus.3–5 An association between SDB and CHD has been reported in several observational and clinic-based cross-sectional studies.6,7 However, studies on the incidence of new CHD among individuals with SDB in nonclinical populations have shown variable results.8–16 In the largest longitudinal study to date, the Sleep Heart Health Study, SDB was associated with incident cardiovascular disease among men 40–70 y old with the most severe SDB (apnea-hypopnea index [AHI] ≥ 30). The association was significant for incident heart failure but not for CHD and was seen only in men.9 A recent systematic review and meta-analysis of incident CHD studies showed an overall trend but not conclusive evidence for an association of incident cardiovascular disease with SDB.17 The meta-analysis result was limited by marked heterogeneity between studies likely caused by differences in study populations, outcome measurements, adjustment for varied confounders, and length of follow-up, warranting recommendation by the authors for further high-quality evidence to determine causality in the association between SDB and cardiovascular disease.17,18 A recent study investigating the role of obstructive sleep apnea (OSA) and CPAP therapy on the incidence of stroke or CHD in women reported that untreated sleep apnea is associated with increased incidence of stroke, and that adequate continuous positive airway pressure (CPAP) treatment reduced the risk.18 We investigated the association between baseline SDB status and new CHD or heart failure incidence among participants followed up to 24 y in the Wisconsin Sleep Cohort Study to further examine the hypothesis that SDB is an independent risk factor for CHD and heart failure in middle-aged men and women.
METHODS
Sample
The Wisconsin Sleep Cohort was established in 1988 as a prospective, population-based study of predictors, outcomes, and natural history of SDB.19,20 Protocols and informed consent documents were approved by the University of Wisconsin Health Sciences Institutional Review Board. In brief, 30- to 60-y-old men and women living in south-central Wisconsin were selected from payroll records of Wisconsin state agencies with job classifications ranging from unskilled to professional. Of the 2,940 individuals invited to undergo a baseline overnight in-laboratory protocol, 1,546 (53%) participated. Compared to the entire sampling frame, cohort participants had a slightly healthier profile and lower death rate.3,19,20
The participants included in the current analysis (n = 1,280) were those who, at the time of the baseline polysomnography study, had not experienced the CHD and heart failure events under investigation, and had at least one polysomnography study for assessment of baseline sleep apnea severity and, thereafter, provided sufficient data to assess CHD and heart failure outcomes subsequent to baseline sleep apnea evaluation. Incident CHD and heart failure information was obtained from self-reported detailed health history questionnaires regarding specific types of physician-diagnosed CHD and heart failure, date of diagnosis, as well as medications or other treatments during: (1) one or more follow-up in-laboratory polysomnography studies scheduled at 4-yearly intervals; and/or (2) mailed health surveys querying all cardiovascular outcomes; and/or death records searches. Health surveys were mailed to all participants in 2008 and during 2010–2013. Deaths were assessed in 2013, as described in the following paragraphs.
Baseline SDB Assessment
Participants underwent a baseline overnight 18-channel polysomnography (Grass model 78; Quincy, MA) at the University of Wisconsin Clinical Research Unit using a standard protocol. Polysomnography-recorded sleep state using electroencephalography, electrooculography, and electromyography; breathing, using respiratory inductance plethysmography (Respitrace; Ambulatory Monitoring, Ardsley, NY); nasal and oral airflow (ProTec thermocouples; Hendersonville, TN); and oxyhemoglobin saturation, using pulse oximetry (Ohmeda Biox 3740, Englewood, CO). Each 30-sec epoch of the polysomnographic recordings was scored for apnea and hypopnea events and sleep stage by trained technicians and reviewed using standard criteria.21–23 Apnea was defined as cessation of nasal and oral airflow for ≥ 10 sec and hypopnea as a discernible reduction in breathing (sum of chest and abdominal excursions) with a reduction in oxyhemoglobin saturation of ≥ 4%.
The AHI, our summary measure of SDB severity, was calculated as the mean number of apnea and hypopnea events per hour of sleep. AHI was categorized according to widely used cutoff points in the literature and clinical practice: normal (AHI = 0), minimal SDB (AHI > 0 to ≤ 5); mild SDB (AHI 5 to ≤ 15); moderate SDB (AHI 15 to ≤ 30); and severe SDB (AHI ≥ 30).19 In addition to SDB severity characterized by the AHI, we also examined the association between percent of sleep time with oxyhemoglobin saturation below 90% and incident CHD or heart failure for participants who had this parameter available from baseline polysomnography studies (n = 740).
CHD and Heart Failure Outcome Assessment
Health history questionnaires provided at the time of polysomnography studies and mailed health surveys included items on cardiovascular disease outcomes, and requested a detailed history of the outcomes including specific cardiovascular disease event and the dates of diagnosis by physician and/or hospitalizations and treatments, if any. Incident CHD and heart failure were defined by: (1) new reports by the participants of any of the following events: myocardial infarction, coronary revascularization procedures, coronary artery bypass graft surgery, percutaneous coronary intervention with angioplasty and/or stent placement, internal cardiac defibrillator placement, congestive heart failure, and; (2) cardiovascular death from death records searches (as determined in the following paragraphs). We further evaluated self-reported CHD and heart failure history by determining the consistency of the reported events at subsequent 4-yearly follow-up visits as well as in the additional health surveys sent in 2008 and 2010–2013. Any inconsistencies were adjudicated by the study physician (KMH), who was unaware of the participants' SDB status. Among those with inconsistencies, i.e., having inconsistent answers of occurrence of CHD or heart failure events from first report to subsequent reports in consecutive follow-up visits, we found six participants who checked “yes” for CHD or heart failure but were adjudicated as not to have CHD or heart failure, and another six who checked “no” for CHD or heart failure who were adjudicated to have CHD or heart failure based on other evidence from reviewing medications and additional written-in comments. We verified the self-reported diagnoses by reviewing if participants were on medications typically used for CHD and heart failure such as beta-blockers, calcium channel blockers, nitrates, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and loop diuretics.
Deaths in the cohort occurring up to May 2013 were identified by matching Social Security numbers with two death record sources: the National Death Index (NDI) and the Wisconsin State Bureau of Health Information and Policy, Vital Records Section. Matches on Social Security number were verified with participants' age and sex. All deaths in Wisconsin, reported by the vital records of Wisconsin, were also identified in the NDI; in addition, deaths occurring outside of Wisconsin were identified by the NDI. Date of death was available on all decedents, and underlying and contributory causes of deaths were available from Wisconsin Vital Statistics. Wisconsin Vital Statistics and the NDI supplied files with data on each individual death, including the cause of death and corresponding description as abstracted from individual death certificates. For all deaths, cause of death was further ascertained and adjudicated by examining each death certificate for primary cause, secondary cause, and underlying conditions by the study physician (KMH) who was blinded to the participants' SDB status.
Covariate Assessment
Trained personnel administered standardized interviews and objective clinical assessments. Information used for this analysis included medication use, alcohol consumption (drinks/week), cigarette smoking (current, former, or never), self-reported hours of weekly physical exercise, usual sleep duration, history of physician-diagnosed diabetes, measured blood pressure, history of physician-diagnosed sleep apnea, and use of CPAP treatment. Body mass index (BMI; weight in kilograms divided by height in meters squared), waist circumference, and age (years), total cholesterol and high-density lipoprotein (HDL) cholesterol at baseline were used as continuous variables.
Statistical Analysis
SAS software (SAS Institute, Inc. Cary, NC, Version 9.1.3) was used. Total number of person-years were accumulated from baseline study to date of incident CHD or heart failure or date of last follow-up. We computed incident CHD or heart failure rates (events/1,000 person-years) and 95% Fisher exact confidence intervals (95% CI) by SDB categories and continuous SDB severity parameterizations at baseline. Cox proportional hazards regression models were used to estimate adjusted hazard ratios (HRs) and 95% CIs using SAS PHREG software. Trends were examined by testing the significance of SDB categories as a linear term and by using log base 2 (AHI+1). Because of the strong dependence of CHD risk on age, Cox regression models were based on age as the time scale, allowing for left truncation (late entry).23 In the regression models, we examined sex, BMI, smoking, alcohol use, HDL, total cholesterol, physical activity, diabetes, hypertension, and sleep duration as potential confounding and/or mediating factors. Interactions of SDB with age, sex, BMI and diabetes in predicting incident CHD or heart failure were examined. In addition to examining associations of baseline SDB severity with CHD or heart failure outcomes, we also performed time-varying exposure regression modeling that included follow-up SDB and covariate assessments to predict CHD or heart failure incidence. In analyses examining baseline SDB severity as a predictor of incident CHD or heart failure, participants who used CPAP devices during baseline polysomnography (n = 9) were also included in the severe SDB category (AHI ≥ 30); sensitivity analyses excluding these subjects were also performed. We examined associations between SDB and CHD or heart failure in the subset of participants (n = 1,131) who were never treated with CPAP—i.e., at baseline or any subsequent assessments. We also analyzed the associations of SDB with incident CHD or heart failure in the entire sample (n = 1,280) including 149 participants who were treated with CPAP at baseline or at follow-up visits.
We further analyzed models examining the association of SDB with incident CHD only (heart failure events were not counted as events in these analyses) in the untreated subset (n = 1,130) as well as in the entire sample including those treated with CPAP (n = 1,279) to examine the effect of CHD only, because self-reports of heart failure might be not as accurate as those of coronary heart disease events.
RESULTS
Participant Characteristics
Characteristics of all study participants (n = 1,280) are described in Table 1. Total person-years observed was 17,978. Approximately one in four participants had at least mild SDB: 14% had mild SDB (AHI 5 to ≤ 15), 5% had moderate SDB (AHI 15 to ≤ 30) and 4% had severe SDB (AHI ≥ 30). The mean age of the participants was 47 y and mean BMI was 30. Participants with SDB were more likely to be male, older, and have higher BMI, hypertension, and diabetes mellitus.
Table 1.
Baseline characteristics of all participants categorized by apnea-hypopnea index severity (n = 1,280).

Incident CHD or Heart Failure, Types and Percent
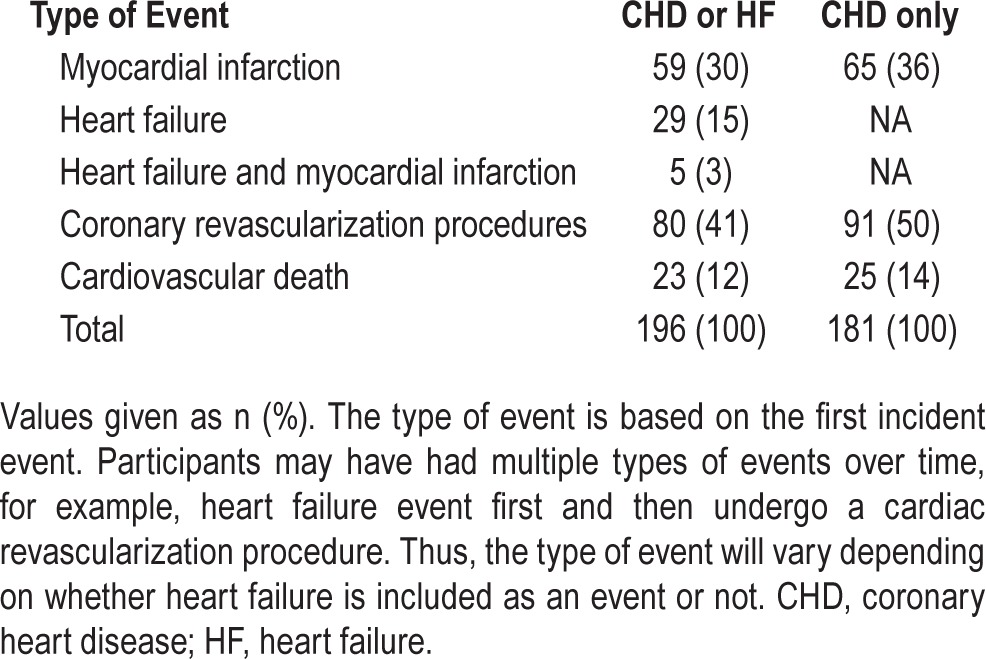
As shown in Table 2, 196 participants experienced first-time CHD or heart failure events during follow-up; this corresponded to an incidence of 10.9 per 1,000 person-years. The majority of incident events reported were myocardial infarction (30%) or coronary artery revascularization procedures including percutaneous coronary interventions (angioplasty and stent placements) and coronary artery bypass graft surgery (41%). Another 15% of events were diagnoses of heart failure and 12% were CHD or heart failure related cardiovascular deaths. One hundred eighty-one events were incident CHD only.
Table 2.
Type of incident event in the combined coronary heart disease or heart failure group and in coronary heart disease-only group.
Association of SDB and Incident CHD or Heart Failure
The unadjusted incidence of combined CHD or heart failure events was 7.1%, 14.6%, 23.1%, 27.5%, and 43.5% in the groups with AHI = 0, AHI > 0 to ≤ 5, AHI 5 to ≤ 15, AHI 15 to ≤ 30, and AHI ≥ 30, respectively, among participants who were not treated with CPAP (n = 1,131) (Table 3). With adjustment for age and sex only, there was a significant association between SDB and increased incidence of CHD or heart failure across all SDB categories, with the highest HR in the AHI ≥ 30 category at 5.4. After further adjusting for BMI and smoking history, the association of SDB with incident CHD or heart failure still showed a significant trend (P = 0.02): HRs (95% CI) were 1.5 (0.9–2.6), 1.9 (1.05–3.5), 1.8 (0.85–4.0) and 2.6 (1.1–6.1) in the minimal, mild, moderate and severe SDB categories, respectively, compared to no SDB. Using Log2AHI [modeled by Log base 2 (AHI+1)], a two-fold increase in AHI was associated with a 1.14-fold (1.03–1.27) higher risk of CHD or heart failure (P = 0.01).
Table 3.
The association of untreated sleep disordered breathing with incident coronary heart disease or heart failure events.
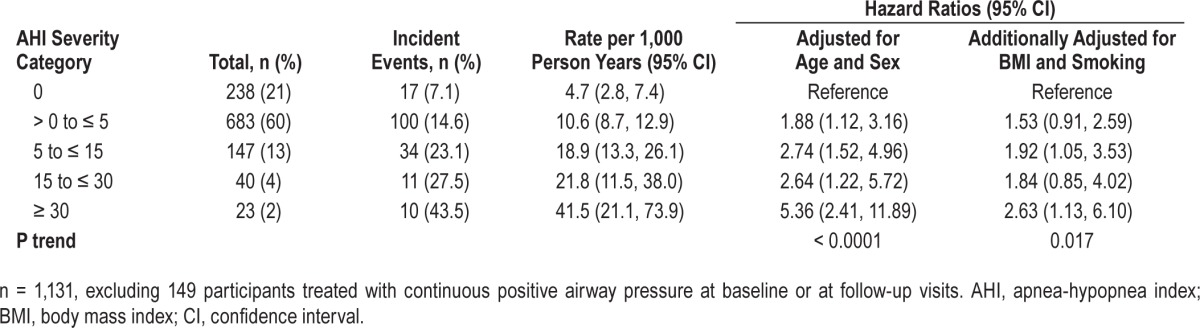
After further adjustment for duration of sleep, a significant trend (P = 0.03) remained. We also added variables for total cholesterol and HDL cholesterol to the model and the HRs for severe SDB remained significant (data not shown).
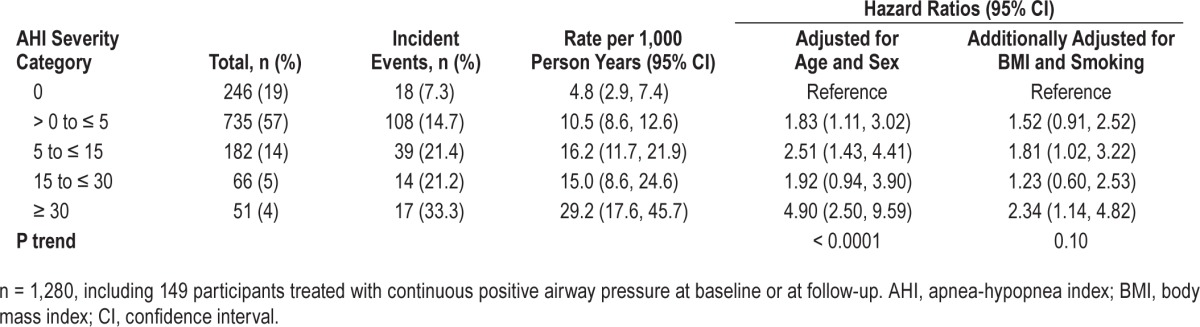
As shown in Table 4, when all participants, including 149 participants who were treated with CPAP or wore the CPAP on the night of the studies, were included (entire sample n = 1,280), the HR for severe SDB predicting incident CHD or heart failure remained significant (HR = 2.3: 95% CI: 1.1–4.8), although the P value for trend in the HR was not (P = 0.1). Using log2AHI, a twofold increase in AHI was associated with 1.10-fold (1.00–1.20) higher risk of CHD or heart failure (P = 0.055). These findings that included participants treated with CPAP are consistent with an expected small underestimation of the association due to potential beneficial effect of CPAP on CHD or heart failure risk in persons with treated SDB.
Table 4.
The association of sleep disordered breathing with incident coronary heart disease or heart failure events in all participants (including participants treated with continuous positive airway pressure).
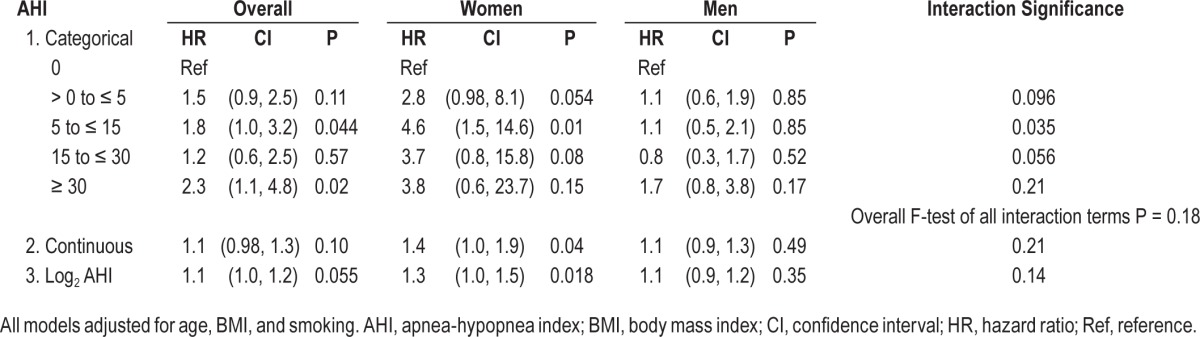
We further analyzed the association of SDB with incident CHD or heart failure stratified by sex in all participants (Table 5). Among 587 women, 57 experienced incident CHD or heart failure events; of 693 men, 139 men experienced incident CHD or heart failure events. The F-test for an overall sex-SDB severity interaction was not significant (P = 0.18).
Table 5.
Association between sleep disordered breathing and incident coronary heart disease or heart failure stratified by sex in all participants (n = 1,280).
Examination of oxyhemoglobin desaturation levels in 740 participants showed a consistent association—more time spent below 90% saturation was associated with higher hazards of incident CHD or heart failure, but the association did not reach statistical significance (P = 0.11). We also examined time-varying SDB exposure models that incorporated both baseline and subsequent, postbaseline SDB assessments. These models did not show a significant association with incident CHD or heart failure (data not shown).
To evaluate whether the association between SDB and CHD or heart failure may be mediated, in part, by elevated blood pressure or metabolic dysregulation, we examined models that additionally adjusted for hypertension and diagnosed diabetes mellitus. If either or both conditions act as intermediary mechanisms by which SDB affects CHD risk, then adjustment for these factors would be expected to attenuate SDB-heart disease associations. When we added hypertension and diabetes mellitus to the baseline SDB model, the HR for incident CHD or heart failure for AHI ≥ 30 was decreased to 1.65 (0.69, 3.98) (P trend = 0.13), suggesting that some of the relationship between SDB, CHD, and heart failure may be mediated or confounded (or both) by hypertension and diabetes mellitus. We also investigated physical activity as a potential confounder for the SDB-heart disease association but found no evidence of confounding. All examined interactions of SDB with age, sex, BMI, and diabetes were not statistically significant.
Association of SDB with Incident CHD Only
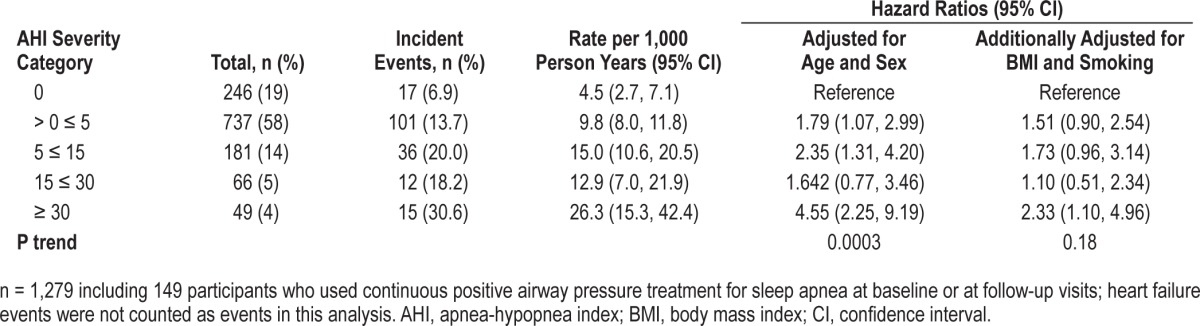
Because the self-reported history of congestive heart failure, compared to CHD, may differ in accuracy, we analyzed the association of incident CHD only, as shown in Table 6. The association of SDB with incident CHD only in untreated SDB (excluding the 149 participants who used CPAP) showed a similar but less significant trend (P = 0.06): the HR for incident CHD in the fully adjusted model was 2.4 (0.99–6.0) for the AHI ≥ 30 category. Using Log2 AHI, a twofold increase in AHI was associated with a 1.11-fold (1.00–1.24) higher risk of CHD in untreated sample (P = 0.053). This association remained significant using the entire sample (n = 1,279), including the 149 participants who were treated or used CPAP: HR was 2.3 (1.1–5.0) for the association between severe SDB and incident CHD (P trend = 0.17) (Table 7). Using log2 AHI, a twofold increase in AHI was associated with a 1.08-fold (0.98–1.19) higher risk of CHD in the entire sample (P = 0.14).
Table 6.
The association of untreated sleep disordered breathing with incident coronary heart disease events only.
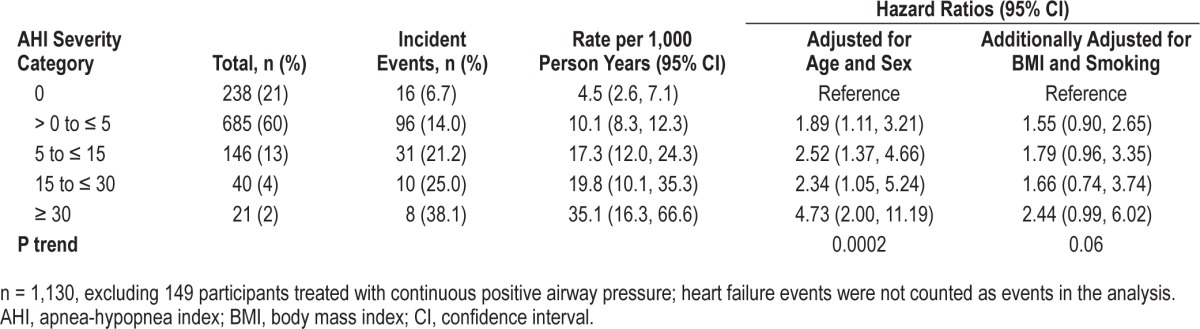
Table 7.
The association of sleep disordered breathing with incident coronary heart disease events only in all participants (including participants treated with continuous positive airway pressure).
DISCUSSION
In our prospective, population-based cohort study of middle-aged women and men, followed up to 24 y, SDB was associated with incident CHD or heart failure after adjusting for age, sex, BMI, and smoking. The association was strongest in the most severe SDB category and remained significant after further controlling for sleep duration, total cholesterol, and HDL cholesterol.
When we controlled for hypertension and diabetes mellitus, the HRs for incident CHD or heart failure no longer achieved statistical significance but trends remained evident. This is consistent with hypertension and diabetes mellitus acting as (partial) mediating factors on a pathway between SDB and CHD or heart failure. It is also plausible that there may be confounding by these factors (or acting as proxies of other confounders that were unmeasured or measured with error); however, there is considerably more evidence consistent with SDB—and disturbed sleep in general—as a cause of hyper-tension and metabolic dysregulation than for hypertension or diabetes mellitus (independent of included covariates such as age and BMI) as causes of SDB.24,25
Our oxyhemoglobin saturation data showed a consistent trend of higher hazards of incident heart disease with more time spent below 90% oxyhemoglobin saturation, but was not statistically significant, likely because of a smaller sample size of participants for which this parameter was calculable at baseline (n = 740). We did not see an association between SDB burden over time in our time-varying exposure models and it is possible that much of the relevant SDB cumulative exposure had already occurred at the time of baseline study for some of our middle-aged adult participants with more severe SDB. An additional explanation of baseline SDB being more predictive of incident heart disease than later SDB assessments is that subjects are more likely to have been medically treated for cardiovascular pathology during the more recent SDB assessments, thus unlinking associations of heart disease with traditional heart disease risk factors, including SDB.
A recent systematic review and meta-analysis of the association of OSA with serious cardiovascular disease events was published based on nine prospective studies done in patients with OSA who had no history of recent myocardial infection or stroke at baseline and had a longitudinal follow up of more than 1 y by Loke et al.17 This analysis showed that OSA appears to be associated with stroke, but the relationship with ischemic heart disease and cardiovascular mortality was not consistent and the authors proposed the need for further research.17 The authors suggested that inconclusive results were possibly due to marked heterogeneity among the mostly clinic-based study population as well as variations in their measurements, duration of follow-up, and definitions of OSA and cardiovascular disease.8–16 Our findings are consistent with other population-based studies showing a significant but weaker HR for the association between SDB and incident CHD compared to those reported from sleep laboratory clinic-based studies. Our data are similar to the only other prospective community-based study to date, the Sleep Heart Health Study (SHHS), which showed an increased incidence of CHD and heart failure among subjects with the most severe SDB.9 However, we found a stronger association than that of SHHS (HR of 2.6 versus 1.13), and, unlike the SHHS findings, our observation of increased incidence of CHD or heart failure was not restricted to heart failure only; we found an increased incidence of CHD or heart failure as well as CHD only in our participants with severe SDB. Also, in contrast to SHHS data, our data showed a trend for a higher association between SDB and incident CHD in women than in men. One important difference between the two studies is that the SHHS study population is older on average than our cohort (and, thus, more cases of heart disease had to be excluded because they were already present at baseline) and the apnea events were more frequent in the younger age group, possibly indicating a survivor bias seen in the older participants—a bias that may differentially affect men and women. Additionally, the referent groups differed between the two studies; SHHS included those with AHI < 5 as their referent group, while we used those with AHI = 0 as the referent group. This difference may partly explain the higher HR in our study.
In our study, the association of SDB with incident CHD or heart failure was attenuated when participants who reported using CPAP were included in the analyses (Tables 4 and 7). If SDB among treated subjects was treated effectively by CPAP, their CHD and heart failure risk may have been lowered and including them among participants with untreated severe SDB could have underestimated an association between SDB and incident CHD or heart failure. Our results showing that the association between SDB and CVD is stronger when CPAP users were excluded support the idea that risk for CHD and heart failure is lower among those treated for SDB.
Our results support the hypothesis that SDB is an independent risk factor for CHD and heart failure. It has been speculated that SDB affects CHD and heart failure via several pathophysiologic mechanisms that arise from the repeated episodes of nocturnal airway obstruction: increased ventilatory effort, negative intrathoracic pressures, sleep fragmentation and arousals with resultant increased sympathetic activity, intermittent hypoxia during apneic and hypoxic episodes, and increased vasoreactivity and oxidative stresses, which may all lead to endothelial dysfunction, vascular inflammation, accelerated atherosclerosis, and resultant CHD and heart failure.26 A recently published community study also suggests that SDB is independently associated with subclinical myocardial injury as measured by high-sensitivity troponin T; they found that high-sensitivity troponin T was associated with risk of death or incident heart failure in all SDB categories in a large cohort of community-dwelling older adults with SDB and cardiovascular disease.27
The strengths of our study include a long-term follow up of over 1,000 community-dwelling participants who were studied using overnight full polysomnography, detailed health history questionnaires at baseline and at regular follow-up intervals, allowing tracking of incident cases of CHD and heart failure, and a complete search of in-state and out-of-state death certificates.
One limitation of our study is the assessment of CHD and heart failure outcomes based on self-reported data. However, we took several approaches to enhance the accuracy of the outcomes information. We ascertained the data from detailed health history questionnaires including the dates of first occurrence of physician-diagnosed CHD and heart failure, medications during in-laboratory follow-up visits repeated at four yearly intervals and additional reporting of CHD and heart failure incidence collected via mailed surveys between 2008 and 2013. We required that the initial self-report of events be consistently reported in all subsequent questionnaires obtained at follow-up visits and mailed surveys. We also cross-checked the new CHD and heart failure events reported with initiation of medications typically used for CHD and heart failure. Inconsistencies were adjudicated and reclassified using participants' other supportive documentation and additional comments written on the questionnaire, in addition to information on the questionnaire about type and date of event occurrence, hospitalization date, cardiac catheterization or surgery dates, as well as correlation with initiation of medications such as loop diuretics, angiontensin-converting enzyme inhibitors, and beta-blockers. Adjudication was performed by the study physician (KMH) blinded to the AHI category and SDB status of the participants. It is also notable that large-sample validation studies (self-report compared to chart review) have found self-report of physician-diagnoses of some cardiovascular outcomes (myocardial infarction and stroke) to be highly specific and moderately sensitive measures.28,29 In these validation studies, self-reported events were not checked for consistency over time as we did in our study. Misclassification of cardiovascular outcomes with high specificity will tend to produce conservative underestimation of associations. Because CHD but not heart failure self-report measures have been shown to have relatively good specificity and sensitivity, we analyzed our self-reported incident data using CHD only as well as with heart failure and found that incident CHD only as well as incident CHD or heart failure were both significantly associated with severe SDB.
Another related limitation of our study is the potential misclassification of causes of death using death certificate information. To reduce the occurrence of this possible misclassification, each death certificate was carefully examined and cause of death adjudicated by the study physician blinded to the decedents' SDB status.
Finally, our study is limited in its power to detect associations among subjects with severe SDB because of the low number of participants in this category. However, the trend for increased incidence across the severity spectrum was significant. Furthermore, the log AHI analysis also confirmed that continuous AHI was associated with increased risk of CHD in participants with untreated SDB (P = 0.05).
Our study results add to the mounting evidence that SDB is associated with incident CHD and heart failure or its precursors. Given recent evidence that effective SDB treatment, such as CPAP, may improve cardiovascular outcomes,30–33 it is important for clinicians to be vigilant not only in the early diagnosis and management of SDB, but also in motivating their patients with severe SDB to adhere to SDB treatment regimens for possible primary prevention of CHD and heart failure.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the National Heart, Lung, and Blood Institute (grant R01HL62252), National Institute of Aging (grants 1R01AG036838 and R01AG14124), and the National Center for Research Resources (grant 1UL1RR025011) at the National Institutes of Health. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the technical expertise of Amanda Rasmuson, Nicole Salzieder, Kathryn Pluff, Kathy Stanbeck, Robin Stubbs, Eric Young, and Mary Sundstrom. The authors are also grateful for Patty Boyle's expert assistance with manuscript preparation.
Footnotes
A commentary on this article appears in this issue on page 659.
REFERENCES
- 1.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep. 2008;31:795–800. doi: 10.1093/sleep/31.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arzt M, Young T, Peppard PE, et al. Dissociation of obstructive sleep apnea from hypersomnolence and obesity in patients with stroke. Stroke. 2010;41:e129–34. doi: 10.1161/STROKEAHA.109.566463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endeshaw YW, Bloom HL, Bliwise DL. Sleep-disordered breathing and cardiovascular disease in the Bay Area Sleep Cohort. Sleep. 2008;31:563–8. doi: 10.1093/sleep/31.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin CM, Bell IR, Guerra S, Quan SF. Obstructive sleep apnea and ischemic heart disease in southwestern US veterans: implications for clinical practice. Sleep Breath. 2005;9:111–8. doi: 10.1007/s11325-005-0025-y. [DOI] [PubMed] [Google Scholar]
- 8.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 12.Munoz R, Duran-Cantolla J, Martinez-Vila E, et al. Severe sleep apnea and risk of ischemic stroke in the elderly. Stroke. 2006;37:2317–21. doi: 10.1161/01.STR.0000236560.15735.0f. [DOI] [PubMed] [Google Scholar]
- 13.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 14.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apneahypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14:131–6. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 16.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 17.Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5:720–8. doi: 10.1161/CIRCOUTCOMES.111.964783. [DOI] [PubMed] [Google Scholar]
- 18.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nunez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and CPAP therapy in the incidence of stroke or coronary heart disease in women. Am J Respir Crit Care Med. 2014;189:1544–50. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 19.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 21.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 22.Rechtscahaffen A, Kales AA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 23.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 24.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 25.Varounis C, Katsi V, Kallikazaros IE, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: a systematic review and meta-analysis. Int J Cardiol. 2014;175:195–8. doi: 10.1016/j.ijcard.2014.04.240. [DOI] [PubMed] [Google Scholar]
- 26.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Querejeta Roca G, Redline S, Punjabi N, et al. Sleep apnea is associated with subclinical myocardial injury in the community. The ARIC-SHHS study. Am J Respir Crit Care Med. 2013;188:1460–5. doi: 10.1164/rccm.201309-1572OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women's Health Initiative. Am J Epidemiol. 2004;160:1152–8. doi: 10.1093/aje/kwh314. [DOI] [PubMed] [Google Scholar]
- 29.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Bazzano LA, Khan Z, Reynolds K, He J. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 31.Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757–64. doi: 10.1001/archinte.167.8.757. [DOI] [PubMed] [Google Scholar]
- 32.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127:2076–84. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 33.Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, Shekelle P. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471–83. doi: 10.7326/0003-4819-159-7-201310010-00704. [DOI] [PubMed] [Google Scholar]


