To the Editor
Chronic idiopathic/spontaneous urticaria (CIU/CSU), which affects ~1% of the United States population, is defined as recurrent hives for >6 weeks (Kaplan and Greaves, 2009). Active CIU/CSU patients uniquely display suppressed basophil FcεRI-mediated histamine release (BHR) (Saini, 2014). This is a feature that improves in disease remission (Eckman et al., 2008; Kern and Lichtenstein, 1976; Oliver, 2014). The pathways leading to basophil FcεRI suppression in CIU/CSU are unclear. There is evidence that IgE plays a role in urticaria. For example, injection of cold urticaria patients’ serum into a healthy subject’s skin transfers the sensitivity to the cold stimulus (Gruber et al., 1988). Clinical trials with monoclonal anti-IgE (omalizumab) in subjects with CIU/CSU have shown rapid symptom relief, which implicates IgE in the disease pathway (Gober et al., 2008; Maurer et al., 2013; Saini et al., 2011). Furthermore, the autoimmune theory of CIU/CSU pathogenesis proposes that serum IgG autoantibodies specific to IgE or the IgE receptor directly activate skin mast cells and basophils in a subset of CIU/CSU subjects (Kaplan and Greaves, 2009). In this study, we examined the ability of active CIU/CSU patients’ serum to transfer FcεRI-mediated BHR suppression to healthy basophils and further test the contributions of IgE, IgG, and complement in the observed basophil mediator suppression.
Healthy adults (n=8, 25% female) had repeated blood collections using an IRB approved protocol. CIU/CSU sera were collected as part of a longitudinal study (Baker et al., 2008). Written informed consent was obtained from all subjects, whether for blood or for sera. Remission sera were collected from CIU/CSU patients with no skin symptoms or medication use for ≥2 months (Baker et al., 2008). For CIU/CSU patients using omalizumab, sera collection occurred before and after the patient had been on therapy for 1 to 4 months with marked clinical improvement (Gober et al., 2008). All serum samples were stored at −20°C.
Basophils were purified from venous blood using percoll density gradients (GE Healthcare, Sweden) that were followed by negative selection as described (Vonakis et al., 2007). Purified basophils (>200,000 basophils/condition) were cultured overnight (37°C, 18–22 hours, 5% CO2) in IMDM media (Gibco, New York) ±15% serum v/v (chosen because 15% does not induce BHR) (Vasagar et al., 2006). Each culture experiment utilized a single individual’s serum (CIU/CSU or autologous). IL-3 (0.1–100 ng/ml) (R&D Systems, Minnesota) was added for basophil survival, with no difference observed in results based on the concentration of IL-3. Post-culture analysis of the histamine content of media supernatants and total basophil histamine content revealed no differences between conditions.
After culture, cells were harvested, stimulated with polyclonal goat anti-human IgE (0.01–1 μg/ml) or fMLP (10−6 M) (Sigma Aldrich, Missouri) at >10,000 basophils/stimulation, and assayed for histamine by fluorimetry (Vonakis et al., 2007). In select experiments, CIU/CSU serum were tested as follows: 1) paired sera from patients before and after remission, 2) serum depleted of IgE by overnight incubation with omalizumabR covalently coupled to Sepharose-CL-4B as previously validated to achieve a 98% IgE depletion rate (Eckman et al., 2008), 3) serum heat inactivated at 56°C for 30 minutes to denature IgE and inactivate complement (Ishizaka et al., 1986), 4) serum IgG-depleted by adsorption with Staphylococcal Protein G Sepharose 4B-CL (Fu et al., 2007) (>93% depletion).
All statistics were performed with Prism 4.0 using the Wilcoxon signed rank test between the two groups being compared at the same concentration of stimulant. Error bars in the graphs indicate the standard error of the mean (SEM).
Healthy donor basophils cultured in active CIU/CSU subjects’ serum exhibited marked suppression of FcεRI-mediated BHR when compared to media (Figure 1a), or media with autologous serum (Figure S1a). In contrast, basophils cultured in media or autologous serum showed no significant difference in subsequent BHR (Figure S1b). Further, fMLP induced BHR did not differ between the media vs CIU/CSU serum or autologous serum vs CIU/CSU serum cultures (data not shown).
Fig 1. Normal basophils cultured in individual CIU/CSU serum show a suppressed BHR profile that reverses in disease remission serum.
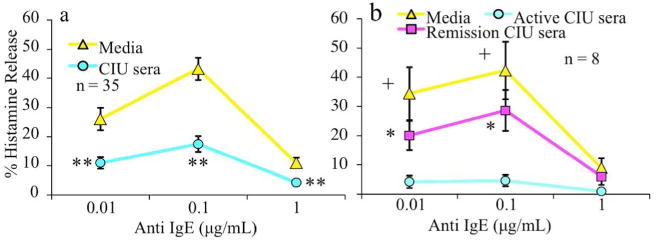
Purified basophils from healthy donors were cultured overnight in media or CIU/CSU serum and assessed for FcεRI mediated BHR. a) CIU/CSU serum cultured basophils showed significant BHR suppression as compared to parallel media cultured basophils (n=35). (**p<0.0001 to matched control) b) Basophils cultured in CIU/CSU patients’ serum obtained during active disease showed marked BHR suppression relative to culture in the same subject’s serum during remission (n=8). No statistical differences were observed between the levels of BHR from cells cultured in serum from CIU/CSU patients in remission or media alone. All media versus active serum statistics were +p<0.05, while all remission versus active serum statistics were *p<0.01.
In remission, CIU/CSU patients show an increase in their basophil HR profile (Eckman et al., 2008). Therefore, we compared the effect of serum culture from CIU/CSU patients during active disease and in remission. Basophils cultured in remission serum displayed significantly less BHR suppression than active serum culture and were similar to culture in media alone (Figure 1b).
Given that omalizumab therapy rapidly reduces symptoms in CIU/CSU patients (Gober et al., 2008; Maurer et al., 2013; Saini et al., 2011), we explored the role of IgE in conveying BHR suppression. IgE depletion by immunoadsorption failed to change the degree of BHR suppression obtained with intact serum (Figure 2a). Similarly, the use of heat-inactivated serum, inactivating IgE and complement, also did not alter BHR suppression after culture (Figure 2b). Serum from patients with CIU/CSU disease before and after clinical improvement on omalizumab therapy (Figure 2c) did not differ in their capacity to transfer BHR suppression. Likewise, basophils cultured in IgG depleted CIU/CSU serum showed similar suppression of HR to sham-depleted serum (Figure 2d).
Fig 2. The source of the suppressive factor is not complement, IgE, or IgG autoantibody.
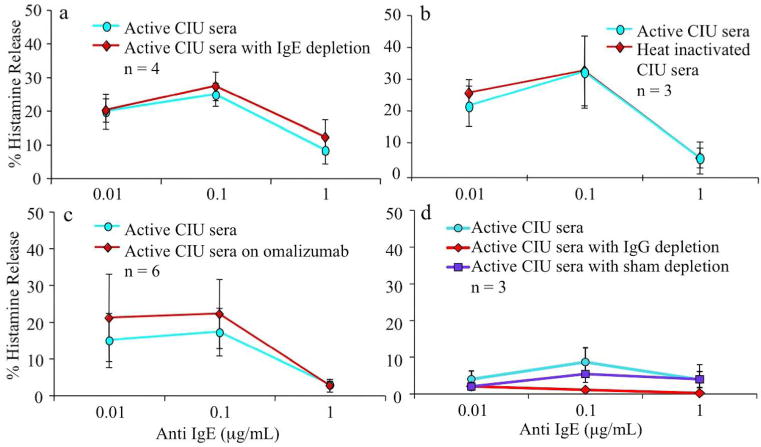
FcεRI mediated BHR suppression does not appear to be affected by a) serum IgE depletion by immunoadsorption (n=4), or b) heat inactivation of IgE and complement (n=3). The 3 sera used in figure 2a contained 33, 212, and 355 ng/ml of IgE and after immunoadsorption, levels were reduced levels to 2% of original values. c) Serum from patients pre and post omalizumab therapy (n=6) showed no differences in the degree of histamine suppression observed. d) IgG was depleted from active CIU/CSU serum using protein G Sepharose, and evaluated in comparison to untreated serum for BHR suppression (n=3). No statistical differences were seen among the active, IgG depleted, or sham depleted sera.
An unclear mechanism suppresses the FcεRI pathway of blood basophils in subjects with active CIU/CSU. We have reported altered expression of inhibitory molecules in the IgE receptor signaling pathway (Vonakis et al 2007). In remission, basophil FcεRI histamine response increases towards a more normal profile (Eckman et al., 2008). To gain insights into factors responsible for basophil FcεRI suppression, we examined the effects of culture of healthy donor basophils with well-characterized CIU/CSU patients’ serum. We consistently observed marked suppression of FcεRI-mediated BHR in cultures using active CIU/CSU patients’ serum, but found the serum from patients in remission had less capacity to suppress basophil HR.
Given the putative roles of IgE in CIU/CSU in disease, IgE depletion and heat denaturation of serum from active CIU/CSU patients was examined for its’ impact on the suppression of donor basophil HR. Such approaches failed to alter the ability of these sera to suppress basophil mediator release. Omalizumab therapy led to rapid symptom relief in CIU/CSU subjects with symptom rebound when therapy was discontinued (Maurer et al., 2013). However, culture with CIU/CSU serum in the presence of therapeutic levels of omalizumab also failed to impair the transfer of BHR suppression. These observations suggest that the suppressive factor in the serum of CIU/CSU patients acts through the IgE receptor pathway, but is not IgE. Furthermore, IgG depletion of active CIU/CSU serum also failed to alter the transfer of mediator suppression, indicating that IgG antibodies are not directly involved in the phenomenon.
While the nature of the serum factor responsible for suppressing BHR in CIU/CSU remains unknown, its significance as a potential disease biomarker for CIU/CSU is apparent, but more work is needed. The suppression factor is present in active disease, reduced as the patient experiences natural disease remission and is independent of IgE and IgG antibodies. Elucidation of the nature of this factor may provide insights into the pathogenesis of CIU/CSU.
Supplementary Material
Acknowledgments
We would like to thank Drs. Becky Vonakis, Don MacGlashan, and John Eckman for their comments, suggestions, and support for this paper.
Grant funding: Dr. Saini received support as Cosner Scholars in Translational Research from Johns Hopkins University. This work was supported by the Asthma and Allergic Disease Research Centers grant U19AI070345 from the National Institutes of Health and AAAAI Bridge Award.
Abbreviations
- CIU/CSU
chronic idiopathic/spontaneous urticaria
- BHR
basophil histamine release
- fMLP
N-formylmethionine-leucyl-phenylalanine
Footnotes
Conflicts of Interest
The authors have no conflicts of interest with any companies having an interest in the topic of our manuscript.
References
- Baker R, Vasagar K, Ohameje N, et al. Basophil histamine release activity and disease severity in chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2008;100:244–9. doi: 10.1016/S1081-1206(10)60449-8. [DOI] [PubMed] [Google Scholar]
- Eckman JA, Hamilton RG, Gober LM, et al. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol. 2008;128:1956–63. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- Fu Q, Bovenkamp DE, Van Eyk JE. A rapid, economical, and reproducible method for human serum delipidation and albumin and IgG removal for proteomic analysis. Methods Mol Biol. 2007;357:365–71. doi: 10.1385/1-59745-214-9:365. [DOI] [PubMed] [Google Scholar]
- Gober L, Sterba P, Eckman J, et al. Effect of Anti-IgE (Omalizumab) in Chronic Idiopathic Urticaria Patients. J Allergy Clin Immunol. 2008;121:S147. [Google Scholar]
- Gruber BL, Baeza ML, Marchese MJ, et al. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–7. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- Ishizaka T, Helm B, Hakimi J, et al. Biological properties of a recombinant human immunoglobulin epsilon-chain fragment. Proc Natl Acad Sci U S A. 1986;83:8323–7. doi: 10.1073/pnas.83.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777–87. doi: 10.1111/j.1365-2222.2009.03256.x. [DOI] [PubMed] [Google Scholar]
- Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest. 1976;57:1369–77. doi: 10.1172/JCI108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Rosen K, Hsieh HJ, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013;368:924–35. doi: 10.1056/NEJMoa1215372. [DOI] [PubMed] [Google Scholar]
- Oliver ET, Sterba PM, Saini SS. Interval Shifts in Basophil Measures correlate with Disease Activity in Chronic Idiopathic Urticaria. Allergy. 2014 doi: 10.1111/all.12578. In press. [DOI] [PubMed] [Google Scholar]
- Saini S, Rosen KE, Hsieh HJ, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567–73 e1. doi: 10.1016/j.jaci.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Saini SS. Chronic spontaneous urticaria: etiology and pathogenesis. Immunol Allergy Clin North Am. 2014;34:33–52. doi: 10.1016/j.iac.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasagar K, Vonakis BM, Gober L, et al. Evidence of In Vivo Basophil Activation in Chronic Idiopathic Urticaria. Clin Exp Allergy. 2006;36:770–6. doi: 10.1111/j.1365-2222.2006.02494.x. [DOI] [PubMed] [Google Scholar]
- Vonakis BM, Vasagar K, Gibbons JSC, et al. Basophil FceRI histamine release parallels expression of Src-homology2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol. 2007;119:441–8. doi: 10.1016/j.jaci.2006.09.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


