Abstract
Background and Study Objectives:
A relationship between sleep and seizures is well-described in both humans and rodent animal models; however, the mechanism underlying this relationship is unknown. Using Drosophila melanogaster mutants with seizure phenotypes, we demonstrate that seizure activity can be modified by sleep deprivation.
Design:
Seizure activity was evaluated in an adult bang-sensitive seizure mutant, stress sensitive B (sesB9ed4), and in an adult temperature sensitive seizure mutant seizure (seits1) under baseline and following 12 h of sleep deprivation. The long-term effect of sleep deprivation on young, immature sesB9ed4 flies was also assessed.
Setting:
Laboratory.
Participants:
Drosophila melanogaster.
Interventions:
Sleep deprivation.
Measurements and Results:
Sleep deprivation increased seizure susceptibility in adult sesB9ed4/+ and seits1 mutant flies. Sleep deprivation also increased seizure susceptibility when sesB was disrupted using RNAi. The effect of sleep deprivation on seizure activity was reduced when sesB9ed4/+ flies were given the anti-seizure drug, valproic acid. In contrast to adult flies, sleep deprivation during early fly development resulted in chronic seizure susceptibility when sesB9ed4/+ became adults.
Conclusions:
These findings show that Drosophila is a model organism for investigating the relationship between sleep and seizure activity.
Citation:
Lucey BP, Leahy A, Rosas R, Shaw PJ. A new model to study sleep deprivation-induced seizure. SLEEP 2015;38(5):777–785.
Keywords: sleep homeostasis, Drosophila melanogaster, seizure
INTRODUCTION
Sleep and seizure have been associated in humans since antiquity1; however, the mechanism underlying this relationship is unknown. Evidence of a link between sleep and seizure in humans is primarily suggested by observational data. Sleep deprivation, for instance, activates paroxysmal epilepti-form activity during routine electroencephalography (EEG).2,3 Moreover, treatment of obstructive sleep apnea in patients with epilepsy has been observed to improve seizure control without alteration of anticonvulsant medications or doses.4,5 Patients with epilepsy are also observed to have increased sleepiness, although the etiology is most likely multifactorial from seizure frequency, medications, or other causes.6 Understanding the relationship between sleep deprivation and seizure may provide insights into potential new antiseizure therapies and the role of sleep in epilepsy.
Drosophila melanogaster is a powerful model organism that has been successfully used to study human physiologic processes such as sleep and disease states such as epilepsy.7–10 However, the relationship between sleep and seizure in Drosophila mutants with a behavioral seizure phenotype is unknown. Research into sleep and the fruit fly has shown not only that flies sleep but that data obtained in the fly can be applied directly to humans. Biomarkers of sleepiness first identified in the fly, for instance, have also been found to be elevated in healthy human subjects after prolonged waking.11–15 Interestingly, there have been no prior investigations into the effect of sleep deprivation on the seizure phenotype in any Drosophila seizure mutants.
Drosophila mutants with a phenotype of seizure-like activity induced in response to both mechanical and temperature stress have been described and are a validated animal model to investigate molecular and cellular networks responsible for seizure phenotypes.16 Mechanical and temperature stress-induced seizures exhibit several features similar to seizures in humans, such as a stereotyped behavioral sequence of spasm-and-paralysis followed by a refractory period when the mutant flies are no longer sensitive to their respective stress disturbance. Further, evidence supports an electrical and neural basis for the stereotyped behavior. An electroconvulsive stimulus across the Drosophila brain replicated similar, stereotyped seizure behavior in multiple bang-sensitive mutants, including bang sensitive and bang senseless. In that study, wild-type Canton-S (Cs) flies showed the same behavioral seizure event in response to electrical stimuli, although of short duration and requiring a greater stimulus.17 Decapitation has also lead to decreased sensitivity to mechanical and temperature stress in bang-sensitive mutants.16 Genetic mutations in a number of ion channels have been found to cause both human epilepsy and Drosophila mutants with a seizure phenotype: potassium channel (humans: KCNA1, poor forming alpha subunit18; Drosophila: seizure, a erg voltage-gated potassium channel19) and sodium channels (humans: SCN1A, a voltage-gated sodium channel20; Drosophila: para, voltage-gated sodium channel21). Finally, Drosophila seizure mutants have also been shown to respond to drugs used in humans to treat epilepsy with decreased seizure duration and frequency. Treatment of bang-sensitive mutants easily shocked, slamdance, or bangsenseless with antiseizure medications such as phenytoin and gabapentin,22 as well as valproate23 and potassium bromide24 altered the seizure-like behavior such as mean recovery time and percentage of flies that displayed bang-sensitive behavior. Drosophila seizure mutants have even been used for anticonvulsant drug screening.25 While these studies do not support a direct correlation to the electrophysiologic basis of seizures in humans, it does support a hypothesis that the mechanically and temperature-induced seizures in the fruit fly are stereotyped behaviors that have an electrophysiologic underpinning affected by drugs used to treat seizures in humans.
In this study, we tested the hypothesis that sleep deprivation of Drosophila seizure mutants alters their seizure susceptibility as in humans, potentially opening up an exciting new avenue of research into the relationship between sleep and epilepsy.
METHODS
Fly Stocks
stress sensitive B (sesB9Ed-4), a bang-sensitive paralytic mutant with loss of function of adenine nucleotide translocase (ANT),26 seizure (seits), a temperature-sensitive paralytic mutant that encodes an erg voltage-gated potassium channel,19 and UAS-sesB31320 (UAS-sesBRNAi#1) and UAS-sesB36661 (UAS-sesBRNAi#2) RNAi lines from the TRIP collection, were obtained from the Bloomington Stock center.27,28 seiP and UAS-seiRNAi were gifts from the Dr. Ben-Shahar lab (Washington University).
Sleep Measurement and Deprivation
Flies were cultured at 25°C in 50% to 60% humidity for a 12 h:12 h light-dark cycle on yeast, dark corn syrup, and agar food. Flies remained on the food used for culturing for all behavioral experiments. Newly eclosed female flies were collected from culture vials daily under CO2 anesthesia and maintained in groups of 20 in standard food vials until they were 2 to 3 days old. Flies were individually placed into 65-mm glass tubes in the Trikinetics activity monitoring system, and sleep parameters were continuously evaluated.7,29 Flies remained in the Trikinetics monitors during baseline sleep, sleep deprivation, and the recovery period. Sleep deprivation was conducted during the 12-h dark period after one full 24-h day of baseline by the Sleep Nullifying Apparatus (SNAP), a device that asymmetrically tilts from −60° to +60° such that the sleeping flies were displaced during the downward movement 10 times per minute while being monitored in the Trikinetics monitor. The SNAP has been found to produce waking without activating stress responses.29
Developmental Sleep Deprivation
As previously described,30 flies were collected several hours after eclosing and placed in 65-mm glass tubes in the Trikinetics activity monitors for 12 h of sleep deprivation during the dark period via the SNAP. After sleep deprivation, flies were left in the Trikinetics activity monitors under a 12 h:12 h light-dark schedule without further sleep deprivation for an additional 2–3 days until undergoing seizure testing.
Behavioral Seizure Testing
Each bang-sensitive paralytic mutant was placed individually into a vial and then vortexed at the maximum setting for 10 sec as has been previously described.9 The time for the fly to right itself and resume normal activity was recorded manually. Temperature-sensitive paralysis behavior was manually assessed by immersing each vial with an individual fly in a 39°C water bath until the fly became paralyzed as previously described.31 Seizure testing occurred within 60 min of the start of the light period (approximately 09:00) for flies immediately after sleep deprivation and 2–3 days following developmental sleep deprivation. Daytime sleep deprivation occurred from 08:00 to 17:00 during the light period, with flies assessed for seizure activity within 60 min (approximately 18:00).
Drug Feeding
Valproic acid sodium salt (valproate) was obtained from Sigma-Aldrich (St Louis, MO) and was added to food at a concentration of 25 mM, a dose previously noted to completely suppress seizure susceptibility in bang senseless and slam-dance mutants.23 sesB9Ed-4/+ flies were exposed to the drug for 12 to 18 h prior to bang-sensitive paralysis behavior testing (approximately 14:00–20:00 to 08:00).
Statistical Analysis
All comparisons were done using a Student t-test or, if appropriate, ANOVA and subsequent modified Bonferroni comparisons unless otherwise stated. All statistically different groups are defined as *P < 0.05.
RESULTS
Disrupting sesB Does Not Alter Sleep
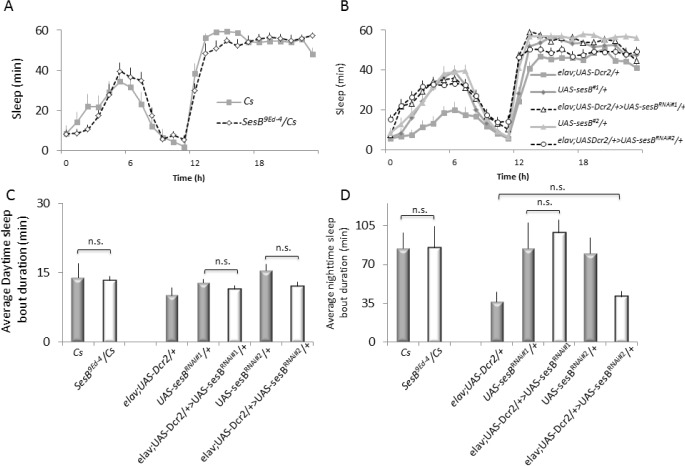
Although we could have evaluated the effects of sleep loss in a variety of bang-sensitive seizure mutants, we chose to examine sesB9Ed-4 flies since we have previously demonstrated that a different sesB mutant was able to maintain normal behavior during sleep deprivation in SNAP.29 The ability of a seizure mutant to maintain normal waking behavior during sleep deprivation is a requirement since it would not be possible to unequivocally determine whether a paralyzed seizure-mutant has truly been awake during the deprivation protocol. With that in mind, we first evaluated sleep in sesB9Ed-4 mutants. sesB9Ed-4 have been defined as haplo-specific lethal mutants and were thus crossed to Canton-S (Cs) and females were tested as F1s.32 sesB9Ed-4/+ flies exhibited sleep parameters that fall well within the observed range of variability measured for independent replicates of Cs flies measured over months.33,34 Indeed, sesB9Ed-4/+ flies obtained 846 ± 37 min of total sleep, while Cs flies slept for 856 ± 50 min. The distribution of sleep across the biological day can be seen for Cs and sesB9Ed-4/+ flies in Figure 1A. Sleep consolidation during the day and night are shown in Figure 1C, 1D, left. sesB9Ed-4/+ also exhibit normal waking activity and sleep homeostasis (Figure S1A supplemental material, and data not shown). Although most sesB alleles are lethal in females, a previous report suggests that hemizygous sesB9Ed-4 escapers can be collected when crossed with the deficiency Df(1)HC133.26 Unfortunately, we did not observe a single male or female escaper in over 1,000 F1s evaluated. Thus to further evaluate the effects of disrupting sesB, we expressed 2 independent RNAi lines from the TRIP collection28 that target different portions of the gene. As seen in Figure 1B, neither elav;UAS-Dcr2/+ > UAS-sesBRNAi#1/+ nor elav;UAS-Dcr2/+ > UAS-sesBRNAi#2/+ (black open markers) displayed alterations in sleep that differed in the same direction from both of their respective parental controls elav;UASDcr2/+, UAS-sesBRNAi#1/+, and UAS-sesBRNAi#2/+ (gray). Similarly, disrupting sesB did not produce consistent effects on other aspects of sleep architecture including sleep consolidation during the day and night (Figure 1C, 1D). Thus, disrupting sesB does not substantially alter sleep.
Figure 1.
Sleep is not disrupted in sesb9Ed-4/+ flies. (A) Sleep in sesb9Ed-4/+ flies falls within the range typically seen for wild-type flies (n = 14–16 flies/ group). (B) Neither elav;Dcr2/+ > sesBRNAi#1/+ nor elav;UAS-Dcr2/+ > sesBRNAi#2/+ (black open markers) displayed alterations in sleep that differed in the same direction from both of their respective parental controls elav;UAS-Dcr2/+, sesBRNAi#1/+, and sesBRNAi#2/+ (n = 32 flies/group). (C) No change in sleep consolidation during the day was observed for either sesb9Ed-4/+ mutants or following RNAi mediated knockdown of sesB. (D) Disrupting sesB did not alter sleep consolidation during the night.
Disrupting sesB Leads to an Increase in Seizure Duration Following Sleep Deprivation
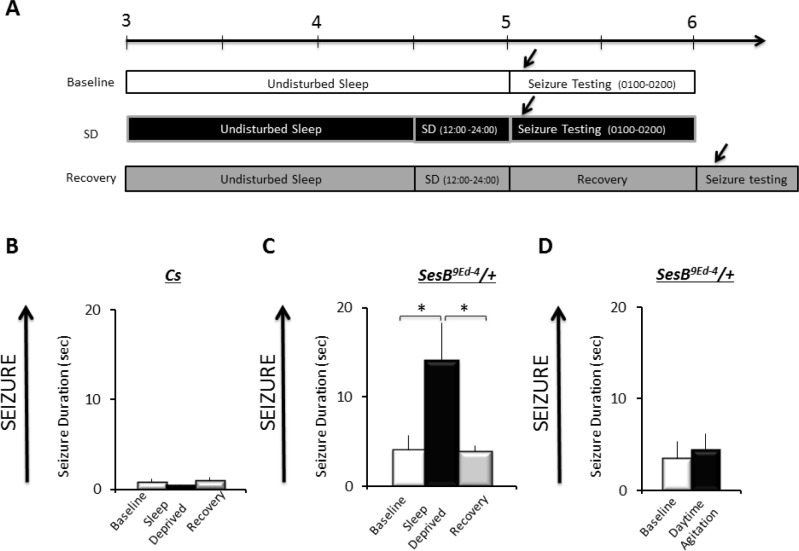
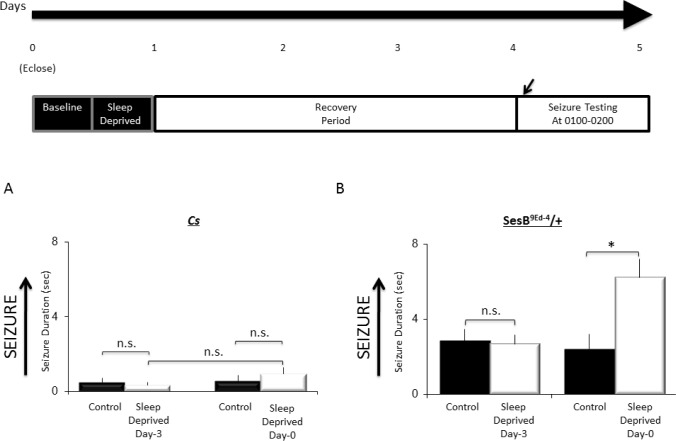
To ensure that the original seizure phenotype for sesB9Ed-4 was not masked by the accumulation of unidentified genetic modifiers, we first confirmed their seizure behavior to be as previously documented.19,26 Following 10-s of stimulation, sesB9Ed-4/+ flies required more time to right themselves and resume normal activity as previously described (data not shown). Thus, we evaluated seizure activity in wild-type and sesB9Ed-4/+ according to the protocol in Figure 2A. Not surprisingly, Cs flies quickly righted themselves and resumed activity during baseline; sleep deprivation did not significantly alter this response (Figure 2B); one-way ANOVA F2,49 = 1.3; P = 0.26. In contrast, 12 h of sleep deprivation increased duration of seizure in sesB9Ed-4/+ flies compared to the non-sleep deprived sesB9Ed-4/+ siblings; seizure duration returned to baseline following 24 h of recovery (Figure 2C; one-way ANOVA F2,227 = 4.9; P = 0.007, *P < 0.05 modified Bonferroni test). Note that the effects of sleep deprivation on seizure duration are made within a genotype. To determine whether the mechanical stimulus was responsible for the change in seizure rather than the loss of sleep per se, sesB9Ed-4/+ were exposed to the deprivation stimulus during their primary wake-period as previously described.29,35 As seen in Figure 2D, flies exposed to the deprivation stimulus during the day did not differ from their untreated controls (two-tailed t-test, P < 0.7). These data indicate that sleep deprivation can increase seizures in susceptible flies.
Figure 2.
Sleep deprivation increases seizure activity in sesb9Ed-4/+ flies. (A) Overview of experimental design. Adult flies were tested for seizure activity during baseline, immediately after 12 h of sleep deprivation (SD), or during recovery a day after being sleep deprived. (B) No seizure activity is observed in Cs flies under baseline, following sleep-deprivation, or during recovery (n = 60–70 flies/group). (C) sesb9Ed-4/+ flies exhibit increased seizure following sleep deprivation and returns to baseline during recovery (60–70 flies/group). (D) No change in seizure activity was observed in sesb9Ed-4/+ flies immediately following exposure to the same mechanical stimulation used to sleep deprive flies during the day (n = 32 flies/group).
To confirm that disrupting sesB can increase seizures, which can be further disrupted by sleep deprivation, we knocked down sesB using the RNAi lines described above. elav;UASDcr2/+ > UAS-sesBRNAi#1/+, elav;UAS-Dcr2/+ > sesBRNAi#2/+ and their parental controls (elav;UAS-Dcr2/+, UAs-sesBRNAi#1/+, and UAS-sesBRNAi#2/+) were evaluated for seizure activity under baseline and then following 12 h of sleep deprivation. A 5 (Genotype) × 2 (Condition: Baseline, Sleep deprived) ANOVA revealed a main effect for Genotype F4,170 = 137.5; P = 1.01E-011, a main effect for condition F1,170 = 32.5; P = 5.07E-08, and a Genotype by Condition interaction F4,170 = 13.6; P = 1.25E-009. Modified Bonferroni comparisons revealed that under baseline conditions both elav;UAS-Dcr2/+ > UAS-sesBRNAi#1/+ and elav;UAS-Dcr2/+ > UAS-sesBRNAi#2/+ exhibited seizures of longer duration than their respective parental controls (Figure 3, White). Importantly, 12 h of sleep deprivation significantly increased seizure duration in both elav;UAS-Dcr2/+ > UAS-ses-B RNAi#1/+ and elav;UAS-Dcr2/+ > UAS-sesBRNAi#2/+ compared to their own baseline; sleep deprivation did not alter seizure duration in parental controls (Figure 3). These data confirm previous reports in knock-down experiments that disrupting sesB can enhance seizure activity. In addition, the findings indicate that sleep deprivation can further enhance seizure activity when sesB is disrupted using 2 different strategies (mutant and RNAi).
Figure 3.
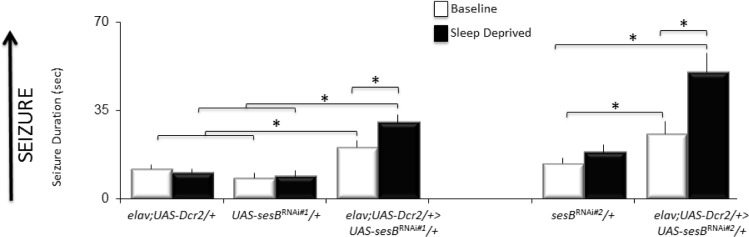
Sleep deprivation increases seizure activity following RNAi knockdown of sesB. During baseline, seizure activity of RNAi lines elav;UASDcr2/+ > sesBRNAi#1/+ and elav;UAS-Dcr2/+ > sesBRNAi#2/+ exhibited increased seizure activity compared to their parental controls (elav;UAS-Dcr2/+, sesBRNAi#1/+, and sesBRNAi#2/+); seizure activity was further increased in elav;UAS-Dcr2/+ > sesBRNAi#1/+ and elav;UAS-Dcr2/+ > sesBRNAi#2/+ following sleep deprivation (n = 16–20 flies/condition). *P < 0.05 modified Bonferroni test. Although not indicated on the graph, the seizure duration of elav;UASDcr2/+ > sesBRNAi#2/+ was also statistically different from the elav;UAS-Dcr2/+ parental control.
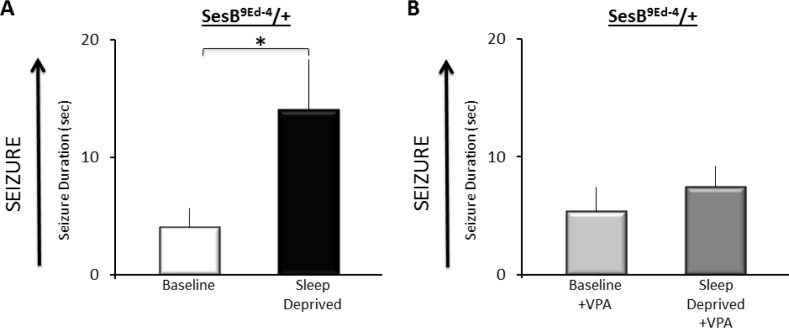
Previous studies have shown that administering bang senseless and slamdance mutants valproic acid (VPA) could completely suppress seizure activity.23 Thus, we asked whether we could modify seizure activity in sesB9Ed-4/+ flies by feeding them 25 mM VPA. sesB9Ed-4/+ were administered VPA or maintained on vehicle. Flies were either kept in Trikinetics tubes to serve as untreated controls or sleep deprived for 12 h. VPA did not alter total sleep time (Figure S1B). As seen in Figure 4, sleep deprived vehicle-fed sesB9Ed-4/+ flies exhibited increased duration of seizure compared to their non-sleep deprived sesB9Ed-4 controls. In contrast, VPA-fed sesB9Ed-4/+ flies did not exhibit an increase in seizure following sleep deprivation when compared to their non-sleep deprived siblings. VPA did not diminish the effectiveness of sleep deprivation (Figure S1C). Thus, VPA can reduce the susceptibility to sleep deprivation-enhanced seizure in sesB9Ed-4/+ mutants.
Figure 4.
Valproic Acid (VPA) reduces sleep deprivation induced seizure activity in sesb9Ed-4/+ flies. Vehicle-fed sesb9Ed-4/+ flies show a significant increase in seizure activity following sleep deprivation compared to untreated siblings. In contrast, seizure activity was not increased following sleep deprivation in sesb9Ed-4/+ flies maintained on 25 mM VPA compared to their untreated, VPA-fed siblings; *P < 0.05 modified Bonferroni test.
Sleep Deprivation Increases Seizure in a Temperature Sensitive-Paralytic Mutant
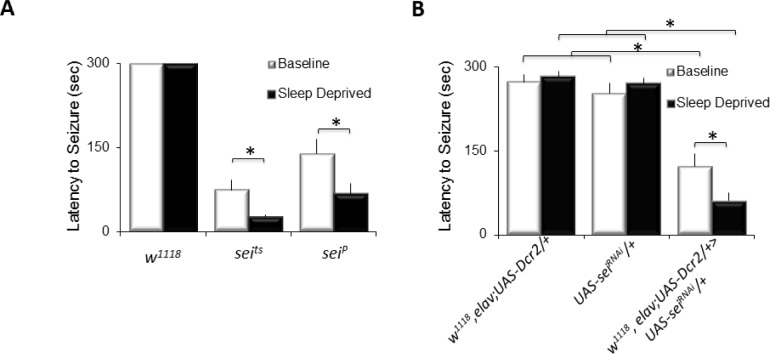
To evaluate the possibility that sleep deprivation can only influence seizure activity in bang-sensitive paralytic mutants, we asked whether sleep loss would increase seizure in a temperature sensitive paralytic, seizure (seits1,seiP). seits1 and seiP mutants were evaluated for seizure activity under baseline and after sleep deprivation by placing them in a 39°C water bath and calculating the amount of time to the onset of paralysis. As seen in Figure 5A, seits and seiP flies rapidly exhibit paralysis when exposed to 39°C. Importantly, sleep deprived seits and seiP flies were found to have a significantly shorter time to the onset of paralysis than non-sleep deprived seits siblings (Figure 5A, black) A 3(Genotype: w1118, seits1, seiP) × 2 (Condition: baseline; sleep deprivation) ANOVA revealed a significant main effect for genotype, F2,88 = 81.6, P = 1.60E-011 and condition, F1,88 = 6.6, P = 0.012). To confirm the results observed with seits and seiP, we evaluated a previously validated RNAi line that targets sei.36 Consistent with previous reports, w1118, elav;UAS-Dcr2/+ > UAS-seiRNAi/+ flies exhibit a reduced latency to seizure when placed at 39°C compared to w1118, elav;UAS-Dcr2/+ and UAS-seiRNAi/+ parental controls, a 3(Genotype) × 2(Condition) reveals a significant main effect for genotype F2,86 = 62, P = 1.711.71E-11. Furthermore, knocking down sei did not alter total sleep time (Figure S1D). These data indicate that the effects of sleep deprivation on seizure activity are not confined to a particular class of seizure mutant and can be observed using mechanical stimuli to induce seizure as well as changes in temperature.
Figure 5.
Sleep deprivation increases seizure in a temperature-sensitive paralytic mutant. (A) No seizure activity was detected during either baseline or following sleep deprivation in w1118 flies exposed to 39°C (n = 10/group). In contrast, both seits1 and seiP became paralyzed quickly when placed in a 39°C water bath (n = 18 flies/group). Sleep deprivation significantly shortens the latency to seizure activity in seits and seiP (n = 18 flies/group, *P < 0.05 modified Bonferroni test.). (B) Seizure activity was rare during baseline (n = 17–18 flies/group) and following 12 h of sleep deprivation in both w1118, elav;UAS-Dcr2/+ and UAS-seiRNAi/+ parental controls (n = 10 flies/group). In contrast, the latency to paralysis was significantly faster in w1118, elav;UAS-Dcr2/+ > UAS-seiRNAi/+ flies during baseline (n = 22 flies/group), and the latency was shortened by sleep deprivation (n = 22 flies/group) *P < 0.05 modified Bonferroni test.
Sleep Deprivation during Early Development Increases Seizure Susceptibility in Adults
We have previously shown that there is a critical window of fly development during which sleep loss results in long-lasting, negative consequences on short-term memory and adaptive behavior.30 To determine whether sleep loss during this stage of development would have long-lasting effects on seizure susceptibility, we sleep deprived Cs or sesB9Ed-4/+ flies for 12 h on the day that they emerged. The flies were then placed back into Trikinetics tubes and remained unperturbed for an additional 72 h before being evaluated for seizure activity. A separate group of Cs or sesB9Ed-4/+ flies were sleep deprived for 12 h when they were 3 days old, then placed back into Trikinetics tubes and evaluated for seizure activity 72 h later. As seen in Figure 6A, no change in seizure activity was observed when Cs flies were sleep deprived on the day they eclosed or if they were deprived at 3 days of age. Similarly, sesB9Ed-4/+ flies that had been sleep deprived when they were 3 days old showed similar seizure activity as their untreated siblings when they were they evaluated 72 h later (Figure 6B). However, when sesB9Ed-4/+ were sleep deprived for 12 h on their first day of adult life, they continued to show an enhancement of seizure activity compared to their non-sleep deprived siblings 72 h later; a 2(Control, Sleep Deprived) × 2(age at sleep deprivation) ANOVA revealed a significant Condition by age interaction F1,135 = 4.66; P = 0.03. Thus, sleep deprivation during a critical window of early development can increase the susceptibility to sleep deprivation-enhanced seizure in sesB9Ed-4/+ mutants.
Figure 6.
Sleep deprivation during a critical window of brain development increases seizure susceptibility in adults. (A) No difference was observed in Cs flies tested for seizure activity after 12 h sleep deprivation when they eclosed (Day-0), or when they were mature on Day-3 and assessed for seizure after 3-d of recovery (n = 32 flies/group). (B) Immature sesb9Ed-4/+ were sleep deprived for 12 h when they eclosed (Day-0), or when they were mature on Day-3 and assessed for seizure after 3-d of recovery (n = 30–50 flies/group). Sleep deprivation did not alter seizure susceptibility in mature sesb9Ed-4/+. In contrast, when immature sesb9Ed-4/+ flies were sleep deprived they continued to display increased susceptibility to seizure 3 days later. *P < 0.05 modified Bonferroni test.
DISCUSSION
In humans, sleep deprivation has long been observed to increase the propensity for seizure and to induce epileptiform activity on EEG. For instance, sleep deprivation increases the frequency of spike-wave discharges in idiopathic generalized epilepsy in humans.37 The underlying physiologic basis for the interaction of sleep and seizure is unknown in humans and other organisms. Current theories hypothesize that circadian rhythms,38 the synchronizing role of thalamo-cortical networks,39 and/or reduced intracortical inhibition40 may be contributory in humans. Increased cortical excitability during sleep loss has also been suggested to reduce the seizure threshold.41 Both epileptic activity and cortical excitability may be modulated by infraslow oscillations during sleep.42 In addition, increased slow wave activity on EEG has been found during both waking and sleeping, especially during NREM sleep, in response to sleep deprivation.43,44 This suggests that sleep deprivation may increase slow wave activity during both waking and recovery sleep and therefore increase the propensity for epileptic activity through decreased inhibition and activation of synchronous cortical networks.45
Despite the electrophysiologic aspects of seizure being diffi-cult to study in Drosophila, we have shown that sleep deprivation increases seizure susceptibility in two distinct Drosophila seizure mutants and that this effect is not dependent on genetic background. Further, the increase in seizure duration seen in sesb9Ed-4/+ flies in response to sleep deprivation is reversible by treatment with the antiseizure drug valproic acid. The results show that Drosophila melanogaster is a model system for studying the relationship between sleep and seizure susceptibility, opening a new avenue of research into this clinically important phenomenon that may lead to an improved understanding of how seizure thresholds are modified by genetic factors, the role of sleep in brain development and epileptogenesis, and how sleep affects seizure through changes cellular metabolism.
Results from our study suggest potential new avenues for research into the mechanism of the relationship between sleep and epilepsy. sesB encodes ANT, the mitochondrial inner membrane ATP/ADP exchanger. Drosophila sesB mutants have been shown to have impaired synaptic transmission at the neuromuscular junction46 and cellular response to oxidative stress.47 In humans, mitochondrial dysfunction and oxidative stress are a possible mechanism in epileptogenesis in both inherited epilepsy such as from a mitochondrial disorder, and acquired focal epilepsy such as temporal lobe epilepsy.48 The anticonvulsant effect of the ketogenic diet is mediated by changing brain metabolism at least in part by altering gene expression including in the mitochondria.49 In a study of extended wakefulness in mice, dynamic changes in components of energy regulation were noted, some in as little as 3 hours of sleep deprivation.50 These findings suggest a potential new avenue of investigation into whether or not the observed effect of sleep deprivation on seizure activity in sesB is mediated through increased energy utilization and oxidative stress in the setting of mitochondrial dysfunction.
We also tested a second Drosophila paralytic mutant, sei, that encodes an erg potassium channel that is a homolog of the human ether-a-go-go-related (hERG) gene and has a mutation that results in a severely truncated protein without the membrane spanning segments due to a premature stop codon.19 hERG is implicated in several human diseases including cardiovascular disease, muscular dystrophy, and epilepsy.51 Surprisingly no direct link between sleep deprivation and seizure propensity has yet been made with the erg potassium channel. Moreover seits1 flies have been found to be normal sleepers.52 Nonetheless potassium channelopathies are well-described as an etiology for human genetic epilepsy53,54 and animal models of acquired epilepsy.55,56 Although there is no evidence of direct interaction with sleep, a missense mutation in a sodium-gated potassium channel gene KCNT1 was recently found in a family with severe autosomal dominant nocturnal frontal lobe epilepsy, an inherited disorder characterized by motor seizures arising from sleep.54 Our newly described model system has the potential to further investigate the relationship between potassium channel dysfunction, epilepsy, and sleep deprivation.
Treatment of sesb9Ed-4/+ flies with valproate prevented the increase in seizure duration in response to sleep deprivation; however, seizure duration under baseline sleep conditions was not reduced compared to untreated controls. There are no prior reports of how seizure duration is affected in sesb9Ed-4 flies treated with valproate. Our dose of valproate 25 mM was based on previous reports from experiments in other seizure mutants and it is possible that this dose is not effective under baseline conditions in sesb9Ed-4 flies. We also may not have treated the sesb9Ed-4/+ flies for a sufficient duration. Genetic differences may also exist between sesb9Ed-4 mutants and other seizure mutants tested with valproate. For instance, direct injection of valproate into the nervous system of bang-sensitive seizure mutants slamdance, easily shocked, and paralyzed was tested to bypass detoxification of ingested compounds and this method of administration significantly reduced seizure thresholds.57 Further, overexpression of human multidrug resistance-associated protein 1 in neurons of in bang senseless mutants blocked the acute and chronic application of phenytoin and the chronic application of valproate.58 These prior studies suggest that genetic differences between sesb9Ed-4 and other Drosophila seizure mutants may account for different drug effects.
Using this new model, we sleep deprived sesb9Ed-4/+ flies immediately after eclosure and showed that there is a prolonged increase in seizure susceptibility even in the absence of subsequent sleep deprivation. Although further investigation is needed, these findings suggest that sleep may play a developmental role in establishing the seizure phenotype in this Drosophila mutant. This finding follows other recent work showing that sleep deprivation during the first full day of adult life impairs brain development and results in lasting deficits in learning and memory30 as well as behavior.59 Although we do not know the mechanism causing this effect, sleep deprivation is known to change gene expression in the brain of rats60 and Drosophila.29 The fruit fly has been used as a model organism to study aspects of seizure susceptibility,10 such as the role of cellular metabolism61,62 and transcriptional changes associated with seizure-induced synaptic connections.63 Applying similar experiments to Drosophila seizure mutants in the setting of early sleep loss may help elucidate the developmental role of sleep in epileptogenesis.
DISCLOSURE STATEMENT
This study was funded by 2 R01 NS051305-09 to Dr. Shaw. The authors have indicated no financial conflicts of interest. Averi Leahy is now at Tufts University School of Medicine, Boston, MA. Regine Rosas is now at Brown University, Providence, Rhode Island.
ACKNOWLEDGMENTS
The authors thank M. Thimgan, S. Dissel, and K. Melnattur for comments and advice.
SUPPLEMENTAL MATERIAL
(A) sesB mutants display normal sleep rebound. The percentage of sleep recovered during 48 h following 12 h of sleep deprivation at night is the same in Cs (white) and SesB9Ed-4/+ flies; t-test P > 0.05. (B) VPA does not alter baseline sleep in sesB9Ed-4/+ mutants. Total sleep time in SesB9Ed-4/+ flies maintained vehicle (Black bar, n = 30) does not differ from siblings maintained on 25 mM VPA (white bar, n = 21); t-test, P > 0.05. (C) VPA does not diminish the effectiveness of the SNAP. The percentage of sleep lost during 12 h of sleep deprivation at night is the same in Cs (white) and SesB9Ed-4/+ flies maintained on 25 mM VPA. (D) Pan neuronal knockdown of seizure does not alter sleep. Total sleep in w1118, elav-GAL4;UAS-Dcr2/+ > UAS -seiRNAi/+ flies (white bar) is not significantly different from either w1118, elav-GAL4;UAS-Dcr2/+ or UAS -seiRNAi parental controls (Black bars) F2,66 = 0.13,P = 0.87, not significant (n.s.), modified Bonferroni test.
REFERENCES
- 1.Temkin O. The falling sickness: a history of epilepsy from the Greeks to the beginnings of modern neurology. 2nd ed. Baltimore: The Johns Hopkins University Press; 1971. [Google Scholar]
- 2.Niedermeyer E, Silva FL. Electroencephalography: basic principles, clinical applications, and related field. 5th ed. Philadelphia: Lippincott, Williams, and Wilkins; 2005. [Google Scholar]
- 3.Gibbs EL, Gibbs FA. Diagnostic and localizing value of electroencephalographic studies in sleep. Publ Assoc Res Nerv Ment Dis. 1947;26:366–76. [Google Scholar]
- 4.Vaughn BV, D'Cruz OF, Beach R, Messenheimer JA. Improvement of epileptic seizure control with treatment of obstructive sleep apnoea. Seizure. 1996;5:73–8. doi: 10.1016/s1059-1311(96)80066-5. [DOI] [PubMed] [Google Scholar]
- 5.Malow BA, Weatherwax KJ, Chervin RD, et al. Identification and treatment of obstructive sleep apnea in adults and children with epilepsy: a prospective pilot study. Sleep Med. 2003;4:509–15. doi: 10.1016/j.sleep.2003.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Manni R, Politini L, Sartori I, Ratti MT, Galimberti CA, Tartara A. Daytime sleepiness in epilepsy patients: evaluation by means of the Epworth sleepiness scale. J Neurol. 2000;247:716–7. doi: 10.1007/s004150070120. [DOI] [PubMed] [Google Scholar]
- 7.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–7. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 8.Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutants in Drosophila melanogaster. Molec Gen Genet. 1973;120:107–14. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 9.Ganetzky B, Wu C-F. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics. 1982;100:597–614. doi: 10.1093/genetics/100.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol. 2000;83:998–1009. doi: 10.1152/jn.2000.83.2.998. [DOI] [PubMed] [Google Scholar]
- 11.Seugnet L, Boero J, Gottschalk L, Duntley SP, Shaw PJ. Identification of a biomarker for sleep drive in flies and humans. PNAS. 2006;103:19913–8. doi: 10.1073/pnas.0609463104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thimgan MS, Gottschalk L, Toedebusch C, et al. Cross-translational studies in human and Drosophila identify markers of sleep loss. PLoS One. 2013;8:e61016. doi: 10.1371/journal.pone.0061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seugnet L, Suzuki Y, Merlin G, Gottschalk L, Duntley SP, Shaw PJ. Notch signaling modulates sleep homeostasis and learning after sleep deprivation in Drosophila. Curr Biol. 2011;21:835–40. doi: 10.1016/j.cub.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seugnet L, Suzuki Y, Thimgan M, et al. Identifying sleep regulatory genes using a Drosophila model of insomnia. J Neurosci. 2009;29:7148–57. doi: 10.1523/JNEUROSCI.5629-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann V, Klaus F, Bodenmann S, et al. Functional ADA polymorphism increases sleep depth and reduces vigilant attention in humans. Cereb Cortex. 2012;22:962–70. doi: 10.1093/cercor/bhr173. [DOI] [PubMed] [Google Scholar]
- 16.Burg MG, Wu CF. Mechanical and temperature stressor-induced seizure-and-paralysis behaviors in Drosophila bang-sensitive mutants. J Neurogenetics. 2012;26:189–97. doi: 10.3109/01677063.2012.690011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Wu CF. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J Neurosci. 2002;22:11065–79. doi: 10.1523/JNEUROSCI.22-24-11065.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Adamo MC, Catacuzzeno L, Giovanni GD, Franciolini F, Pessia M. K+ channelepsy: progress in the neurobiology of potassium channels and epilepsy. Front Cell Neurosci. 2013;7:134. doi: 10.3389/fncel.2013.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus SA, Warmke JW, Ganetzky B. The Drosophila erg K+ channel polypeptide is encoded by the seizure locus. J Neurosci. 1997;17:875–81. doi: 10.1523/JNEUROSCI.17-03-00875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Escayg A, MacDonald BT, Meisler MH, et al. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet. 2000;24:343–5. doi: 10.1038/74159. [DOI] [PubMed] [Google Scholar]
- 21.Parker L, Padilla M, Du Y, Dong K, Tanouye MA. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics. 2011;187:523–34. doi: 10.1534/genetics.110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds ER, Stauffer EA, Feeney L, Rojahn E, Jacobs B, McKeever C. Treatment with the antiepileptic drugs phenytoin and gabapentin ameliorates seizure and paralysis of Drosophila bang-sensitive mutants. J Neurobiol. 2004;58:503–13. doi: 10.1002/neu.10297. [DOI] [PubMed] [Google Scholar]
- 23.Kuebler D, Tanouye M. Anticonvulsant valproate reduces seizure-susceptibility in mutant Drosophila. Brain Res. 2002;958:36–42. doi: 10.1016/s0006-8993(02)03431-5. [DOI] [PubMed] [Google Scholar]
- 24.Tan JS, Lin F, Tanouye MA. Potassium bromide, an anticonvulsant, is effective at alleviating seizures in the Drosophila bang-sensitive mutant bang senseless. Brain Res. 2004;1020:45–52. doi: 10.1016/j.brainres.2004.05.111. [DOI] [PubMed] [Google Scholar]
- 25.Stilwell GE, Saraswati S, Littleton JT, Chouinard SW. Development of a Drosophila seizure model for in vivo high-throughput drug screening. Eur J Neurosci. 2006;24:2211–22. doi: 10.1111/j.1460-9568.2006.05075.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YQ, Roote J, Brogna S, Davis AW, Barbash DA, Nash D, Ashburner M. stress sensitive B encodes an adenine nucleotide translocase in Drosophila melanogaster. Genetics. 1999;153:891–903. doi: 10.1093/genetics/153.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni JQ, Markstein M, Binari R, et al. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietzl G, Chen D, Schnorrer F, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–7. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 29.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–91. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 30.Seugnet L, Suzuki Y, Donlea JM, Gottschalk L, Shaw PJ. Sleep deprivation during early-adult development results in long-lasting learning deficits in adult Drosophila. Sleep. 2011;34:137–46. doi: 10.1093/sleep/34.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson FR, Wilson SD, Strichartz GR, Hall LM. Two types of mutants affecting voltage-sensitive sodium channels in Drosophila melanogaster. Nature. 1984;308:189–91. doi: 10.1038/308189a0. [DOI] [PubMed] [Google Scholar]
- 32.Nash D, Janca FC. Hypomorphic lethal mutations and their implications for the interpretation of lethal complementation studies in Drosophila. Genetics. 1983;105:957–68. doi: 10.1093/genetics/105.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw PJ, Franken P. Perchance to dream: solving the mystery of sleep through genetic analysis. J Neurobiol. 2003;54:179–202. doi: 10.1002/neu.10167. [DOI] [PubMed] [Google Scholar]
- 34.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–72. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 35.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–7. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng X, Valakh V, DiAntonio A, Ben-Shahar Y. Natural antisense transcripts regulate the neuronal stress response and excitability. eLife. 2014;3:e01849. doi: 10.7554/eLife.01849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halasz P, Filakovszky J, Vargha A, Bagdy G. Effect of sleep deprivation on spike-wave discharges in idiopathic generalized epilepsy: a 4 x 24 h continuous long term EEG monitoring study. Epilepsy Res. 2002;51:123–32. doi: 10.1016/s0920-1211(02)00123-7. [DOI] [PubMed] [Google Scholar]
- 38.Quigg M, Straume M, Menaker M, Bertram EH. Temporal distribution of partial seizures: comparison of an animal model with human partial epilepsy. Ann Neurol. 1998;43:748–55. doi: 10.1002/ana.410430609. [DOI] [PubMed] [Google Scholar]
- 39.Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 2005;28:317–24. doi: 10.1016/j.tins.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Kreuzer P, Langguth B, Popp R, et al. Reduced intra-cortical inhibition after sleep deprivation: a transcranial magnetic stimulation study. Neurosci Lett. 2011;493:63–6. doi: 10.1016/j.neulet.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 41.Scalise A, Desiato MT, Gigli GL, et al. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–8. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- 42.Vanhatalo S, Palva J, Holmes M, Miller J, Voipio J, Kaila K. Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. PNAS. 2004;101:5053–7. doi: 10.1073/pnas.0305375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes the EEG during sleep and wakefulness. Sleep. 1993;16:100–13. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- 44.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 45.Romcy-Pereira RN, Leite JP, Garcia-Cairasco N. Synaptic plasticity along the sleep-wake cycle: implications for epilepsy. Epilepsy Behav. 2009;14(Suppl 1):47–53. doi: 10.1016/j.yebeh.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Trotta N, Rodesch CK, Fergestad T, Broadie K. Cellular bases of activity-dependent paralysis in Drosophila stress-sensitive mutants. J Neurobiol. 2004;60:328–47. doi: 10.1002/neu.20017. [DOI] [PubMed] [Google Scholar]
- 47.Terhzaz S, Cabrero P, Chintapalli VR, Davies SA, Dow JA. Mislocalization of mitochondria and compromised renal function and oxidative stress resistance in Drosophila SesB mutants. Physiol Genomics. 2010;41:33–41. doi: 10.1152/physiolgenomics.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88:23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–35. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- 50.Nikonova EV, Naidoo N, Zhang L, et al. Changes in components of energy regulation in mouse cortex with increases in wakefulness. Sleep. 2010;33:889–900. doi: 10.1093/sleep/33.7.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He FZ, McLeod HL, Zhang W. Current pharmacogenomic studies on hERG potassium channels. Trends Mol Med. 2013;19:227–38. doi: 10.1016/j.molmed.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Reduced sleep in Drosophila shaker mutants. Nature. 2005;434:1087–92. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 53.Scheffer IE, Berkovic SF. The genetics of human epilepsy. Trends Pharmacol Sci. 2003;24:428–33. doi: 10.1016/S0165-6147(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 54.Heron SE, Smith KR, Bahlo M, et al. Missense mutations in the sodium-gated potassium channel gene KCNT1 cause severe autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet. 2012;44:1188–90. doi: 10.1038/ng.2440. [DOI] [PubMed] [Google Scholar]
- 55.Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D. Acquired dendritic channelopathy in temporal lobe epilepsy. Science. 2004;305:532–5. doi: 10.1126/science.1097065. [DOI] [PubMed] [Google Scholar]
- 56.Poolos NP, Johnston D. Dendritic ion channelopathy in acquired epilepsy. Epilepsia. 2012;53(Suppl 9):32–40. doi: 10.1111/epi.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Howlett IC, Tanouye MA. Seizure-sensitivity in Drosophila is ameloriated by doral vessel injection of the antiepileptic drug valproate. J Neurogenetics. 2013;27:143–50. doi: 10.3109/01677063.2013.817574. [DOI] [PubMed] [Google Scholar]
- 58.Bao GS, Wang WA, Wang TZ, et al. Overexpression of human MRP1 in neurons causes resistance to antiepileptic drugs in Drosophila seizure mutants. J Neurogenetics. 2011;25:201–6. doi: 10.3109/01677063.2011.620662. [DOI] [PubMed] [Google Scholar]
- 59.Kayser MS, Yue Z, Sehgal A. A critical period of sleep for development of courtship circuitry and behavior in Drosophila. Science. 2014;344:269–74. doi: 10.1126/science.1250553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cirelli C, Faraguna U, Tononi G. Changes in brain gene expression after long-term sleep deprivation. J Neurochem. 2006;98:1632–45. doi: 10.1111/j.1471-4159.2006.04058.x. [DOI] [PubMed] [Google Scholar]
- 61.Fergestad T, Bostwick B, Ganetzky B. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics. 2006;173:1357–64. doi: 10.1534/genetics.106.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fergestad T, Olson L, Patel KP, Miller R, Palladino MJ, Ganetzky B. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics. 2008;178:947–56. doi: 10.1534/genetics.107.082115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guan Z, Saraswati S, Adolfsen B, Littleton JT. Genome-wide transcriptional changes associated with enhanced activity in the Drosophila nervous system. Neuron. 2005;48:91–107. doi: 10.1016/j.neuron.2005.08.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) sesB mutants display normal sleep rebound. The percentage of sleep recovered during 48 h following 12 h of sleep deprivation at night is the same in Cs (white) and SesB9Ed-4/+ flies; t-test P > 0.05. (B) VPA does not alter baseline sleep in sesB9Ed-4/+ mutants. Total sleep time in SesB9Ed-4/+ flies maintained vehicle (Black bar, n = 30) does not differ from siblings maintained on 25 mM VPA (white bar, n = 21); t-test, P > 0.05. (C) VPA does not diminish the effectiveness of the SNAP. The percentage of sleep lost during 12 h of sleep deprivation at night is the same in Cs (white) and SesB9Ed-4/+ flies maintained on 25 mM VPA. (D) Pan neuronal knockdown of seizure does not alter sleep. Total sleep in w1118, elav-GAL4;UAS-Dcr2/+ > UAS -seiRNAi/+ flies (white bar) is not significantly different from either w1118, elav-GAL4;UAS-Dcr2/+ or UAS -seiRNAi parental controls (Black bars) F2,66 = 0.13,P = 0.87, not significant (n.s.), modified Bonferroni test.