Abstract
Traumatic pericallosal aneurysm (TPA) is typically seldom yet potentially lethal. Because of its rarity, also complicated by the unpredictable delayed-onset, TPA is more difficult to be diagnosed promptly. Due to the sporadic reports and diverse opinions on the priority of surgical treatment, a consensus about effective management of TPA has not been reached. Here we report a 55 year-old male patient with TPA, who received an emergent craniotomy to clip the pseudoaneurysm and remove the hematoma under intense intracranial pressure (ICP) monitoring. A satisfactory clinical outcome was achieved at a 3-month follow-up. Thereafter, a review was conducted to evaluate the outcomes of different managing modalities.
Keywords: Trauma, aneurysm, surgery
Introduction
Traumatic pericallosal aneurysm (TPA) was rarely encountered in clinical practice. To date, there have been fewer than 30 cases reported worldwide (articles restricted to English) [1-4]. In addition, the unpredictable delayed-onset rebleeding makes it less possible to be diagnosed promptly. Due to the sporadic reports and different opinions on the priority of surgical treatment, a consensus about effective management of TPA has not been reached. Here we report a case in which a TPA was found after a fall accident and treated surgically, and to evaluate the clinical outcomes of open surgery compared to endovascular coiling. From the outcomes of the previous reports, both surgical and endovascular therapy may be effective in managing TPA. However, surgical treatment accompanied by close monitoring of intracranial pressure might contribute to a better prognosis than endovascular intervene in severe TPA combined with coma.
Case presentation
A 55-year-old man was admitted to local hospital for an inadvertent fall accident from a ladder. The patient showed no coma, no vomiting, or other severe clinical manifestations, and received an emergent head CT scan (Figure 1A). With both normal examination results, he was discharged after receiving routine debridement. Six days later, the patient developed a sudden disturbance of consciousness and paralysis of the right limbs, and he was transferred to our emergency department 24 hours after the new symptoms. Physical examination showed a Glascow Coma Scale (GCS) of 8 (E2V2M4), and unstable vital signs (Heart rate 49 bpm, Blood pressure 173/98 mmHg, Respiration rate 14 bpm). An emergent head CT scan revealed a hemorrhage of more than 30 ml in the left corpus callosum and an absent supratentorial brain ditch (Figure 1B). An emergent cerebral catheter angiography showed an aneurysm located at the bifurcation of the left pericallosal artery (Figure 1C, 1D). After informed consent was obtained, a ventriculostomy was created for ICP monitoring, and an emergent surgery was operated to evacuate intracranial hematoma, clip and resect the ruptured aneurysm after full exposure of surgical field. The post-surgical CT showed that the hematoma was evacuated and the intracranial pressure decreased (Figure 1E). Dextran was used to promote brain microcirculation and hemostatic chemicals were avoided to prevent thrombotic complications. The patient recovered gradually. The distal anterior cerebral artery was preserved and no ischemia signs were found by CT angiography (Figure 1F). The histological examination of resected sac confirmed that there was no endothelial cell or vessel wall tissue (Figure 1G). After three months of designed rehabilitation, the patient achieved a satisfactory recovery without neurological deficit (Figure 1H).
Figure 1.
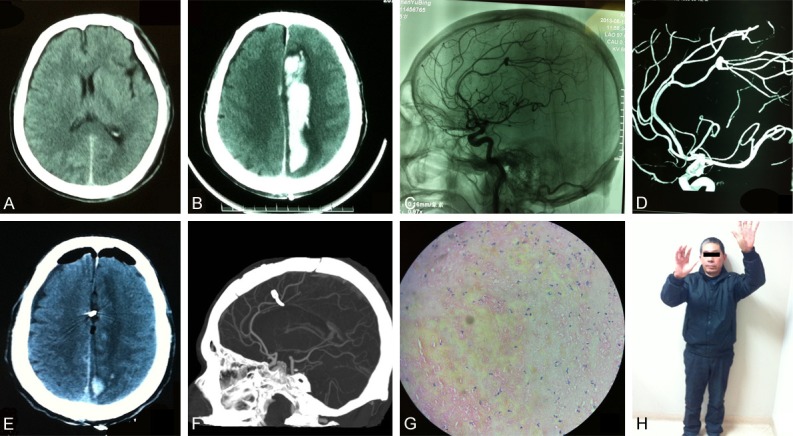
Surgical treatment of traumatic pericallosal aneurysm. (A) An emergent head CT scan after a fall accident showed no hemorrhage or bone fracture; (B) One week later, a hematoma of more than 30 ml in the left corpus callosum was found in a cerebral CT scan; (C, D) A pericallosal aneurysm was confirmed by non-subtracted angiography (C) and three-dimensional angiography (D); (E) The post-operational CT showed that the hematoma was evacuated; (F) The distal anterior cerebral artery was shown in post-operational CT angiography; (G) No endothelial cell or vessel wall tissue was found in the resected sac by histology. (H) The patient achieved a satisfactory recovery at three-month follow up.
Discussion
The incidence of traumatic intracranial aneurysms is reported constituting 0.4% to 9% of intracranial aneurysms [5-7], and TPA is one of a much more seldom kind with some distinctions to the other types [8,9]. The mechanisms of formation of TPA are poorly understood, and a possible explanation is that it might result from shearing forces between the vessel and the inferior margin of the falx cerebri.
Similar to the other traumatic intracranial aneurysms, TPA shows similar clinical features such as delayed onset, continued deterioration of neurological symptoms, and poor clinical outcomes. Nonetheless, TPA presents different characteristics in clinical manifestation and management strategies as a result of its thin-wall and fragility. Its delicacy and direction of projection are frequently associated with higher rate of premature rupture. Therefore, physicians should be vigilant when encountered with TPA in clinical practice.
The literature review data include patients’ clinical presentations, trauma type, location, and outcomes (Table 1). From January 1950 to December 2013, 25 TPA cases have been described with sufficient information under a broad search strategy with the terms: ‘pericallosal artery’ AND (injury OR trauma OR dissection).
Table 1.
Literature review of traumatic pericallosal aneurysm
| Case | Sex | Age | Rupture | Trauma type | Coma history | Time of Onset | Location | Treatment | Prognosis | Reporter |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 10 y | Yes | Vehicle | Yes | 30 d | L. periA | Surgery | N/A | Steinborn et al. (2010) |
| 2 | M | 18 y | Yes | Vehicle | No | 17 d | L. periA | Surgery | Well recovered | Hsieh et al. (2007) |
| 3 | M | 23 y | Yes | Vehicle | Yes | 46 d | L. periA | Surgery | Well recovered | O’Brien et al. (1984) |
| 4 | F | 2 y | Yes | Transoral penetrating injury | No | 21 d | R. periA | Surgery | Mild left lower limb weakness | Shih et al. (2002) |
| 5 | M | 18 y | Yes | Vehicle | Yes | 15 d | ACA & R. periA | Surgery | Well recovered | Yuge et al. (1990) |
| 6 | F | 50 y | Yes | External Ventricular Drain Placement | No | N/A | L. periA | Surgery | Well recovered | Ji et al. (2010) |
| 7 | M | 10 y | No | Ventriculoperitoneal shunt placement | No | 2 d | R. periA | Surgery | Residual weakness of left leg | Tubbs et al. (2006) |
| 8 | M | 24 y | Yes | Gun-shot wound | No | 14 m | L. periA | Surgery | Occasional tonic-clonic seizure | Soria et al. (1988) |
| 9 | M | 6 y | Yes | Brain surgery | No | 4 y | R. periA | Surgery | Uneventfully | Dunn et al. (2007) |
| 10 | F | 29 y | Yes | Vehicle | No | N/A | L. periA | Surgery | N/A | Tokuno et al. (1994) |
| 11 | F | 4 y | Yes | Vehicle | Yes | 63 d | R. periA | Surgery | Uneventful | Asari et al. (1976) |
| 12 | F | 33 y | Yes | Vehicle | Yes | 1 m | L. periA | Surgery | Uneventful recovery with no neurological deficit | Suhara et al. (2008) |
| 13 | M | 6 w | Yes | Shaken baby syndrome | No | 3 w | R. periA | Surgery | N/A | Lam et al. (1996) |
| 14 | F | 32 y | Yes | Roller-coaster | Yes | 8 d | L. periA | Surgery | Satisfactory recovery | Senegor et al. (1991) |
| 15 | M | 46 y | Yes | Slip | N/A | N/A | R. periA | Endovascular | Well recovered | Yang et al. (2007) |
| 16 | M | 25 y | Yes | Vehicle | N/A | N/A | R. periA | Endovascular | Well recovered | |
| 17 | M | 5 y | No | Vehicle | Yes | 14 d | R. periA | Endovascular | Mild subjective dysarthria | Sim et al. (2008) |
| 18 | F | 17 y | Yes | Bomb | Yes | 10 d | R. periA | Endovascular | Minor residual paresis in right arm | Cohen et al. (2008) |
| 19 | F | 35 y | Yes | Brain surgery | No | 2 d | L. periA | Endovascular | Die of glioblastoma | Cohen et al. (2005) |
| 20 | M | 0.5 y | Yes | Gun-shot wound | Yes | 6 d | R. periA | Endovascular | Well recovered | |
| 21 | M | 3 w | Yes | Shaken baby syndrome | No | 8 m | R. periA | Endovascular | No progress | Levine et al. (2004) |
| 22 | M | 48 y | Yes | Slip | Yes | 3 w | R. periA | Endovascular firstly, Surgery secondly | Mild left side weakness | Yuen et al. (2007) |
| 23 | M | 28 y | Yes | Vehicle | Yes | At accident | R. periA | Endovascular | Recovered gradually | Van Rooij et al. (2013) |
| 24 | M | 22 y | Yes | Vehicle | Yes | 17 d | R. periA | Endovascular | Recovered slowly | |
| 25 | M | 33 y | Yes | Vehicle | Yes | At accident | R. periA | Intubated and ventilated | Die of trauma | Opeskin et al. (1995) |
periA, pericallosal aneurysm; ACA, Anterior Cerebral Artery; N/A, not available; R, right; L, left.
Vehicle-involved injury is the most common incentive (12/25, 48%), followed by iatrogenic injury in 4 cases (16%). Other incentives include: gunshot wound (2/25, 8%), shaken baby syndrome (2, 8%), slip (2, 8%), roller-coast (1, 4%), transoral penetrating injury (1, 4%), and bomb explosion (1, 4%). The patients are relatively young with an average age of 20.7 years old (range 3 weeks~50 years). The incubation periods (from trauma to established diagnosis) vary from1 day to 4 years, consistent with the previously reported unpredictability of TPA. Of all the TPAs, 92% (23/25) of them ruptured, confirming its fragility. Poor outcome inclination finds expression in 13 patients manifested with coma or unconsciousness before they are admitted to the hospital. In the literature, 15 cases of TPA are treated with surgery, and 10 cases are managed endovascularly. Surgery achieves full recovery in 8 of 13 cases (61.5%) while endovascular management achieves full recovery in 4 of 10 cases (40%). In the critically ill patients accompanied with coma, surgery makes satisfactory recovery in all five cases, while the endovascular therapy makes satisfactory recovery in 1 out of 6 cases (16.7%). No death related to surgery or endovascular intervention was found.
With the extremely low but unknown incidence of pericallosal pseudoaneurysm and discrepancy in diagnostic and management strategies, it is still challenging for clinicians to make diagnosis and treatment plan when encountered with TPA caused by craniofacial trauma. Angiography is essential to diagnosis; however, it is noted to distinguish traumatic aneurysm from the spontaneous aneurysm that develops before trauma and possesses different treatment characteristics. The angiography image of TPA is frequently associated with irregular shape, inconspicuous aneurysm neck and contrast agent retention, conducing to the differential diagnosis.
Both surgical and endovascular trapping or occlusion have proven to be fairly reliable options for most patients with traumatic intracranial pseudoaneurysm [1,5,10]. However, TPA is intractable either by direct clipping or by endovascular embolization, because its parent artery is thinner and more delicate than the other intracranial arteries and the TPA wall is prone to lack true collagenous layer. Sim et al. reported one young child with non-ruptured pericallosal pseudoaneurysm treated with endovascular trapping, and discussed the limitations of surgery, such as a higher possibility of premature rupture, limited operative field, and inflexibility when facing anatomical variations, drawing a conclusion that endovascular treatment is a favorable option for the TPA [11]. We agree with their point of view in managing TPA endovascularly when there is no or little hematoma, no markedly increased intracranial pressure, no progressing deterioration of neurological function, or poor artery condition. However, pericallosal artery is part of the distal anterior cerebral artery, which multiplies difficulty in placing micro-catheter. What’s more, the delicate aneurysmal wall cannot keep the coils to remain in position. Yuen et al. reported a case with rapid regrowth of pseudoaneurysm after endovascular coiling, achieved a satisfactory prognosis after surgical treatment [8].
Advances in endovascular techniques and development of the microcatheter contribute to the widely used endovascular intervention to traumatic pseudoaneurysms over the past decade, and some successful cases treated with endovascular therapy have been reported [1,2]. However, in distal branch of intracranial arteries, such as pericallosal artery, surgery still should be considered as an irreplaceable option. We found that the endovascular group bears a higher rate (55.56%, 5/9) of minor neurological deficits than surgery group (30.77%, 4/13). The neurological deficits include residual weakness or paresis of limb, subjective dysarthria, and even worse, the regrowth of the aneurysm [8]. More importantly, in the severe TPA accompanied with coma, endovascular treatment seems to result in less satisfactory outcomes than surgery (full recovery rate 16.7% versus 100%). In our speculation, surgery benefits more in remitting the increased ICP, which is related to the coma situation. Therefore, in our and many reporters’ opinion (60%, 15/25), open surgery should be the first option for TPA, especially when combined with large hematoma or other inapplicable condition for endovascular management [8,12-14]. In addition, ICP plays a key role in the occurrence of coma and is strongly recommended to be under monitor while surgery is taken.
In our case, a ruptured pseudoaneurysm was found while a more than 30 ml hematoma causing deteriorating symptoms; after weighing benefits and losses, we chose surgery over endovascular therapy and helped the patient to achieve a well clinical outcome. Tetsuyoshi et al. recommended horizontal contralateral approach for a comfortable operative field, the minimum retraction of the brain, and the easy securing of the parent artery [15]. In our patient, we found that hematoma was located in the upper of the left corpus callosum, and no obvious return of left bridging vein was showed in the angiography, so we chose anterior interhemispheric approach. Although in principle, the first one third of the bridging vein above the sagittal sinus vein can be sacrificed, we tried to save the bridging vein, cut some small ones and isolated the trunk from the arachnoid membrane to avoid bleeding. During exposure, peripheral hematoma was firstly removed to lower the cerebral tension, and the blood pressure was monitored to avoid pseudoaneurysm rupture caused by the fluctuation of blood pressure. When near the core of hematoma and begin to clip the pseudoaneurysm, we confirmed that the parent artery and perforating vessels were not occluded. Low molecule dextran was administered to improve cerebral microcirculation, and infection prevention was applied. We did not use hemostatic drug and mannitol to avoid thrombosis, and ICP was consistently monitored.
Conclusion
TPA is easily neglected and misdiagnosed in patients with trauma. When one’s symptoms and signs were difficult to explain by primary injury, the traumatic aneurysm ought to be considered and angiography should be performed promptly. Tendency from the literature review suggested that surgical clipping might be a technically feasible and safe alternative modality in pericallosal pseudoaneurysm, especially those combined with coma. When encountered with a severe TPA, ICP monitoring can be used to forecast future episodes of intracranial hypertension, providing valuable information for clinical decision.
Acknowledgements
We acknowledge the patient for his cooperation in our follow-up and agreement for us to share his pictures.
Disclosure of conflict of interest
None.
References
- 1.Cohen JE, Gomori JM, Segal R, Spivak A, Margolin E, Sviri G, Rajz G, Fraifeld S, Spektor S. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 1998;63:476–485. doi: 10.1227/01.NEU.0000324995.57376.79. [DOI] [PubMed] [Google Scholar]
- 2.Van Rooij WJ, Van Rooij SB. Endovascular treatment of traumatic pericallosal artery aneurysms. A case report. Interv Neuroradiol. 2013;19:56–59. doi: 10.1177/159101991301900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinborn M, Schäffeler C, Kabs C, Kraus V, Rüdisser K, Hahn H. CT and MR imaging of primary cerebrovascular complications in pediatric head trauma. Emerg Radiol. 2010;17:309–315. doi: 10.1007/s10140-010-0860-4. [DOI] [PubMed] [Google Scholar]
- 4.Opeskin K. Traumatic pericallosal artery aneurysm. Am J Forensic Med Pathol. 1995;16:11–16. doi: 10.1097/00000433-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mao Z, Wang N, Hussain M, Li M, Zhang H, Zhang Q, Zhang P, Zhi X, Ling F. Traumatic intracranial aneurysms due to blunt brain injury: a single center experience. Acta Neurochir (Wien) 2012;154:2187–2193. doi: 10.1007/s00701-012-1487-x. [DOI] [PubMed] [Google Scholar]
- 6.Larson PS, Reisner A, Morassutti DJ, Abdulhadi B, Harpring JE. Traumatic intracranial aneurysms. Neurosurg Focus. 2000;8:1–9. doi: 10.3171/foc.2000.8.1.1829. [DOI] [PubMed] [Google Scholar]
- 7.Dario A, Dorizzi A, Scamoni C, Cerati M, Balcone Grimaldi G. Iatrogenic intracranial aneurysm. Case report and review of the literature. J Neurosurg Sci. 1997;41:195–202. [PubMed] [Google Scholar]
- 8.Yuen CM, Kuo YL, Ho JT, Liao JJ. Rapid regrowth of a successfully coiled traumatic pericallosal aneurysm. J Clin Neurosci. 2007;14:1215–1219. doi: 10.1016/j.jocn.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Suhara S, Wong AS, Wong JO. Post-traumatic pericallosal artery aneurysm presenting with subdural haematoma without subarachnoid haemorrhage. Br J Neurosurg. 2008;22:295–297. doi: 10.1080/02688690701687678. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz MB, Kopitnik TA, Landreneau F, Ramnani DM, Rushing EJ, George E, Purdy PP, Samson DS. Multidisciplinary approach to traumatic intracranial aneurysms secondary to shotgun and handgun wounds. Surg Neurol. 1999;51:31–41. doi: 10.1016/s0090-3019(98)00029-9. [DOI] [PubMed] [Google Scholar]
- 11.Sim SY, Shin YS, Yoon SH. Endovascular internal trapping of traumatic pericallosal pseudoaneurysm with hydrogel-coated self-expandable coil in a child: a case report. Surg Neurol. 2008;69:418–422. doi: 10.1016/j.surneu.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Tokuno T, Ban S, Shingu T, Yamamoto T. [Traumatic anterior cerebral artery aneurysm difficult to distinguish from congenital cerebral aneurysm: case report] . No Shinkei Geka. 1994;22:1073–1076. [PubMed] [Google Scholar]
- 13.Lam CH, Montes J, Farmer JP, O’Gorman AM, Meagher-Villemure K. Traumatic aneurysm from shaken baby syndrome: case report. Neurosurgery. 1996;39:1252–1255. doi: 10.1097/00006123-199612000-00041. [DOI] [PubMed] [Google Scholar]
- 14.Cohen JE, Rajz G, Itshayek E, Shoshan Y, Umansky F, Gomori JM. Endovascular management of traumatic and iatrogenic aneurysms of the pericallosal artery. Report of two cases. J Neurosurg. 2005;102:555–557. doi: 10.3171/jns.2005.102.3.0555. [DOI] [PubMed] [Google Scholar]
- 15.Horiuchi T, Nitta J, Nakagawa F, Hongo K. Horizontal contralateral approach for the distal anterior cerebral artery aneurysm: technical note. Surg Neurol. 2009;72:65–68. doi: 10.1016/j.surneu.2008.02.021. [DOI] [PubMed] [Google Scholar]


