Abstract
The integration of cells with their extracellular environment is facilitated by cell surface adhesion receptors, such as integrins, which play important roles in both normal development and the onset of pathologies. Engagement of integrins with their ligands in the extracellular matrix, or counter receptors on other cells, initiates the intracellular assembly of a wide variety of proteins into adhesion complexes such as focal contacts, focal adhesions and fibrillar adhesions. The proteins recruited to these complexes mediate bidirectional signalling across the plasma membrane and as such help to coordinate and / or modulate the multitude of physical or chemical signals to which the cell is subjected. The protocols in this unit describe two approaches for the isolation or enrichment of proteins contained within integrin-associated adhesion complexes together with their local plasma membrane / cytosolic environments from cells in culture. In the first protocol integrin-associated adhesion structures are affinity isolated using microbeads coated with extracellular ligands or antibodies. The second protocol describes the isolation of ventral membrane preparations that are enriched for adhesion complex structures. The protocols permit the determination of adhesion complex components by subsequent downstream analysis by Western blotting or mass spectrometry.
Keywords: Integrins, cell adhesion, adhesion complexes, extracellular matrix, affinity purification, ventral membranes
INTRODUCTION
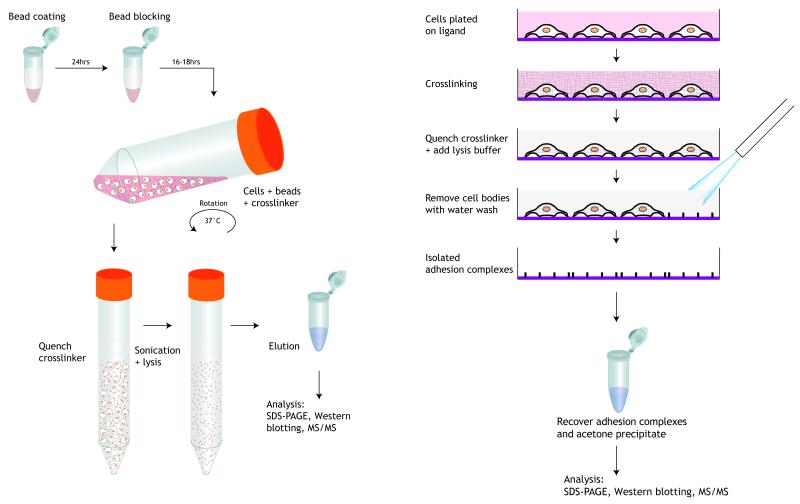
This unit describes two methods for the isolation of adhesion complexes: the first for complexes formed upon cell attachment to ligand-coated microbeads, the second for complexes formed on the bottom of a cell that is attached to an extracellular matrix (ECM)-coated tissue culture dish. Cell-ECM attachment is mediated by a connection termed the adhesion nexus that consists of clustered transmembrane adhesion proteins, typically integrins, and associated adaptor and signalling proteins (Wehrel-Haller, 2012). These complexes couple ECM fibres to the intracellular cytoskeleton as well as acting as scaffolding and signalling hubs to mediate a diverse array of cellular processes such as migration and proliferation (Wolfenson et al. 2013). Biochemical analysis of adhesion complex composition has primarily been performed in a candidate based manner and has focused on the immunoprecipitation of individual adhesion components. Whilst greater than 200 proteins, termed the adhesome (Zaidel-Bar et al. 2007; Winograd-Katz et al. 2014), have been reported to be associated with adhesion complexes, isolation of the adhesion nexus has proved problematic due to the inherent instability and inaccessibility of integrin-associated complexes. Therefore conventional coimmunoprecipitation approaches are not suitable for their global analysis. The signalling complex components linked to transmembrane receptors such as integrins are highly dynamic, part of the insoluble cytoskeletal fraction of the cell under standard extraction conditions, and disassociate in stringent lysis buffers. This unit describes two strategies that stabilise adhesion complexes in live cells from their native cell culture environment using membrane-permeable crosslinker, coupled with removal of the cell body and cytoplasmic proteins resulting in the enrichment of adhesion complexes from cells attached to integrin ligands such as the ECM component fibronectin (Figure). The preparation of cells for spreading on ECM-coated plates in Basic Protocol 2 is essentially identical to that described in Unit 9.1 (Cell-Substrate Adhesion Assays).
Figure 9.8.1.
Left: Flow chart for isolation of integrin-based adhesion complexes: Basic Protocol 1
Right: Flow chart for isolation of integrin-based adhesion complexes: Basic Protocol 2
BASIC PROTOCOL 1 - MICROBEAD-BASED ISOLATION OF INTEGRIN ADHESION COMPLEXES FOR PROTEOMIC ANALYSIS
The method described here is for K562 cells attached to fibronectin as an ECM substrate for 1 hour (Figure). This approach will primarily isolate complexes associated with the integrin α5β1. The same basic protocol has been used for VCAM-1 binding to α4β1 (Humphries et al. 2009; Byron et al. 2011; Byron et al. 2012) and can be used for a range of cell lines (that can be kept in suspension for short periods), integrin ligands or antibodies, and spreading times.
Materials
Cells (as appropriate; K562 cells used as example)
Complete, FBS-containing cell culture medium (as appropriate)
Dynabeads® M-450 Tosylactivated (Dynal Biotech, cat no.140.13, available from Thermo Fisher Scientific). Supplied at 4 × 108 beads/ml in distilled H2O.
1mg/ml bovine fibronectin solution (F1141; Sigma-Aldrich) or other integrin-specific or control ligands or antibodies. For basic protocol 1 the stock solution of fibronectin and other ligands are diluted as stated in steps 2-4
0.1M sodium phosphate buffer, pH 7.4 to 8
1% (w/v) bovine serum albumin (BSA; A7638; Sigma-Aldrich) in 0.1M sodium phosphate buffer, pH 7.4 to 8
0.1% (w/v) bovine serum albumin (BSA; A7638; Sigma-Aldrich) in PBS−
0.1% (w/v) bovine serum albumin (BSA; A7638; Sigma-Aldrich) in 0.2M Tris-Cl, pH 8.5
Dulbecco’s modified Eagle’s medium containing 25 mM HEPES (DMEM-HEPES; D6171; Sigma-Aldrich; also available from Thermo Fisher Scientific)
0.2% (w/v) bovine serum albumin (BSA; A7638; Sigma-Aldrich) in DMEM-HEPES
PBS− ; Calcium and magnesium-free Dulbecco’s phosphate-buffered saline (CMF-DPBS)
Trypsin-EDTA solution (available from various molecular biology suppliers)
Manganese chloride (Sigma-Aldrich)
DTBP crosslinker (20665; Thermo Fisher Scientific)
1 × RIPA Buffer (for microbead assay; see recipe)
5 × reducing sample buffer (See Recipe)
Cytoskeletal stabilizing buffers (prepare as described in Reagents and Solutions):
CSK− (see recipe)
CSK− with Tris (see recipe)
CSK+ (see recipe)
0.1% (w/v) crystal violet
10% (v/v) acetic acid
Temperature controlled shaking incubator for bead coating and recovery of adhesion complexes (Thermomixer compact, Eppendorf).
Magnetic particle separators (MPC; Dynal, Thermo Fisher Scientific): MPC-S for 1.5ml microcentrifuge tubes and MPC-6 for 14 ml conical tubes.
15- and 50-ml conical centrifuge tubes (e.g., BD Falcon)
Refrigerated centrifuge
Platform shaker
Microscope slides and coverslips
Light microscope
96-well plates (3596; Corning Costar)
96-well plate reader spectrophotometer
Bioruptor UCD-200 (Diagenode) or Sonicator VibraCell VCX 500, (Sonics & Materials)
Additional reagents and equipment for basic cell culture techniques including trypsinization and counting cells (UNIT 1.1)
Basic Protocol Steps
Prepare cells
- 1. Passage cells appropriately such that there will be approximately 1 × 108 log-phase cells per experimental condition on the day of adhesion complex isolation.For K562 cells, we recommend seeding 1.0–1.5 × 107 log-phase cells into fresh culture medium three days before the ligand affinity purification assay.
Coating of beads with ligand
- 2. In summary, and unless otherwise determined, we aim to coat 5 × 107 beads per experimental condition with ligand at 200 μg/ml at least 2 days prior to adhesion complex isolation (bead binding assay). To achieve this, resuspend stock bead suspension thoroughly by vortexing and inverting, then transfer sufficient beads (5 × 107 beads per condition) to a 1.5-ml Eppendorf and separate using a magnet (MPC, Dynal) for 1 min.E.g. for four conditions use 0.5 ml of beads (4 × 5 × 107 = 2 × 108 beads).A variety of magnetic beads types are available and have been used with this protocol. We recommend by starting with Dynabeads® M-450 Tosylactivated (Dynal, Invitrogen) .
3. Discard supernatant and wash twice with initial bead volume of 0.1 M sodium phosphate buffer, pH 7.4–8.
- 4. Resuspend beads in 0.1 M sodium phosphate buffer, pH 7.4–8 (minus ligand volume) such that 15–25 μg of ligand is present per 5 × 107 beads at 4–8 × 108 beads/ml. Then add ligand to 200 μg/ml and mix thoroughly by vortexing and inverting.If resuspending beads from the stock solution this equates to the same volume as taken for each condition from the stock. E.g. for four conditions of fibronectin-coated beads, resuspend 2 × 108 beads in 400 μl of PB then add 100 μl of 1 mg/ml stock fibronectin (Sigma-Aldrich) resulting in a final concentration of fibronectin of 200 μg/ml in 500 μl final volume.
5. Rotate beads in a shaking incubator (Thermomixer compact, Eppenforf UK), 1400 rpm, 25°C, 15 min.
- 6. Add BSA to beads (0.01–0.5% w/v).E.g. to 500 μl of beads, add 100 μl of 1% (w/v) BSA in phosphate buffer to give 0.167% (w/v) final concentration. BSA is added to aid orientation and presentation of ligands to cells.
7. Continue incubation, 1400 rpm, 25 °C, 24 h.
Block Beads
8. Separate coated beads using a magnetic particle separator for 1 min.
9. Discard supernatant and wash beads twice with initial bead volume of 0.1% BSA in PBS–, incubating 5 min on ice during each wash, then separate using a magnetic particle separator
10. To deactivate remaining free tosyl groups, add 0.5–1 ml of 0.1% (w/v) BSA in 0.2 M Tris-HCl, pH 8.5.
11. Rotate beads in a Thermomixer for 16 hr (overnight) at 1400 rpm, 25 °C.
12. Ensure that all stock solutions are prepared in advance for the bead-cell incubation step on the following day - e.g. 5% (w/v) Triton X100 detergent for preparing RIPA buffer, DMEM-HEPES, 1 M sucrose, 100 mM PIPES, pH 6.8 (for preparing the cytoskeletal stabilization buffers), 0.1% (w/v) crystal violet,and protease/phosphatase inhibitors for solutions requiring them (see Reagents and Solutions).
Incubate beads with cells
Aim to achieve a bead:cell ratio of 1:2 (i.e. 5 × 107 beads:1 × 108 cells).
13. Prewarm 12.5 ml of DMEM-HEPES and 20 ml of 0.2% (w/v) BSA in DMEM-HEPES per condition to 37 °C.
14. Separate blocked beads from step 11 using the magnetic particle separator for 1 min.
- 15. Discard bead supernatant and wash beads twice with 0.5 ml of 0.1% (w/v) BSA in PBS–, incubating on ice during each wash, 5 min.If using beads for bead-cell incubation immediately discard supernatant and wash once with 0.5 ml of DMEM-HEPES. Alternatively, store coated, blocked and washed beads in 0.1% (w/v) BSA in PBS–, 4 °C (up to one month). This is the manufacturers’ recommendation and works well for antibody-coated beads, however for ligands such as fibronectin we recommend for best results that the beads are used immediately.
- 16. If using adherent cells, wash flasks with 25 ml of PBS–, then detach cells with 25 ml of 1x trypsin/EDTA, 37 °C, incubating for 5 min (see UNIT 1.1 for additional detail on trypsinization). Decant detached cells into 25 ml complete (i.e. FBS-containing) medium to quench trypsin.Please note that due to the time taken to detach cells with trypsin or collect cells by centrifugation, it may be necessary to start steps 16 to 19 before step 14
17. For both suspension and adherent cells, spin down cells for 4 min at 280 × g, room temperature, discard supernatant, and wash pellet with 25–50 ml of prewarmed DMEM-HEPES.
18. Count cells using a haemocytometer (UNIT 1.1).
19. Spin down cells for 4 min at 280 × g, room temperature, discard supernatant and resuspend pellet in a minimum of 10.5 ml of prewarmed 0.2% (w/v) BSA in DMEM-HEPES supplemented with 0.2mM Mn2+ (as manganese chloride) per condition, ideally to give a concentration of 1 × 107 cells/ml.
20. Rest cells for 15 to 30 min at 37 °C in a tissue culture incubator while final preparation of the beads is performed.
21. Resuspend washed beads to 2 × 108 beads/ml in 0.2% (w/v) BSA in DMEM-HEPES.
- 22. Add 9.75 ml of rested cells (i.e. ~1 × 108 cells) to a 50-ml conical tube for each experimental condition and add 5 × 107 beads in DMEM-HEPES from step 15 or step 21.This will result in a bead:cell ratio of 1:2 (i.e. 5 × 107 beads:1 × 108 cells). This ratio can be optimised by user depending on cell type used in the assay.
- 23. Tilt gently to mix.Optional: Wrap Parafilm around the lid of each Falcon tube to prevent leakage.
- 24. Rotate cells/beads at a near-horizontal position (to ensure thorough mixing) on a shaking platform at 70 rpm, 37 °C, 30 min.Rest the conical tubes on the edge of a square petri dish to prevent leakage.Some cell types will attempt to engulf ligand-coated beads. To avoid this, bead-cell incubations can be performed at room temperature .
- 25. Remove DTBP crosslinker from dessicator in the refrigerator and allow it to reach room temperature before use (15-30 minutes).The DTBP crosslinker needs to be stored at 4°C and equilibrated to room temperature prior to use to prevent deterioration of the reagent.
- 26. Optional: Prepare a total cell lysate of each cell line used to verify expression of proteins by Western blotting:
- Spin down 500 μl of unused cells (i.e. 5 × 107 cells) for 4 min at 250 × g, room temperature
- Wash pellet with 500 μl of PBS–, then spin down cells for 4 min at 250 × g, room temperature
- Lyse pellet in 500 μl of 1x RIPA buffer (supplemented with protease and phosphatase inhibitors as described in Reagents and Solutions) on ice, 30 min.
- Spin down insoluble debris for 15 min at 21,900 × g, 4 °C.
- Collect supernatant and add reducing sample buffer to 1x.
- Store total cell lysate at –80 °C until required.
Crosslink proteins
- 27. After 20 min of cell/bead incubation, prepare DTBP crosslinker. Weigh sufficient DTBP powder to make up a 100 mM stock.For example, for two conditions at 3 mM final DTBP concentration, you will need 300 μl of 100 mM stock per 10-ml cell/bead condition = 2 × 300 μl = 600 μl required. Therefore, make up enough for 700 μl stock, weighing out 21.63 mg DTBP.CAUTION: For safety, wear a face mask while handling the DTBP powder.
- 28. Make up 100 mM DTBP stock in DMEM-HEPES (no BSA) immediately before use, then add appropriate volume of DTBP stock to cells/beads after 30min of bead/cell incubation.Do not add BSA to this solution as this will quench the DTBPFor example, for 3 mM final DTBP concentration, add 300 μl of 100 mM stock DTBP per 10-ml cell/bead condition.Do not allow DTBP stock to turn cloudy before use.
29. Continue with the cell/bead incubation with shaking at 70 rpm on a platform shaker, 37 °C, for a further 30 min.
30. Meanwhile, prepare cytoskeletal (CSK) buffers by making 6.25 ml CSK–, 6.25 ml CSK– with Tris and 25 ml CSK+ per condition. Prechill all buffers on ice.
31. Following cell/bead incubation with crosslinker, add 200 μl 1 M Tris-HCl, pH 8.5, to each condition (20 mM final Tris-HCl concentration if added to 10-ml cell/bead volume) to quench DTBP and incubate 2 to 3 min at room temperature.
32. Gently transfer cells/beads to 15-ml conical centrifuge tubes using a 3-ml plastic Pasteur pipette. Separate bead-bound cells using the magnetic particle separator (MPC-6) for 2 to 3 min.
33. Gently remove and discard supernatant. Keep cells/beads and reagents on ice wherever possible during the following washing and lysis.
34. Wash used 50-ml conical tubes with 5 ml of CSK– with Tris and use this solution to wash cells/beads by gentle pipetting. Separate cells/beads using magnetic particle separator, then discard supernatant.
35. Wash with 5 ml of CSK– by gently pipetting up and down. Separate cells/beads using magnetic particle separator.
Assess bead-cell binding
- 36. Remove 5 μl of cells/beads, place sample on a microscope slide and cover with a coverslip for analysis of bead–cell binding by light microscopy.At this point it will be possible to observe and quantify by counting the number of bead-bound cells (see UNIT 1.1). Experiments performed with control ligands and / or in the presence of anti-functional antibodies can be used to ascertain the specificity of bead-cell binding. Cells and beads may need to be diluted ten-fold to enable accurate counting.
- 37. Optional: To permit an alternative assessment of bead-cell binding, remove an additional 10μl aliquot in triplicate of cells/beads and transfer to wells of a 96-well Costar plate for a crystal violet assay.This assay is based on that described in Unit 9.1 (Humphries, 1998, Cell-Substrate Adhesion Assays):
- Make wells up to 100 μl with 0.2% (w/v) BSA in DMEM5.Optional: Use 20 μl or 100 μl of cells/beads in triplicate for increased signal.
- Also, add a standard curve of a known number of untreated cells to the 96-well plate in triplicate (0%, 25%, 50%, 75%, and 100%) in a total volume of 100 μl of 0.2% (w/v) BSA in DMEM5.
- Add 10 μl of 50% (v/v) gluteraldehyde to wells at room temperature and incubate 30 min at room temperature to fix cells.
- Wash 96-well plate four times with 200 μl of PBS–.
- Stain samples with 50 μl of 0.1% (w/v) crystal violet for 1 h or overnight at room temperature.
- Aspirate crystal violet.
- Wash wells once with 200 μl of distilled water, then four times, each time with 400 μl distilled water.
- Destain with 100 μl of 10% (v/v) acetic acid for 5 min at room temperature.
- Read plate on a 96-well plate reader at absorbance of 570 nm.
Isolate adhesion complexes
38. Separate cells/beads using a magnetic particle separator, then discard supernatant.
39. Resuspend cells/beads in 4 ml CSK+ and transfer two 2-ml aliquots to 15-ml conical centrifuge tubes for lysis, on ice.
- 40. Sonicate samples using a prechilled Bioruptor UCD-200* (power setting M, 30 sec on/30 s off, five times). Aim for a total lysis time of no more than 45 min.Alternatively a probe-based sonicator fitted with a 6mm diameter tip can be used with a 20% amplitude setting for four 5 sec applications in a 2-5ml volume (VibraCell VCX 500, Sonics & Materials). Cells should be kept at 4°C between applications of sonication
- 41. Remove 5 μl of beads, place sample on a microscope slide and cover with a coverslip for analysis of cell lysis by light microscopy.At this point it will be possible to assess the extent of cell disruption by sonication. If sonication has been performed sufficiently only cell debris and beads will be visible. If whole cells bound to beads are still visible the sonication requires optimisation and either extended sonication or increased sonication amplitude is required.
42. Separate beads using the magnetic particle separator for 2 to 3 min. Gently remove and discard supernatant.
43. Wash four times with 5 ml of CSK+ by gently pipetting up and down, separate beads using the magnetic particle separator, then discard supernatant.
- 44. Remove 5 μl of beads, place sample on a microscope slide and cover with a coverslip for analysis of cell lysis and washing by light microscopy.At this point it will be possible to further assess the extent of cell disruption by sonication and washing. If sonication and washing have been performed sufficiently only beads will be visible.
45. Gently resuspend beads in 1 ml of CSK+ and transfer to 1.5-ml microcentrifuge tubes.
46. Separate beads using the magnetic particle separator (MPC-S) for 2 to 3 min. Gently remove and discard supernatant.
47. Elute proteins from beads using 150 to 300 μl of 2x reducing sample buffer with shaking at 1400 rpm in a shaking incubator at 70 °C for ~30 min.
48. Cleave DTBP by incubating samples in a shaking incubator for 5 min at 1400 rpm at 95 °C
49. Separate beads using the magnetic particle separator for 2 to 3 min. Gently remove and retain adhesion complex-containing supernatant.
50. Store samples at –80 °C until required for analysis.
BASIC PROTOCOL 2 - ISOLATION OF INTEGRIN-ASSOCIATED ADHESION COMPLEXES FROM FIBROBLASTS ATTACHED TO A 2-D FIBRONECTIN SUBSTRATE
The method described here is for fibroblasts spread on a fibronectin substrate for 2 hours to allow robust formation of adhesion structures (Fig 9.8.1). This approach will primarily isolate complexes associated with the integrin α5β1 and αVβ3; however, the same basic protocol can be used for a range of cell lines, ECM components and spreading times.
Materials
10 μg/ml bovine fibronectin (F1141; Sigma Aldrich) in Dulbecco’s phosphate-buffered saline (DPBS; with Ca2+ and Mg 2+) prepared fresh from stock solutions immediately before use
10 μg/ml apotransferrin (T5391; Sigma Aldrich) or 10 μg/ml poly-D-Lysine (P6407; Sigma Aldrich, Mr 70,000 to 150,000) in Dulbecco’s phosphate-buffered saline (DPBS; with Ca2+ and Mg 2+)
PBS− : Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ (CMF-DPBS)
Heat Denatured BSA solution (See Recipe)
Human foreskin fibroblast (HFF) cells growing in 10-cm plates at 70% to 80% confluence
Dulbecco’s modified Eagle’s medium (D5796; Sigma Aldrich), serum-free
DTBP crosslinker (Thermo Fisher 20665)
Dulbecco’s modified Eagle’s medium containing 25 mM HEPES (DMEM-HEPES; Invitrogen or Sigma-Aldrich D6171)
1M Tris-HCl, pH8
Modified RIPA buffer (See Recipe)
Adhesion recovery solution (See Recipe)
Acetone −20°C
5 × reducing sample buffer (See Recipe)
100 × 20mm (10-cm) sterile tissue culture plates (Corning Costar, cat. no. 430167,)
Cell scrapers (Greiner Bio-One GmbH, cat. no. 541 070)
Light Microscope
Water tap with 8mm plastic tubing attached
Refrigerated benchtop microcentrifuge capable of holding 1.6ml tubes and centrifugation at 22,000xg
Additional reagents and equipment for basic cell culture techniques including trypsinization and counting cells (UNIT 1.1)
Basic Protocol Steps
Plate cells on Fibronectin
- 1. Coat six 10-cm-diameter tissue culture plates per condition with either 5ml of 10 μg/ml fibronectin solution or 5ml of 10 μg/ml control ligand, (e.g. apotransferrin or poly-D-Lysine solution) overnight at 4°CSix plates of cells should provide enough material to run three SDS-PAGE gels per condition for further analysis e.g. Western blotting. However, the amount of material recovered may differ for each experimental set-up and therefore number of plates required should be assessed initially and may have to be altered dependent upon the cell type or ECM ligand used.Alternatively plates can be coated for 1-2 hours at room temperature.
2. Wash plates twice with PBS then add 5ml heat-denatured BSA solution for 30 minutes at room temperature to block unoccupied sites on plates to promote ECM-specific cell anchorage.
- 3. Trypsinize (see UNIT 1.1) twelve 10-cm plates of Human Foreskin Fibroblast (HFF) cells at 70-80% confluency, then resuspend in 60ml serum-free DMEM-HEPES and place in 37°C incubator for 30 minutes to recover.These suggested cell numbers are for a pairwise comparison of integrin ligand versus control. A suitable starting point for number of cells added is 0.5 −1 × 106 cells per 10-cm-diameter plate.
4. Remove BSA from plates (see step 2) and wash twice with PBS
5. Add 5ml of cell suspension to each plate, then incubate at 37°C for 2 hours prior to cross-linking.
Isolate adhesion complexes
- 6. At a time point 15-30 minutes prior to crosslinking, take DTBP out of the desiccator in the refrigerator to equilibrate to room temperature and pre-warm DMEM-HEPES at 37°CThe DTBP crosslinker needs to be stored at 4°C and equilibrated to room temperature prior to use to prevent deterioration of the reagent.
- 7. Weigh out sufficient DTBP to prepare a 6mM solution (1.85mg/ml)Weigh a sufficient amount for 5-10 ml crosslinker solution per plate
8. Wash plates twice with 5ml of pre-warmed DMEM-HEPES to remove non-adherent cells
9. Resuspend DTBP in DMEM-HEPES at a concentration stated in step 7 and add to cells at 5 to 10 ml per plate. Incubate 5 min at 37°C
- 10. Quench DTBP by adding 150μl of 1M Tris-HCl, pH8, directly to crosslinking medium and incubate for 2 min at room temperatureIt is important to quench excess DTBP crosslinking reagent to prevent non-specific crosslinking reactions occurring.
- 11. Remove quenched crosslinking medium then place the plate of crosslinked cells on ice and wash once with cold PBSFor processing a large number of plate, those awaiting treatment can be left in cold PBS while other plates are dealt with.
12. Add 5ml of modified RIPA buffer per plate. Leave for 2 to 3 minutes to ensure sufficient cell lysis. Check crosslinking efficiency under the microscope, cells should still be visible.
- 13. Remove cell bodies by washing plate with tap water directed through an 8-mm-diameter tube using a flow rate of approximately 150ml per secondWash each plate for approximately 10 sec. Washing should be performed in a methodical fashion and standardised for each assay. In our experience, washes performed by directing the water flow horizontally, vertically then circularly into the middle and back out again within the plate is a good starting point.
- 14. Add 5ml cold PBS, then check under a microscope to ensure cell bodies and nuclei are efficiently removed - i.e. no longer visible.If cell bodies and nuclei are still visible, the washing procedure needs to be optimised.
15. Remove PBS then drain off excess liquid for 1 min on ice. Remove as much as possible to avoid dilution of the adhesion recovery buffer used in the next step.
- 16. Add 100μl Adhesion Recovery Solution to one plate then scrape and collect solution.Remove solution, add to next plate and repeat to combine recovery of adhesion complexes from multiple plates for each condition. Try to avoid introduction of bubbles during manipulations. Users may wish to assess the losses due to inefficient recovery across multiple plates during this procedure. An alternative protocol for the isolation of adhesion complexes is based on the denudation of cells as described in UNIT 10.9 (Beacham et al, Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts). As an alternative to the addition of modified RIPA in step 12, cells are treated with extraction buffer (20mM NH4OH/0.5% (w/v)Triton-X100 in PBS) and then subjected to probe sonication (see Basic Protocol 1) until cell lysis has occurred in all areas of the plate (1 to 2 minutes per plate depending on the cell type). Plates are then washed five times with cold extraction buffer followed by five times with cold PBS before recovery of adhesion complexes as in step 15.
Precipitate adhesion complex proteins with acetone
Acetone precipitation of the recovered adhesion complex proteins allows the reduction of the volume of the isolated protein sample to enable a manageable and standardised volume for performing SDS-PAGE and Western blotting or sample preparation for mass spectrometry.
- 17. Add 4 volumes of −20°C acetone to each sample (i.e. 800μl acetone to 200μl sample)At this point samples collected may need to be aliquoted into separate 1.5-ml microcentrifuge tubes
- 18. Incubate at −80°C overnightDry ice maybe used for this step and a 4-hr incubation may be sufficient.
19. Collect precipitated proteins by centrifugation in a bench top refrigerated microcentrifuge for 20 minutes at 16000xg, 4°C
20. Take off supernatant without disturbing the pellet
- 21. Wash with same volume of acetone and repeat the centrifugation from step 20. Take off supernatant and dry the protein pellet in fume hood at room temperature for about 20 min.Repeat this wash step if necessary.
- 22. Resuspend pellet in 90μl per condition of 2x reducing SDS-PAGE Sample Buffer and then heat at 70°C in a shaker at 1000rpm for 20 minutes.90μl allows you to run three lots of 30μl on gels for further analysis while maintaining a large enough volume to efficiently solubilise all the proteins precipitated. This volume can be altered and, in general, two 10-cm plates will give you sufficient material to perform one SDS-PAGE gel and Western blot.
REAGENTS AND SOLUTIONS
Use deionized or distilled water in all recipes and protocol steps.
Adhesion recovery solution
125mM Tris-HCl, pH6.8
1% (w/v) SDS
150mM dithiothreitol (DTT)
Prepare fresh immediately before use.
Cytoskeletal stabilising (CSK) buffers
CSK– buffer:Combine 10 mM PIPES (pH 6.8), 50 mM sodium chloride, 150 mM sucrose, 3 mM magnesium chloride and 1 mM manganese chloride.
CSK–buffer with Tris:Supplement CSK– buffer with 20 mM Tris-HCl (pH 8.5).
CSK+ buffer: Supplement CSK– buffer with 0.5% (w/v) Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 0.5 mM AEBSF and 2 mM sodium orthovanadate. Prepare all CSK buffers immediately before use from stock solutions (see Table 9.8.1)
Table 9.8.1.
Preparation of Cytoskeletal Stabilizing (CSK) buffers
|
|
|
| 7.5 ml of 1 M sucrose | All CSK buffers |
| 0.5 ml of 5 M NaCl | |
| 5 ml of 100 mM PIPES, pH 6.8 | |
| 150 μl of 1 M MgCl2 | |
| 1 ml of 1 M Tris-HCl, pH 8.5 |
CSK− with Tris only |
| 5 ml of 5% (w/v) Triton X-100 | CSK+ only |
| 50 μl of 10 mg/ml leupeptin | |
| 50 μl of 10 mg/ml aprotinin | |
| 50 μl of 0.5 M AEBSF | |
| 1 ml of 10 mM Na3VO4 | |
| Make up to 50 ml with dH2O |
All CSK buffers |
Heat-denatured BSA solution
Prepare 1% BSA (w/v) in PBS and heat to 80°C for 15 min. Allow solution to cool before use. Store up to 1 week at 4°C
For further details see UNIT 9.1 (Humphries, 1998, Cell-Substrate Adhesion Assays)
Reducing sample buffer, 5 x
To prepare 5× stock, combine:
125 mM Tris-HCl, pH 6.8
25% (w/v) glycerol
10% (w/v) SDS
0.01% (w/v) bromophenol blue
20% (v/v) 2-mercaptoethanol
Store up to 1 year without 2-mercaptoethanol; add that compound fresh for each use
Radioimmunoprecipitation assay (RIPA) buffer for Basic Protocol 1 (microbead assay)
To prepare 1× stock, combine:
50 mM Tris-HCl, pH 8.0
5 mM disodium EDTA
150 mM sodium chloride
1% (w/v) Triton X-100
1% (w/v) sodium deoxycholate
0.1% (w/v) SDS
10 μg/ml leupeptin
10 μg/ml aprotinin
0.5 mM AEBSF
2 mM sodium orthovanadate
Radioimmunoprecipitation assay (RIPA) buffer, modified for Basic Protocol 2 (2-D fibronectin substrate assay)
50mM Tris-HCl, pH7.6
150mM NaCl
5mM disodium EDTA, pH8
0.5% (w/v) SDS
1% Triton X-100
1% sodium deoxycholate
Make up as a 5x stock and dilute in distilled H2O to 1x before use
Store 5x stock up to 1 year at 4°C
COMMENTARY
Background information
The specific isolation of adhesion complexes in their native state allowing systematic analysis of complex composition has long been sought after in the field of integrin research. Approaches utilising imaging and biochemical techniques to identify interactors of known adhesion proteins has identified in excess of 200 proteins, termed the adhesome, that potentially associate with focal adhesions under a wide range of conditions (Zaidel-Bar et al. 2007; Winograd-Katz et al. 2014). Therefore, to gain insights into the context specific composition of adhesion complexes, and to further our knowledge of how adhesion complexes change and signal in a global fashion in response to various stimuli, the development of methods to enrich for integrin-based adhesion complexes has been undertaken by our group and others (Humphries et al. 2009; Kuo et al. 2011; Schiller et al. 2011; Schiller et al. 2013). Initially the microbead based method described here in Basic Protocol 1 was established to provide a relatively rapid method to positively purify or enrich for proteins associated with integrins upon ligand binding. This approach was adopted as it was easier to control with the use of uncoated magnetic beads, or beads coated with antibodies against alternative cell surface receptors or mutants of ligand unable to bind integrin. Comparison with a negative control isolation enabled the demonstration of the specific enrichment of isolated components to adhesion complexes (Humphries et al. 2009; Byron et al. 2011; Byron et al. 2012). Due to the highly labile nature of the adhesion structures the isolation strategy required the prior stabilisation of proteins complexes with chemical crosslinkers to retain them on the beads. Therefore the comparison complexes isolated from integrin ligand-coated beads with those from control-coated beads, along with use of the cell-permeable cross-linker DTBP, allowed for the robust isolation of both α5β1-FN and α4β1-VCAM-1 specific complexes using the Basic Protocol 1. The isolated adhesion complexes were subsequently analysed by mass spectrometry to determine complex composition (Humphries et al. 2009).
However, a number of issues required the establishment of an alternative method for the isolation of adhesion complexes; some canonical adhesome components e.g. FAK were not observed in complexes recruited to microbeads, and some cells types (primary human fibroblasts, mouse embryonic fibroblasts and epithelial cells) routinely engulfed the microbeads when incubated at 37°C. Therefore the development of the 2D isolation method described in Basic Protocol 2 was subsequently undertaken. The method outlined in Basic Protocol 2 essentially evolved from the combination of methods used for the generation of cell derived matrices (Preparation of Extracellular Matrices Produced by Cultured and Primary Fibroblasts UNIT 10.9; Beacham et al., 2006) and incorporated cell-permeable crosslinker to stabilise adhesion complex components (Basic Protocol 1). Methods similar to Basic Protocol 2 have been independently established and published by other groups (Kuo et al. 2011; Schiller et al. 2011; Schiller et al. 2013) and have been used to generate samples for Western blotting and / or subsequently analysed by mass spectrometry. The main differences between the two groups’ approaches were that Schiller et al 2011 utilised a control ligand (Poly-L-Lysine) to determine specificity along with the use of cross-linker and RIPA buffer lysis followed by water wash to remove cell bodies, whereas Kuo et al 2011 did not use a negative control and utilised hypotonic shock in the absence of cross linker followed by a PBS wash to remove cell bodies. Despite these differences in approach, the datasets generated in these studies, along with a number of datasets generated in our lab, have significant overlap in the proteins identified by mass spectrometry. This demonstrates that the basic protocols described generate reproducible adhesion isolations that are suitable for the investigation of numerous questions related to adhesion biology.
Critical parameters and trouble shooting
The choice of negative control is important if the user wishes to gain confidence in the specificity of the recruitment of components to adhesion complexes. Indeed, one of the main sources of variation between proteins identified by mass spectrometry in the complexes, in addition to the cell types chosen for the study, appears to be the choice of negative control (i.e. Poly-L-Lysine or apotransferrin; Humphries lab, unpub. observ.). To enable confident comparisons to be made between experiments it is therefore advisable that once a negative control has been chosen by the user all subsequent experiments continue to use the same reagent. The advantage of using apotransferrin as a control to identify non-specific proteins is that specific enrichment of the transferrin receptor can be confirmed when compared to those spread on an ECM ligand, and as such a comparison is made between two different specific positive isolations of receptors. However, cell attachment to apotransferrin is far less efficient than that of attachment to Poly-L-Lysine so the number of cells used may have to be increased accordingly to end up with similar total isolated protein levels in each sample. The use of a negative control is largely dependent on the question being asked by the investigator: should you wish to know whether proteins are present in adhesions or not, then use of a negative control to identify non-specific proteins is recommended. However, should you be attempting to see how adhesion proteins change in response to a particular stimuli then a negative control may not be required as it could be assumed that non-specific proteins are unlikely to respond to a given stimuli in a consistent manner across experimental repeats, and as such these proteins would be lost from subsequent data analysis. Also the time required in culture for the assay of interest to be performed will dictate if a control is workable; cells in culture for more than 2-3 hours will start to produce and assemble their own ECM environment which will override the control ligand.
In our experience there are a number of key options to consider during the design of experiments using the methods to isolate adhesion complexes:
- The choice of control
- Poly-D/L-Lysine or apotransferrin for the 2D isolation method (Basic Protocol 2)
- Antibodies or control ligands for the microbead assay (Basic Protocol 1)
- The use of crosslinkers or not
- Users should define whether the use of crosslinkers as required as for some cell types they are not essential
- Crosslinker incubation and duration. Starting conditions are suggested above but they should be optimised for each experiment
- 30 mins at 3mM DTBP for static adhesion comparison
- Less than 5 mins for studies of dynamics i.e. time courses
The choice of cell type. A variety of cell types have been successfully used e.g. mouse and human fibroblasts, U20S osteosarcoma cells, A375 melanoma cells, HeLa cells, primary mammary epithelial cells, human mesenchymal stem cells.
The choice of ECM ligand used as this will dictate the integrin heterodimers recruited.
- The choice of cell disruption technique. For the 2-D isolation method (Basic Protocol 2) this includes:
- Mechanical options: sonication versus water wash.
- Chemical options: pH (NH4OH) versus detergents (SDS).
Another issue is how to estimate how much protein has been isolated. This is not without its difficulties as the recovery of adhesion complexes is typically performed in solutions that do not permit standard protein estimation methods such as BCA or Bradford assay. Also the amount of protein recovered from adhesion complexes is typically low, further reducing the options available to reliably measure protein concentration. One way to circumvent this is to construct a standard curve of total cell lysate proteins (of known concentration) from the cell line used in the assay by performing SDS-PAGE. Running the sample of adhesion complexes of unknown concentration alongside the standard curve, the use of standard Coomassie blue staining protocols together with an Odyssey infrared (IR) imaging system (LI-COR Biosciences) can then allow an estimation of the sample concentration and therefore yield of adhesion complex isolation. For the 2-D isolation method this typically results in 5 to 10μg adhesion complexes to be isolated per 10-cm diameter dish.
Anticipated results
The constituents of the isolated protein complexes should be verified by Western blotting. Users should observe specific recruitment of integrins and other canonical adhesion complex components (talin, vinculin, paxillin or ILK) to isolations from integrin ligands compared to the chosen negative control. If using a positive isolation of an alternative receptor as a control e.g. apotransferrin then observing the reciprocal enrichment of the transferrin receptor to complexes isolated from apotransferrin compared to integrin ligands will demonstrate the specific recruitment of proteins to each condition. Additionally, Western blotting for components that do not classically compartmentalise with adhesion complexes can be used to estimate co-purifying contaminants. We have used a variety of reagents for this purpose such as lamin B1 (nuclear), BAK (mitochondrial) and heat shock proteins (HSP90 and mtHSP70). Western blotting for these non-adhesion proteins is essential during method optimisation when their presence can be used to indicate the purity of the isolated complex and therefore the efficiency of the various protocol options being assessed.
A typical first attempt can result in a minimal or absent signal. If this occurs possible reasons could be either insufficient starting material or over effective cell disruption. It is wise to run a total cell lysate prepared from the same cell type on the same SDS PAGE gel. This step will allow you to check if the antibody for Western blotting works and if the protein is expressed in the cells type chosen. This is especially important when probing samples for the presence of non-adhesion related proteins as it is reassuring to see Western blotting band signals in the total cell lysate but not in the isolated adhesion complex samples. Alternatively, excessive signal observed in negative control samples indicate that effective cell disruption has not been performed and further sonication or cell washing is required.
Time Considerations
Typical considerations regarding timings for the above protocols centre on the time to prepare sufficient cells for analysis, time for preparation of reagents, and the time taken to perform the protocols themselves. Depending on the growth rate of the cells to be used it is usually necessary to set up sufficient cells about a week before the assay is to be planned. Apart from the microbead coating, which requires 48 hours of preparation, most reagents can be prepared in advance. The protocols themselves usually take a whole working day to perform and this time, and the use of equipment needed for them, should be planned in advance.
Acknowledgments
This work was supported by the Wellcome Trust (grant 092015 to M.J.H.), a Wellcome Trust Institutional Strategic Support Fund award (grant 097820 to the University of Manchester), a University of Manchester Faculty of Life Sciences Career Development Award (to A.B.) and a Biotechnology and Biological Sciences Research Council studentship (to J.R.)
Literature Cited
- Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 2006;33:10.9.1–10.9.21. doi: 10.1002/0471143030.cb1009s33. [DOI] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Bass MD, Knight D, Humphries MJ. Proteomic analysis of integrin adhesion complexes. Sci. Signal. 2011;4:pt2. doi: 10.1126/scisignal.2001827. [DOI] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Craig SE, Knight D, Humphries MJ. Proteomic analysis of α4β1 integrin adhesion complexes reveals α-subunit-dependent protein recruitment. Proteomics. 2012;12:2104–2114. doi: 10.1002/pmic.201100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ. Cell-substrate adhesion assays. Curr. Protoc. Cell Biol. 1998;00:9.1.1–9.1.11. doi: 10.1002/0471143030.cb0901s00. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes identifies RCC2 as a duel regulator of Rac1 and Arf6. Sci.Signal. 2009;2:ra51. doi: 10.1126/scisignal.2000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J-C, Han X, Hsiao C-T, Yates JR, III, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Friedel CC, Boulegue C, Fässler R. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep. 2011;12:259–266. doi: 10.1038/embor.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Théry M, Mann M, Fässler R. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 2013;15:625–636. doi: 10.1038/ncb2747. [DOI] [PubMed] [Google Scholar]
- Wehrle-Haller B. Structure and function of focal adhesions. Curr. Opin. Cell Biol. 2012;24(1):116–124. doi: 10.1016/j.ceb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat. Rev. Mol. Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell. 2013;24:447–458. doi: 10.1016/j.devcel.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat. Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]