SUMMARY
Objectives.
The work has the objective to analyze the literature on the degradation of the adhesive interface. In particular the study is focused on the role of the metalloproteinase in the hydrolytic degradation of collagen matrix in the bonded interface. The survey will concern also the latest innovations to improve and increase the link between dentin and the restorative materials through the MMPs inhibitors.
Methods.
The research has been carried out in the MEDLINE database by choosing keywords as “metalloproteinases” and “dentin bond” and “degradation”. In vitro studies were included in the research, excluding studies with no human and deciduous teeth. Language was limited to English.
Results.
The collagenolytic enzymes in mineralized dentin have been demonstrated to have an important role in dental hard tissue pathologies, including the degradation of the hybrid layer.
Conclusion.
The preservation of the collagen matrix integrity is a key issue in the attempts to improve the dentin bonding durability.
Keywords: metalloproteinases, dentin bond, MMPs inhibitors
Introduction
Most of the studies carried out in adhesive dentistry in recent years have aimed to simplify the clinical procedures (1).
The modern adhesive techniques provide for a reduction in the number of steps during the application of the adhesive: these allow, in addition to simplification, a reduction of working time and make the process less operator-dependent (2).
Despite the countless studies and the discovery of new and more versatile materials, adhesive interface still remains the weak point of the tooth-restoration complex. One of the most important factors that determine the stability in time of restoration is the hybrid layer, that is influenced by nanoleakage, dentin micropermeability and degradation of metalloproteinases (MMPs). All these factors contribute to the degeneration of the hybrid layer and the failure of the restoration (1, 2).
After dentin bonding with resins collagen fibrils are exposed at the bottom of the hybrid layer owing to imperfect resin impregnation of the demineralized dentin matrix. Exposed collagen fibrils might be affected by MMPs inducing hydrolytic degradation, which might result in reduced bond strength (3).
To perform a systematic review of the literature have been chosen initially the basic parameters, the criteria of inclusion and exclusion that each item should have respected in order to be considered proportionate to the objectives of this research. These parameters and criteria have been chosen choosing as a guide the most important reviews of the literature on the subject of host derived metalloproteinases. The research has been carried out in the MEDLINE database by choosing keywords as “metalloproteinases” and “dentin bond” and “degradation”, placing within the limits of the research all the criteria of inclusion and exclusion previously selected. Only jobs performed in vitro and on human and permanent teeth has been considered, eliminating all those articles relating to animal experiments. The selected language has been limited to English to ensure a proper understanding of the text.
Collagenolytic activity of dentine: the matrix metalloproteinases
The MMPs are a class of zinc and calcium dependent endopeptidases, which are trapped within the mineralized dentin matrix during tooth development. The release and subsequent activation of these endogenous enzymes during dental restorative procedures are believed to be responsible of the dentin-adhesive bonding failure (4, 5). In fact it was observed in an in vitro study the thinning and the disappearance of collagen fibrils from not fully infiltrated hybrid layers after dentin treatment with adhesive (6). Despite the different adhesive procedures, the result is often an incomplete hybridization of the dentin surface, so the collagen fibrils remain unprotected from factors promoting hydrolytic degradation, as the residual solvent of the adhesive or the water not removed from the dentin surface (7).
Recent studies have revealed the contribution of host protease in the degradation of matrix collagen in the pathogenesis of dentin caries and periodontal disease, with potential implications in dentine bond interface (8, 9). The nanoleakage may also occur in the absence of gaps along the adhesive dentin interface, this suggests that the degradation of not fully infiltrated dentin by host proteinases may proceed in absence of bacterial enzymes (10). The matrix of etched dentin may be slowly degraded over time by proteolytic enzymes derived from dentine itself, in the absence of bacteria (11). Pashley et al. have showed that intrinsic collagenolytic activity in human mineralized dentin can be inhibited by specific inhibitors of proteases (12).
The presence of collagenolytic and gelatinolytic activity in partially demineralized dentin is an indirect evidence of the existence of matrix metalloproteinases (MMP) in human dentin (Tab. 1) (13); in fact, the presence of gelatinases (MMP-2 and MMP-9), collagenase (MMP-8) and enamelysine (MMP-20) and in demineralized dentin has been demonstrated through zymography and Western blotting techniques (14–17).
Table 1.
List of known MMPs and their substrates (from Lynch and Matrisian, 2002).
| MMP | Alternative name | ECM substrate | Non-matrix substrates |
|---|---|---|---|
| MMP-1 | Collagenase-1 | Collagen I/II/III/VII/X/XI, gelatin, entactin, aggrecan, fibronectin, laminin, tenascin, vitronectin | Perlecan, IGFBP-2/3, ProTNF-α, α1-AC, α2-MG, α1-PI |
| MMP-2 | Gelatinase A | Collagen I/III/IV/V/VII/X/XI, tenascin decorin, gelatin, elastin, fibronectin, laminin, aggrecan, vitronectin | TGF-β, TGF-β2, IL-1β, MCP-3, SDF-1, IGFBP-3/5, TNF-α, FGF-R1, α1-AC, α1-PI |
| MMP-3 | Stromelysin-1 | Collagen III/IV/V/VII/IX/X/XI, elastin, laminin, fibronectin, gelatin, aggrecan entactin, decorin, tenascin, vitronectin | Perlecan, HB-EGF, IL-1β, plasminogen, E-cadherin, IGFBP-3, TNF-α, α1-AC, α2-MG, α1-PI |
| MMP-7 | Matrilysin | Collagen I/IV, aggrecan, laminin, fibronectin, gelatin, entactin, decorin, elastin, tenascin, vitronectin | FASL, β4 integrin, E-cadherin, HB-EGF, plasminogen, TNF-α, α1-PI |
| MMP-8 | Collagenase-2 | Collagen I/II/III, aggrecan | α2-MG, α1-PI |
| MMP-9 | Gelatinase B | Collagen IV/V/XI/XIV, decorin, gelatin, elastin, laminin, aggrecan, vitronectin | TGF-β2, IL-1β, TNF-α, IL-2Ra, plasminogen, α1-AC, α2-MG, α1-PI |
| MMP-10 | Stromelysin-2 | Collagen III/IV/V, aggrecan, elastin, laminin, fibronectin, gelatin | ND |
| MMP-11 | Stromelysin-3 | ND | IGFBP-1, α2-MG, α1-PI |
| MMP-12 | Metalloelastase | Collagen I/IV, aggrecan, decorin, gelatin, elastin, fibronectin, laminin, vitronectin, entactin | Plasminogen, α2-MG, α1-PI |
| MMP-13 | Collagenase-3 | Collagen I/II/III/VI/IX/X/XIV, gelatin, fibronectin, aggrecan | α2-MG |
| MMP-14 | MT1-MMP | Collagen I/II/III, gelatin, fibronectin, laminin, entactin, vitronectin, aggrecan | CD44, transglutaminase, α2-MG, α1-PI |
| MMP-15 | MT2-MMP | Aggrecan, entactin, fibronectin, laminin, tenascin | Transglutaminase |
| MMP-16 | MT3-MMP | Collagen III, fibronectin, gelatin | Transglutaminase |
| MMP-17 | MT4-MMP | Gelatin | α2-MG, TNF-α |
| MMP-18 | Collagenase-4 (Xenopus) | Collagen I | ND |
| MMP-19 | RASI | Collagen I/IV, fibronectin, gelatin, tenascin, laminin, aggrecan, entactin, COMP | ND |
| MMP-20 | Enamelysin | Collagen X VIII, aggrecan, amelogenin, COMP | ND |
| MMP-21 | XMMP (Xenopus) | No known substrates | ND |
| MMP-22 | CMMP (chicken) | Gelatin | ND |
| MMP-23 | CA-MMP (cysteine array MMP) | ND | ND |
| MMP-24 | MT5-MMP | Collagen I, gelatin, fibronectin, laminin | ND |
| MMP-25 | MT6-MMP | Collagen IV, gelatin, fibronectin | ND |
| MMP-26 | Matrilysin-2 | Collagen IV, fibronectin, gelatin | α1-PI |
| MMP-27 | Endometase | ND | ND |
| MMP-28 | Epilysin | ND | ND |
Recently has been discovery cysteine cathepsins in normal and carious dentin (18–20). Cysteine cathepsins are papain-like endopeptidases that participate in intracellular proteolysis within the lysosomal compartments of living cells (21). These endogenous enzymes are latent forms of MMPs (pro-MMPs) via the cysteine-switch mechanism that exposes the catalytic domain of these enzymes that were blocked by pro-peptides (22). Cathepsins are responsible for the digestion of collagen fibrils exposed at the adhesive interface (22, 23).
The collagen degradation occurs at the bottom of hybrid layer and was also observed in vivo studies (24). Unfortunately, it was not yet established a relationship between the different etch&rinse techniques and the degradation of dentin hybrid layers. Presumably, the dentin conditioning with phosphoric acid could inhibit the MMPs that are entrapped in the mineralized dentin. In fact Mazzoni et al. observed the potential role of dentin adhesives in proteolysis activation using a modelling approach in which the relative dentin proteolytic activities were quantified before and after the sequential application of orthophosphoric acid and total-etch adhesive. The total-etch adhesives can activate dentin MMPs that have been previously neutralized by orthophosphoric acid etchant (11, 25).
The gelatinolytic and collagenolytic activity of dentin can be neutralized from MMPs inhibitors, in fact to reduce the aging of bonding interfaces and to increase the stability of the hybrid layer, the protease inhibitors as additional primers might be recommended (25, 26).
MMPs inhibitors
Chlorhexidine (CHX)
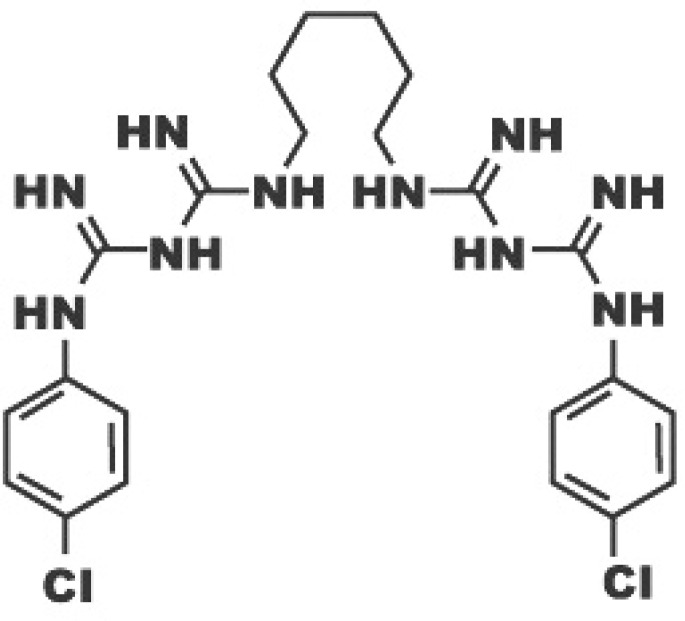
Chlorhexidine (Fig. 1) is now used in adhesive dentistry as an inhibitor of MMPs (27); this chemoterapeutic agent is commonly used in Periodontology as antiseptic agent in mouthrinse (28, 29). CHX is the first candidate to be tested in attempts to inhibit collagenolytic enzymes in dentin (30, 31). CHX had been demonstrated to effectively inhibit MMP-2, -9 and -8 (32). CHX, applied on etched human dentin, has MMPs inhibitory properties: its capacity leads to the protection of the integrity of the hybrid layer collagen after application of the etch & rinse adhesive, confirming the indirect involvement of MMPs in the degradation of collagen (33). The effective concentrations have varied between 0.002% and 4%, 0.2% and 2%. The best concentration common used is the 2% (23).
Figure 1.
Chemical structure of chlorhexidine.
The application of chlorhexidine improves the integrity of the hybrid layer achieving the complete inhibition of the proteolytic enzymes (25, 34). In fact, when phosphoric acid is applied without the subsequent application of chlorhexidine, the collagenolytic activity of mineralized dentin is not inhibited, while the use of CHX after dentin etching strongly inhibited this activity (33). This is asserted in study in which are compared the effect of the application of a total-etch adhesive, the Adper Scotchbond 1XT (SB1XT), with and without 0,2–2% CHX pre-treatment for 30s on the etched surface Zymograms showed that application of SB1XT to human dentin powder increases MMP-2 activity, while CHX pre-treatment inhibited all dentin gelatinolytic activity, irrespective from the tested concentration. CHX significantly lowered the loss of bond strength and nanoleakage seen in acid-etched resin-bonded dentin artificially aged for 2 years (35).
For etch-and-rinse adhesives, CHX may be applied to the demineralized dentin directly or incorporated into an acid conditioner prior to the application of adhesives, which has been shown to be effective for reducing degradation of resin-dentin bonds after in vivo aging (26, 36, 37).
Recently, Scaffa et al. discovered the power of chlorhexidine to inhibit cysteine cathepsins which may also contribute to its effect on preserving hybrid layer collagen (19).
EDTA
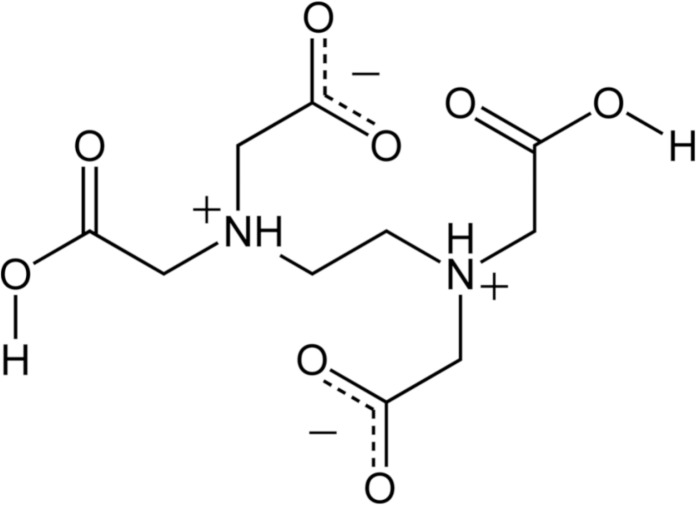
EDTA (ethylenediaminetetraacetic acid) (Fig. 2) is an endodontic chelant with the capacity to inactivate endogenous MMP activity in human dentin (30). In fact, Thompson et al. measured the MMPs activity of dentin demineralized beams with phosphoric acid, which also activated endogenous MMPs, and divided into 4 experimental groups on the basis of exposure time to 17% EDTA (0, 1, 2, or 5 minutes). The study asserted that 17% EDTA significantly inhibits endogenous MMP activity of human dentin within 1–2 minutes. This might minimize hybrid layer degradation after resin bonding procedures (38).
Figure 2.
Chemical structure of EDTA (ethylenediaminetetraacetic acid).
Carbodiimide
Carbodiimide (1-Ethyl-3-[3-dimethylamino propyl] carbodiimide Hydrochloride, or EDC) (Fig. 3) is a stable cyanamide isomer. The EDC is able to improve the stability of the dentine bondin thanks to its cross-linking capacity. Its mechanism of collagen cross-linking involves the activation of the carboxylic acid groups of glutamic and aspartic acid residues (39). The EDC has great potential to enhance the stability of the interface, most likely due to increased mechanical properties of the dentin matrix and slower degradation rates of collagen (40, 41). So it is possible to assert that EDC can produce long-term inactivation of MMPs in acid-etched dentin matrices contributing to bond strength preservation over time. As assayed with the zymography the EDC pretreatment inhibited dentin endogenous MMPs.
Figure 3.
Chemical structure of EDC.
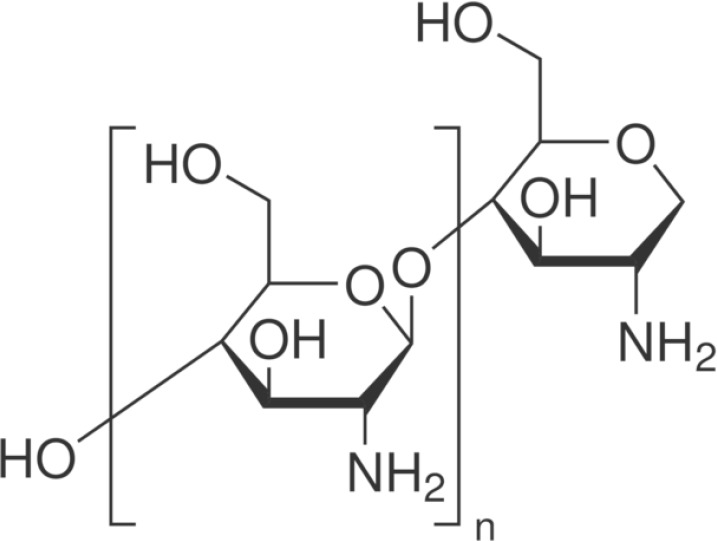
Chitosan
Chitosan (Fig. 4) is a natural polysaccharide biopolymer composed by β-(1–4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). Chitosan owns antibacterial property related partly to the interaction between positively charged chitosan and negatively charged bacterial cell surface that would decrease bacterial cell permeability, resulting in cell death (42). The modification of the bonding substrate with chitosan and riboflavin increases the mechanical properties, enhanced the mechanical stability of demineralized dentin substrates against hydrolytic and collagenolytic degradation of the MMPs. The gradual increase in chitosan contents permits a major obliteration of interfibrillar-spaces that might adversely affect bonding to dentin (43).
Figure 4.
Chemical structure of chitosan.
ZnO
Synthetic peptidomimetic inhibitors with zinc chelator properties can be used to inhibit the active site of the catalytic domain, thus inhibiting the activity of MMPs (44). Addition of ZnO particles to dental adhesives produced a minor dentine collagen degradation and an increasing of resin-dentine bonds durability: so it is possible to preserve the adhesives bonding efficacy overtime (45).
Tetracyclines
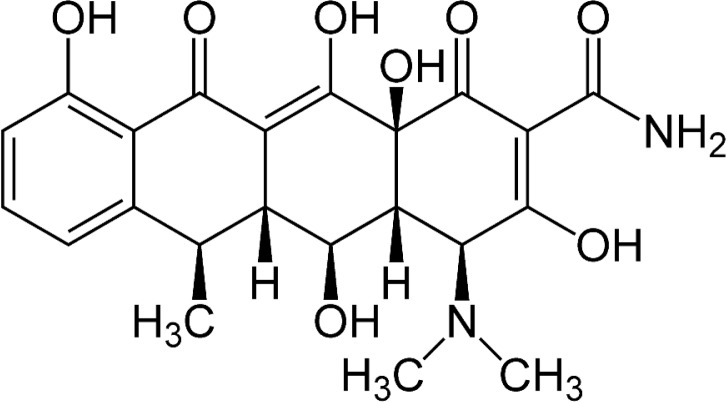
Tetracyclines are antibiotics commonly used in the treatment of periodontitis. Tetracyclines and their semisynthetic forms, doxicycline (DO) (Fig. 5), have the ability to inhibit MMPs collagen degradation activity (46, 47). The dentin surface pre-treatment, after the etching procedures, with an aqueous solutions of 2% doxycycline can inhibit collagenases and gelatinases, determining an improvement of dental bonding. DO cannot be used with acetone based adhesive systems, because lower bond strength values as well as higher silver nitrate penetration were observed within the hybrid layer. Also the derivatives and chemically modified analogs (chemically modified tetracyclines, CMTs) showed the potential to inhibit the collagenolytic degradation of the MMPs (48). The most potent CMTs against collagenases is CMT-3 (aka Metastat, COL-3) but it is also effective against gelatinases, and is particularly effective in inhibiting MMPs in dentinal caries lesions (10).
Figure 5.
Chemical structure of doxicicline.
Galardin
Galardin (Fig. 6) is a synthetic MMPs inhibitor (49, 50). Galardin has a collagen-like backbone and a hydroxamate structure (R-CO-NH-OH) which chelates the zinc-ion located in the catalytic domain of MMPs. The bonding surface treated with 0.2 mM galardin water solution showed a slowed decline of bond strength and reduced the amount of nanoleakage, but it did not completely block these phenomena (49).
Figure 6.
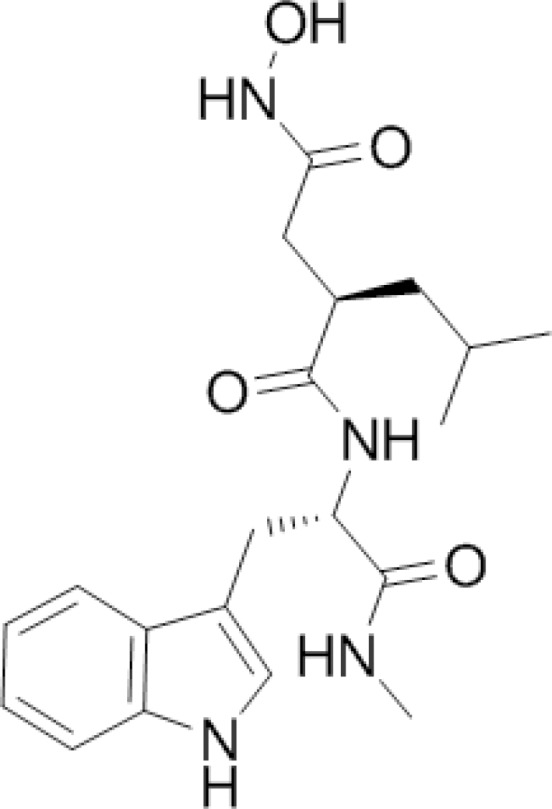
Chemical structure of galardin.
Proanthocyanidins
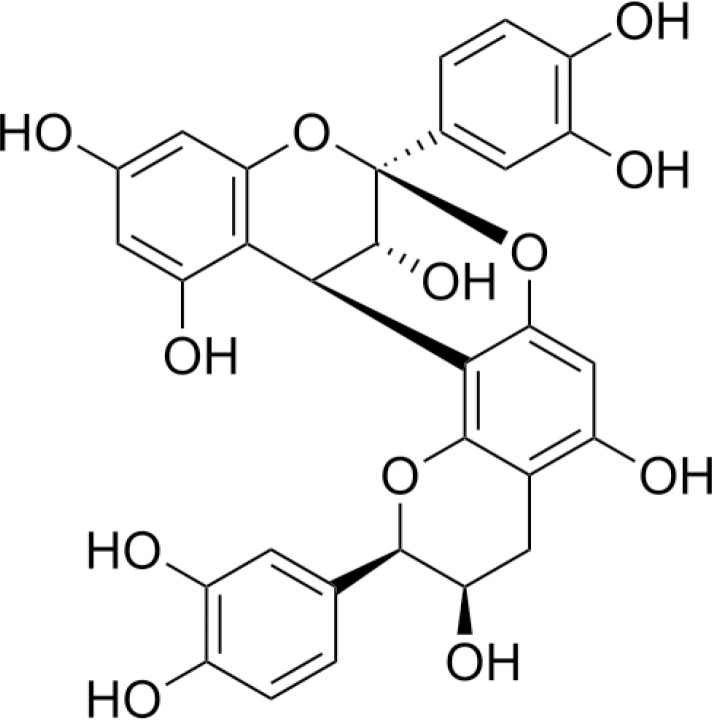
It has also been discovered that proanthocyanidins (PA) (Fig. 7), a grape seed extract, has the ability to inhibit MMP proteolytic activities. PA has the ability to enhance the resistance to collagen biodegradation and lead to a better conservation of the hybrid layer after enzymatic degradation compared to the pure model adhesive (51, 52). PA can effectively cross-link collagen and improve its biological stability in time periods as short as 10s. The use of PA pre-treatment in the demineralized dentin is clinically feasible and is a promising approach to improving the durability of current dentin bonding systems (52).
Figure 7.
Chemical structure of proanthocyanidins.
Conclusion
The dentin adhesive procedures have undergone revolutionary changes in recent decades. Despite the countless studies and the discovery of new more versatile materials, even today, the adhesive interface remains the weak point of the adhesive tooth-restoration complex. Unfortunately, it is also the most vulnerable part of the restoration, because it is influenced by nanolekeage, micropermeability and by the degradation of metalloproteases. The gelatinolytic and collagenolytic activity of dentin can be neutralized from protease inhibitors, which means that inhibition of MMPs could preserve the integrity of the hybrid layer. A partial solution to the problem of hybrid layer deterioration may be the incorporation of appropriate MMPs inhibitors into adhesive bonding systems (25). Water sorption of adhesive interfaces most likely remains the principal mechanism of bond degradation, while endogenous enzymes appear to contribute to bond degradation of only total etch adhesives (30).
References
- 1.Vaidyanathan TK, Vaidyanathan J. Recent Advances in the Theory and Mechanism of Adhesive Resin Bonding to Dentin: A Critical Review. J Biomed Mater Res B Appl Biomater. 2009;88(2):558–78. doi: 10.1002/jbm.b.31253. [DOI] [PubMed] [Google Scholar]
- 2.Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Leandra R, De Stefano Dorigo E. Dental adhesion review: Aging and stability of the bonded interface. Dent Mater. 2008;24(1):90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang SC, Kern M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int J Oral Sci. 2009 Dec;1(4):163–76. doi: 10.4248/IJOS.09044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. Journal of Dental Research. 2005;84:118–32. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 5.Moon PC, Weaver J, Brooks CN. Review of matrix metalloproteinases’ effect on the hybrid dentin bond layer stability and chlorhexidine clinical use to prevent bond failure. Open Dent J. 2010 Jul 20;4:147–52. doi: 10.2174/1874210601004010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, et al. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005;26(34):6863–72. doi: 10.1016/j.biomaterials.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Sulkala M, Larmas M, Sorsa T, Salo T, Tjaderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002;81(9):603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 9.Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52(2):121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur J Oral Sci. 2006;114(2):160–6. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjaderhane L, Toledano M. Reactivation of quenched endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 12.Pashley DH, Tay FR, Yiu CKY, Hashimoto M, Breschi L, Carvalho R, et al. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83(3):216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 13.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70(9/10):561–573. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 14.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Suppa P, Papa S, et al. Zymographic analysis and characterization of MMP-2 and -9 isoforms in human sound dentin. J Dent Res. 2007;86(5):436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 15.De Munck J, Mine A, Van den Steen PE, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Enzymatic degradation of adhesive-dentin interfaces produced by mild self-etch adhesives. Eur J Oral Sci. 2010 Oct;118(5):494–501. doi: 10.1111/j.1600-0722.2010.00758.x. [DOI] [PubMed] [Google Scholar]
- 16.Sulkala M, Larmas M, Sorsa T, Salo T, Tjäderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002;81(9):603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 17.Boushell LW, Kaku M, Mochida Y, Bagnell R, Yamauchi M. Immunohistochemical localization of matrixmetalloproteinase-2 in human coronal dentin. Arch Oral Biol. 2008;53(2):109–16. doi: 10.1016/j.archoralbio.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nascimento FD, Minciotti CL, Geraldeli S, Carrilho MR, Pashley DH, Tay FR, et al. Cysteine cathepsins in human carious dentin. J Dent Res. 2011;90:506–511. doi: 10.1177/0022034510391906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tersariol IL, Geraldeli S, Minciotti CL, Nascimento FD, Pääkkönen V, Martins MT, et al. Cysteine cathepsins in human dentin-pulp complex. J Endod. 2010;36:475–481. doi: 10.1016/j.joen.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Scaffa PM, Vidal CM, Barros N, Gesteira TF, Carmona AK, Breschi L, et al. Chlorhexidine inhibits the activity of dentin cysteine cathepsins. J Dent Res. 2012;91:420–425. doi: 10.1177/0022034511435329. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson DP. Cysteine peptidases of mammals: their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit Rev Oral Biol Med. 2002;13:238–275. doi: 10.1177/154411130201300304. [DOI] [PubMed] [Google Scholar]
- 22.Tallant C, Marrero A, Gomis-Rüth FX. Matrix metalloproteinases: fold and function of their catalytic domains. Biochim Biophys Acta. 2010;1803:20–28. doi: 10.1016/j.bbamcr.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Optimizing dentin bond durability: control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013 Jan;29(1):116–35. doi: 10.1016/j.dental.2012.08.004. Epub 2012 Aug 16. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshiro K, Inoue S, Sano H, De Munck J, Van Meerbeek B. In vivo degradation of resin–dentin bonds produced by a self-etch and an etch-and-rinse adhesive. Eur J Oral Sci. 2005;113(4):341–8. doi: 10.1111/j.1600-0722.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 25.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res. 2009 Dec;88(12):1101–6. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 26.Carrilho MR, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjaderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. J Dent Res. 2007a;86(6):529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 27.Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45(9):757–65. doi: 10.1016/s0003-9969(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 28.Perdigao J, Denehy GE, Swift EJ. Effects of chlorhexidine on dentin surfaces and shear bond strengths. Am J Dent. 1994;7(2):81–4. [PubMed] [Google Scholar]
- 29.Hase JC, Ainamo J, Etemadzadeh H, Astrom M. Plaque formation and gingivitis after mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidinee digluconate and placebo for 4 weeks, following an initial professional cleaning. J Clin Periodont. 1995;22(7):533–9. doi: 10.1111/j.1600-051x.1995.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 30.Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, Tezvergil-Mutluay A, Carrilho M, Carvalho RM, Tay FR, Pashley DH. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent Mater. 2013 Oct;29(10):999–1011. doi: 10.1016/j.dental.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011 Aug;90(8):953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6:437–9. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Tjaderhane L, Toledano M. Reactivation of quenched endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–6. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical breakdown of dentin hybrid layers in vivo. J Dent Res. 2005;84(8):741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 35.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjäderhane L, Ruggeri A, Jr, Tay FR, Dorigo Ede S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010 Apr;26(4):320–5. doi: 10.1016/j.dental.200911.153. Epub 2009 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricci HA, Sanabe ME, de Souza Costa CA, Pashley DH, Hebling J. Chlorhexidine increases the longevity of in vivo resin-dentin bonds. Eur J Oral Sci. 2010 Aug;118(4):411–6. doi: 10.1111/j.1600-0722.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 37.Osorio R, Yamauti M, Osorio E, Ruiz-Requena ME, Pashley D, Tay F, Toledano M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur J Oral Sci. 2011 Feb;119(1):79–85. doi: 10.1111/j.1600-0722.2010.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JM, Agee K, Sidow SJ, McNally K, Lindsey K, Borke J, et al. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. J Endod. 2012;38:62–65. doi: 10.1016/j.joen.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedran-Russo AK, Vidal CM, Dos Santos PH, Castellan CS. Long-term effect of carbodiimide on dentin matrix and resin-dentin bonds. J Biomed Mater Res B Appl Biomater. 2010 Jul;94(1):250–5. doi: 10.1002/jbm.b.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzoni A, Angeloni V, Apolonio FM, Scotti N, Tjäderhane L, Tezvergil-Mutluay A, Di Lenarda R, Tay FR, Pashley DH, Breschi L. Effect of carbodiimide (EDC) on the bond stability of etch-and-rinse adhesive systems. Dent Mater. 2013 Oct;29(10):1040–7. doi: 10.1016/j.dental.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Mazzoni A, Apolonio FM, Saboia VP, Santi S, Angeloni V, Checchi V, Curci R, Di Lenarda R, Tay FR, Pashley DH, Breschi L. Carbodiimide inactivation of MMPs and effect on dentin bonding. J Dent Res. 2014 Mar;93(3):263–8. doi: 10.1177/0022034513516465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elsaka S, Elnaghy A. Effect of addition of chitosan to self-etching primer: antibacterial activity and push-out bond strength to radicular dentin. J Biomed Res. 2012 Jul;26(4):288–94. doi: 10.7555/JBR.26.20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fawzy AS, Nitisusanta LI, Iqbal K, Daood U, Beng LT, Neo J. Chitosan/Riboflavin-modified demineralized dentin as a potential substrate for bonding. J Mech Behav Biomed Mater. 2013 Jan;17:278–89. doi: 10.1016/j.jmbbm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Toledano M, Yamauti M, Osorio E, Osorio R. Zinc-inhibited MMP-mediated collagen degradation after different dentine demineralization procedures. Caries Res. 2012;46(3):201–7. doi: 10.1159/000337315. [DOI] [PubMed] [Google Scholar]
- 45.Toledano M, Yamauti M, Ruiz-Requena ME, Osorio R. A ZnO-doped adhesive reduced collagen degradation favouring dentine remineralization. J Dent. 2012 Sep;40(9):756–65. doi: 10.1016/j.jdent.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Stanislawczuk R, Costa JA, Polli LG, Reis A, Loguercio AD. Effect of tetracycline on the bond performance of etch-and-rinse adhesives to dentin. Braz Oral Res. 2011 Sep-Oct;25(5):459–65. doi: 10.1590/s1806-83242011000500014. [DOI] [PubMed] [Google Scholar]
- 47.Sorsa T, Tjäderhane L, Konttinen YT, Lauhio A, Salo T, Lee HM, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of Medicine. 2006;38:306–21. doi: 10.1080/07853890600800103. [DOI] [PubMed] [Google Scholar]
- 48.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 49.Hao JL, Nagano T, Nakamura M, Kumagai N, Mishima H, Nishida T. Galardin inhibits collagen degradation by rabbit keratocytes by inhibiting the activation of pro-matrix metalloproteinases. Exp Eye Res. 1999;68:565–572. doi: 10.1006/exer.1998.0637. [DOI] [PubMed] [Google Scholar]
- 50.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, Visintini E, Cadenaro M, Tay FR, De Stefano Dorigo E, Pashley DH. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010 Jun;26(6):571–8. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Green B, Yao X, Ganguly A, Xu C, Dusevich V, Walker MP, Wang Y. Grape seed proanthocyanidins increase collagen biodegradation resistance in the dentin/adhesive interface when included in an adhesive. J Dent. 2010 Nov;38(11):908–15. doi: 10.1016/j.jdent.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Chen M, Yao X, Xu C, Zhang Y, Wang Y. Enhancement in dentin collagen’s biological stability after proanthocyanidins treatment in clinically relevant time periods. Dent Mater. 2013 Apr;29(4):485–92. doi: 10.1016/j.dental.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]