Abstract
Aims: To investigate the effects of caspase-3 silencing on the proliferation and apoptosis of rat bone marrow mesenchymal stem cells (MSCs) under hypoxia. Methods: Rat bone marrow MSCs were transfected with a recombinant shRNA lentivirus targeting caspase-3 expression. Protein expression of caspase-3 was measured by western blotting. Cell proliferation was measured with MTS, and the cell cycle was analyzed by flow cytometry. The apoptosis rate was measured at various time points under hypoxia. Apoptotic morphology was assessed by Hoechst 33258 staining. mRNA levels of caspase-3, Bcl-2, and Bax were measured by real-time PCR. Results: Western blotting showed that the rat MSCs were stably transfected with the shRNA targeting caspase-3 by a significant reduction of caspase-3 expression. Silencing of caspase-3 expression resulted in a significant increase of MSC proliferation (P < 0.05), an increase of cells in S-phase (52.66 ± 0.30%), and a significant decrease of apoptotic MSCs (P < 0.05). These effects exhibited a slow increase during hypoxic culture. Furthermore, caspase-3 silencing significantly down-regulated mRNA expression of caspase-3 (P < 0.01) and Bax (P < 0.01), and up-regulated Bcl-2 mRNA expression (p < 0.01), thereby increasing the ratio of Bcl-2/Bax (P < 0.05). Conclusion: Caspase-3 silencing modulates the cell cycle of MSCs, promotes cell proliferation, and enhances the anti-apoptotic capacity of MSCs under hypoxia in vitro.
Keywords: Bone marrow mesenchymal stem cells, gene silencing, caspase-3, cell proliferation, apoptosis
Introduction
Bone marrow mesenchymal stem cells (MSCs) are ideal seed cells for transplantation therapy of myocardial infarction [7-9] because of their abundant availability [1], low immunogenicity [2], immunosuppressive effects [3], multi-potency [2,4], and paracrine effects [5,6]. However, the hypoxic and ischemic microenvironment in the transplantation region results in massive apoptosis after MSC transplantation, followed by limited proliferation and survival, which is the major bottleneck for clinical application [10]. Apoptosis is programmed cell death controlled by tightly regulated genes. Caspase-3 is a key enzyme in the signaling pathway of apoptosis [11]. By inhibiting the expression of caspase-3 in MSCs, it would be possible to decrease MSC apoptosis in the infarction area to extend their survival time. In a preliminary study, we designed and evaluated three pairs of siRNAs targeting caspase-3 to be introduced into MSCs by transient transfection to effectively inhibit caspase-3 expression. In the present study, we constructed a recombinant lentiviral vector containing the optimal shRNA targeting caspase-3 to transfect MSCs and investigate the effects of caspase-3 silencing on their proliferation and cell cycle. We also cultured MSCs in serum-free medium under hypoxia to mimic the in vitro hypoxic/ischemic microenvironment and measure their anti-apoptotic capability and expression of apoptosis genes. This study will provide new insights to increase the therapeutic effects of MSCs on myocardial infarction.
Materials and methods
Reagents and apparatus
The study protocol was approved by the Research Ethics Board at Sun Yat-sen Memorial Hospital, Sun Yat-sen University. The major reagents were fetal bovine serum (FBS; Hyclone, USA), DMEM/F12 (Hyclone, USA), mouse anti-rat CD29 antibody (BioLegend, USA), mouse anti-rat CD45 antibody (eBioscience, USA), mouse anti-rat CD90 antibody (BioLegend), GV115 lentiviral vector (Shanghai Genechem, China), rabbit anti-rat caspase-3 monoclonal antibody (Anbo, USA), anaerobic culture agent (MGC, Japan), oxygen indicator (MGC), MTS kit (Promega, USA), cell cycle kit (KeyGEN Biotech, Nanjing), SYBR Green PCR Master Mix (TOYOBO, Japan), Trizol (Invitrogen, USA), Annexin V-APC/PI apoptosis kit (Keygen, Netherlands), and Hoechst 33258 dye (Beyotime Institute of Biotechnology, China). The major apparatus included an inverted phase contrast microscope (Olympus, Japan), inverted fluorescence microscope (Olympus), constant temperature incubator (Thermo Fisher, USA), enzyme-linked immunosorbent assay (ELISA) reader (Thermo Fisher), quantitative PCR reader (ABI, USA), and flow cytometer (BD, USA).
Culture and identification of MSCs
Three-week-old Sprague-Dawley rats of either sex (100~120 g) were provided by the Experimental Animal Centre, North Campus of Sun Yat-Sen University. Under anesthesia, the animals were sacrificed by cervical dislocation and soaked in 75% ethanol for 5 min. The bilateral femora and tibias were dissected with removal of the epiphysis. The bone marrow cavity was repeatedly flushed with culture medium to collect a cell suspension that was centrifuged (1500 r/min for 10 min) and resuspended in DMEM/F12 supplemented with 10% FBS. The cells were seeded at a density of 1 × 107 cells/mL and cultured at 37°C with 5% CO2. The medium was changed at 48 h after seeding and then every 3 days until the cells reached 80-90% confluence. Then, the cultured cells were digested with 0.25% trypsin and passaged at 1:3. MSCs were purified by medium changes and passaging, and identified by measuring the expression of CD29, CD45, and CD90 at passage 2 by flow cytometry.
Construction of the lentiviral vector and transfection
Based on preliminary results, we selected a targeting sequence with the optimal interfering effect against caspase-3 expression (5’-GCCGACTTCCTGTATGCTTAC-3’) and designed two complementary shRNAs: 5’-CCGGGCCGACTCCTGTATGCTTACCTCGAGGTAAGCATACAGGAAGTCGGCTTTTTG-3’; 5’-AATTCAAAAAGCCGACTTCCTGTATGCTTACCTCGAGGTAAGCATACAGGAAGTCGGC-3’. The shRNAs were annealed and ligated to the linearized GV115 vector to transform DH5α competent cells. The plasmid was extracted and verified by enzymatic digestion and sequencing. Then, 293T cells were used to package the lentivirus. At 30-50% confluence, MSCs were infected with the lentivirus. Cells infected with a lentivirus carrying an empty vector were used as a control. Fluorescence expression was measured after 3-4 days. The experiment included transfection, empty vector, and blank control groups.
Measurement of caspase-3 protein expression by western blot analysis
The protein concentration of MSC lysates was measured using the bicinchoninic acid method. The samples were then subjected to 10% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. The membrane was blocked with a 5% skim milk solution and probed with rabbit anti-rat primary antibodies (anti-caspase-3, 1:1000; anti-GAPDH, 1:1000) at 37°C for 2 h. The membrane was rinsed three times with TBST for 5 min each wash and then incubated with a secondary goat anti-rabbit IgG (1:5000) at 37°C for 1 h. The membrane was rinsed as described and developed with DAB for semi-quantitative analysis of the protein bands by QuantityOne software.
Cell proliferation detection with MTS
The three groups of cells at the logarithmic growth phase were resuspended to a density of 1 × 105 cells/mL, seeded at 100 μL/well, and then incubated at 37°C with 5% CO2. The cells were then harvested at 24, 48, 72, 96, and 120 h, and combined with MTS at a ratio of 1:10. After 4 h, the optical density (OD) at 490 nm was measured with the ELISA reader. The cell proliferation rate = (A 490 valueA 0)/A 0 × 100%, where A 0 is the A 490 value at day 1.
Cell cycle analysis by flow cytometry
The cells were collected, rinsed twice with pre-cooled PBS, and fixed at 4°C overnight in pre-cooled 70% ethanol. Then, the cells were rinsed with PBS and incubated in PBS containing 50 µg/mL brominated acrylic ingot, 100 µg/mL RNase A, and 0.2% Triton X-100 at 4°C in the dark for 30 min. A total of 20,000-30,000 cells were measured by standard flow cytometry procedures, and the data were analyzed to determine the cell cycle distribution by ModFit software.
Hypoxic cell culture
The cells were rinsed with PBS and resuspended in serum-free DMEM/F12. Then, the cells were placed in an incubator with an anaerobic gas generation agent and oxygen indicator at 37°C with 5% CO2.
Measurement of the apoptosis rate by flow cytometry
Cells were collected at 0, 6, 12, 24, and 48 h after hypoxic culture, rinsed with PBS, and resuspended at a density of 5 × 105/mL. Then, the cells were combined with 1.25 μL Annexin V-APC and incubated in the dark at room temperature for 15 min. The cells were then mixed with 10 μL of a propidium iodide (PI) solution and incubated at room temperature for 5 min. Finally, the cells were analyzed by flow cytometry.
Apoptosis measurement with Hoechst staining
Cells cultured under hypoxia for 48 h were re-seeded in 12-well plates and cultured to confluence. The cells were then fixed with 4% formaldehyde for 10 min, washed with PBS, and air-dried. Then, a 1 µg/mL Hoechst 33258 working solution was applied to the cells, followed by incubation at room temperature for 10 min. The cells were then rinsed, mounted with anti-fade mounting medium, and observed by fluorescence microscopy.
Detection of caspase-3, Bcl1-2 and bax mRNA expression by real-time PCR
Cells cultured under hypoxia for 48 h were collected to extract total RNA using Trizol. cDNA was synthesized from the total RNA by reverse transcription. A fluorescence quantitative PCR system with SYBR Green dye was used for real-time PCR. β-actin was used as a reference gene. The primers are listed in Table 1. The reaction conditions were preheating at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 15 s, and extension at 73°C for 32 s.
Table 1.
Primer sequences
| Primer | Sequences |
|---|---|
| Caspase-3-F: | 5’-AATTCAAGGGACGGGTCATG-3’ |
| Caspase-3-R: | 5’-TGACACAATACACGGGATCTG-3’ |
| Bcl-2-F: | 5’-ATCCAGGATAACGGAGGCTG-3’ |
| Bcl-2-R: | 5’-CAGGTATGCACCCAGAGTGA-3’ |
| Bax-F: | 5’-GGCGAATTGGAGATGAACTG-3’ |
| Bax-R: | 5’-TGCCATCAGCAAACATGTCA-3’ |
| β-actin-F: | 5’-AGGGAAATCGTGCGTGACAT-3’ |
| β-actin-R: | 5’-GAACCGCTCATTGCCGATAG-3’ |
Statistical analysis
The data were expressed as the means ± SD. SPSS 13.0 was used for inter-group comparisons with one-way analysis of variance and post-hoc comparisons. A value of P < 0.05 was considered significant.
Results
Cell culture and identification
After the first medium change, large cells were adherent and some cells had a short rod-like shape. At 3-4 days, the adherent cells increased in number to form colonies. At 9-10 days, the cells formed a flake-like confluent layer and were arranged in order along the long axis of the soma as a spiral shape (Figure 1). Immunophenotyping showed that the positive rates of CD29 and CD90 were 93.31% and 93.45%, respectively, while the positive rate of CD45 (a marker of hematopoietic cells) was 5.69% (Figure 2), suggesting that the harvested cells were MSCs.
Figure 1.
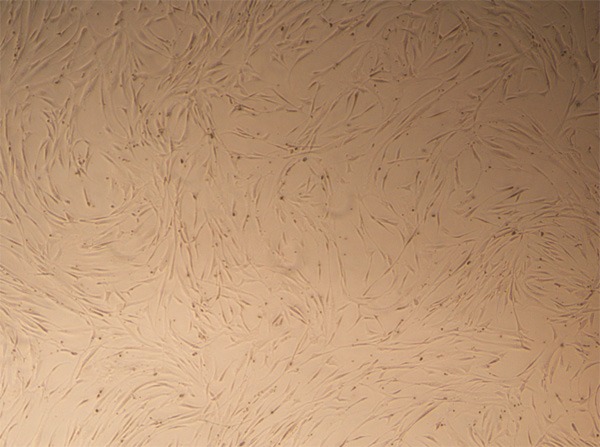
Morphology of MSCs. Passage 3 MSCs were morphologically consistent and showed a long spindle shape, a radial or spiral configuration, and growth as colonies (phase contrast microscopy, ×40).
Figure 2.
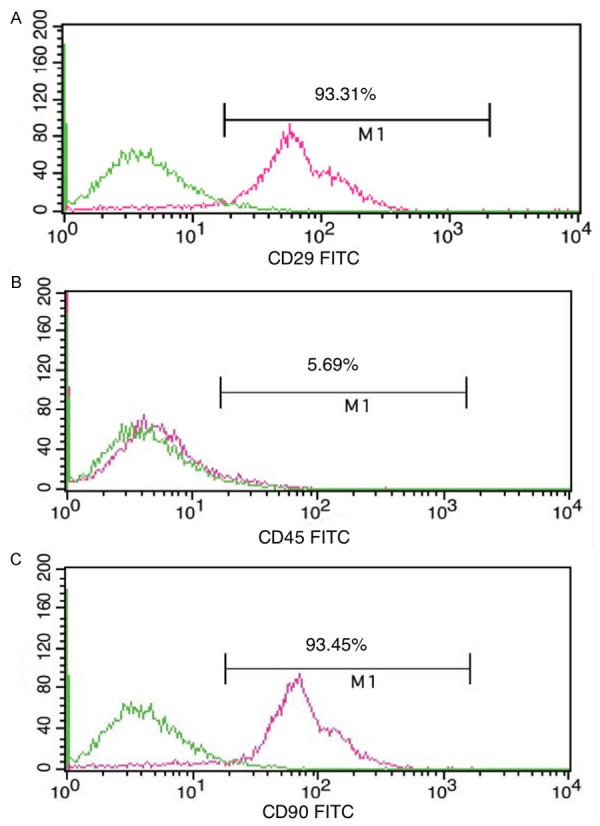
Identification of MSCs. Immunophenotyping showed the positive rates of CD29, CD90, and CD45.
Construction of the shRNA lentiviral vector and transfection
The 7.5 kb vector did not contain an XhoI restriction enzyme site, whereas the inserted shRNA contained such a site. Therefore, only the vector with the insert could be cut by XhoI. Figure 3 shows the successful cloning of the shRNA insert that was confirmed by sequencing.
Figure 3.
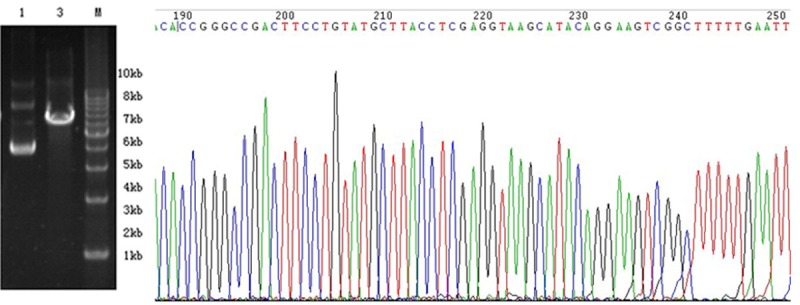
Enzymatic digestion and shRNA sequencing. Successful cloning of the shRNA insert was confirmed by sequencing.
MSCs showed green fluorescence under a fluorescence microscope at 3-4 days after infection with the lentivirus carrying the enhanced green fluorescent protein gene (Figure 4). During culture, the fluorescence intensity gradually increased, and the cells were well stacked without changes in morphology. When the transfection efficiency reached 80-90%, the cells were used for subsequent experiments.
Figure 4.
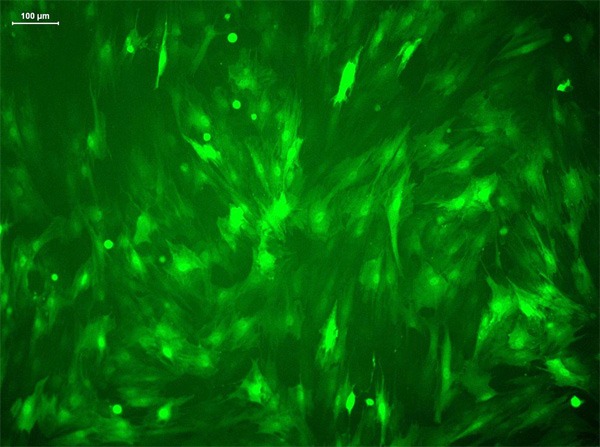
Transfection of MSCs. MSCs expressed EGFP after transfection (fluorescence microscopy, ×400).
Expression of caspase-3 protein
In densitometric analysis, the expression of caspase-3 protein was 0.19 ± 0.07, 0.49 ± 0.16, and 0.48 ± 0.14 in transfection, empty vector, and blank control groups, respectively (F = 5.262, P = 0.048). Compared with empty vector and blank control groups, caspase-3 protein expression in the transfection group was decreased by 61% and 60%, respectively. This result suggested that the recombinant plasmid GV115-shRNA-caspase-3 was successfully expressed in MSCs and significantly inhibited caspase-3 expression (Figure 5).
Figure 5.
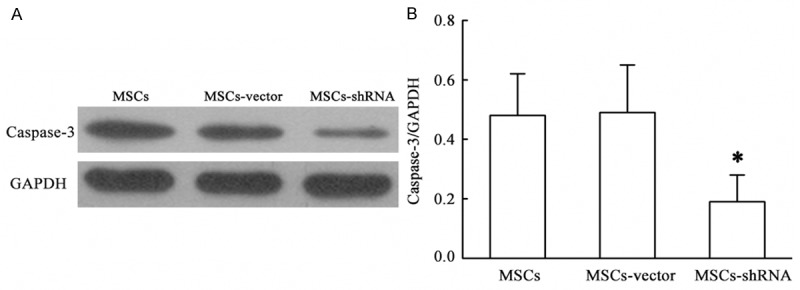
Expression of caspase-3 protein. The results are expressed as the means ± SD (n = 3). *P < 0.05 compared with MSCs or MSCs-vector.
Cell proliferation
The proliferation rate was calculated by measuring OD values at various time points. Except for day 1, the proliferation rates of the transfection group at all other time points were significantly higher than those of the control group (F = 14.332, 11.825, 93.073, and 54.163, all P < 0.05; Figure 6). There was no significant difference between empty vector and blank control groups (P > 0.05). These results suggest that the lentivirus itself had no effect on the proliferation of MSCs and there was specific enhancement of growth by caspase-3 silencing.
Figure 6.
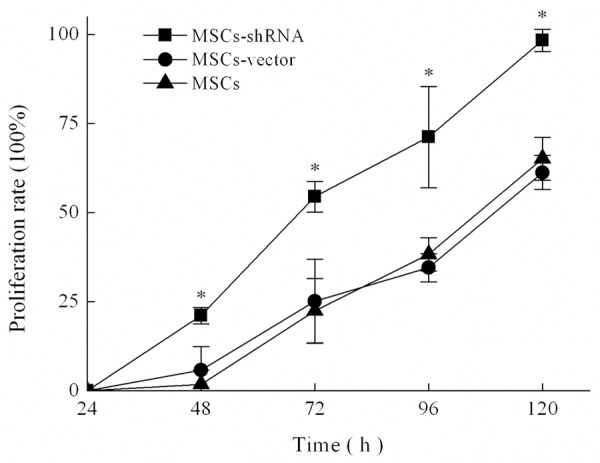
Cell proliferation curves. The results are expressed as the means ± SD (n = 3). *P < 0.05 compared with MSCs or MSCs-vector.
Cell cycle analysis
As shown in Table 2, the percentage of cells at S-phase in the transfection group was significantly increased compared with that in empty vector and blank control groups (F = 1836.707, P < 0.01), while there was a significant decrease of the percentage of cells at G0/G1 and G1/G2 phases (F = 234.182 and 25.908, respectively; P < 0.05). No significant difference was observed between control groups (P > 0.05; Table 2 and Figure 7). MSCs were mainly undergoing S-phase after caspase-3 silencing.
Table 2.
Cell cycle distribution (%)
| Group | Cell cycle | ||
|---|---|---|---|
|
| |||
| G0/G1 | S | G1/G2 | |
| MSCs | 55.39 ± 0.45 | 31.07 ± 0.44 | 12.16 ± 2.70 |
| MSCs-vector | 56.29 ± 0.61 | 34.58 ± 0.62 | 10.51 ± 2.34 |
| MSCs-shRNA | 46.50 ± 0.74* | 52.66 ± 0.30* | 0.84 ± 0.48* |
Means ± SD; n = 3,
P < 0.05 compared with MSCs or MSCs-vector.
Figure 7.
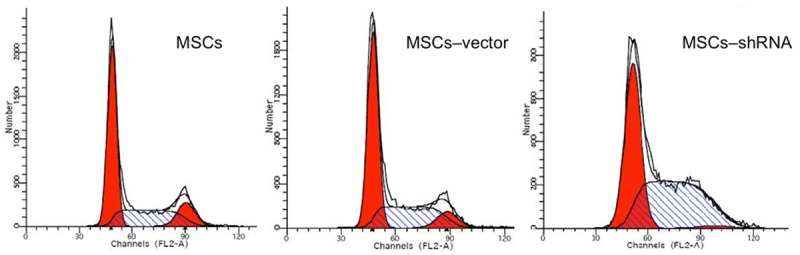
Cell cycle analysis. The percentage of cells at S-phase in the MSCs-shRNA group was significantly increased compared with that of MSCs and MSCs-vector.
Apoptosis rate at various time points under hypoxia
The apoptosis rates measured by Annexin V-APC/PI double staining at 0, 6, 12, 24, and 48 h of hypoxia are shown in Figures 8 and 9. The apoptosis rate of all groups gradually increased under hypoxia. However, the increase of apoptosis in the transfection group was lower than that in the control group at the various time points (F = 80.228, 42.876, 206.870, 730.213, and 521.220, respectively, P < 0.01). This result suggested that caspase-3 silencing enhances the tolerance of MSCs to a hypoxic environment.
Figure 8.
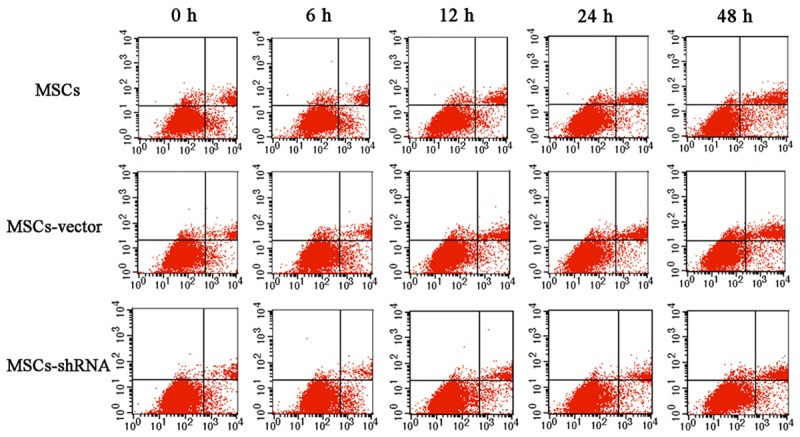
Scatter diagram of MSC apoptosis. The apoptosis rates measured by annexin V-APC/PI double staining at 0, 6, 12, 24, and 48 h of hypoxia.
Figure 9.
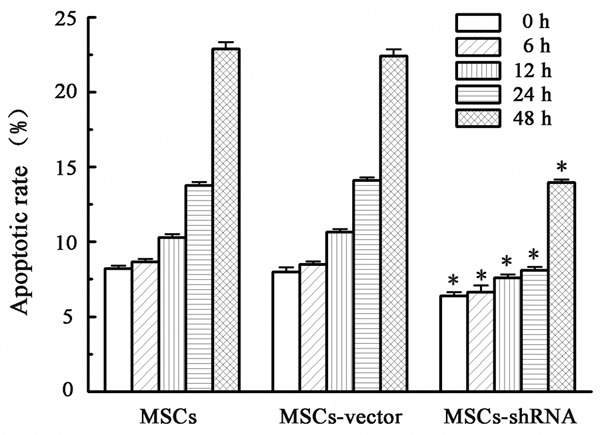
Apoptotic rates at various time points under hypoxia and serum deprivation (%). The results are expressed as the means ± SD (n = 3). *P < 0.05 compared with MSCs or MSCs-vector.
Apoptosis detection by Hoechst 33258 staining
After staining with Hoechst 33258, nuclei exhibited blue fluorescence under a fluorescence microscope, whereas the nuclei of apoptotic cells showed agglomerated nuclear chromatins and condensation (Figure 10). At 400× magnification, six different fields in each group were selected to calculate the number of apoptotic cells. The results indicated that the apoptosis rate of the transfection group under regular culture conditions was 15.00 ± 1.73% compared with that in the empty vector group (25.67 ± 3.05%) and blank control group (23.67 ± 1.16%; F = 21.171, P = 0.002). There was no significant difference between the two control groups (P > 0.05). The apoptosis rate under hypoxic conditions after 48 h in the transfection group was 29.67 ± 2.08% compared with that in the empty vector group (42.33 ± 6.43%) and blank control group (45.00 ± 6.56%; F = 6.812, P = 0.029). There was no significant difference between the two control groups (P > 0.05).
Figure 10.
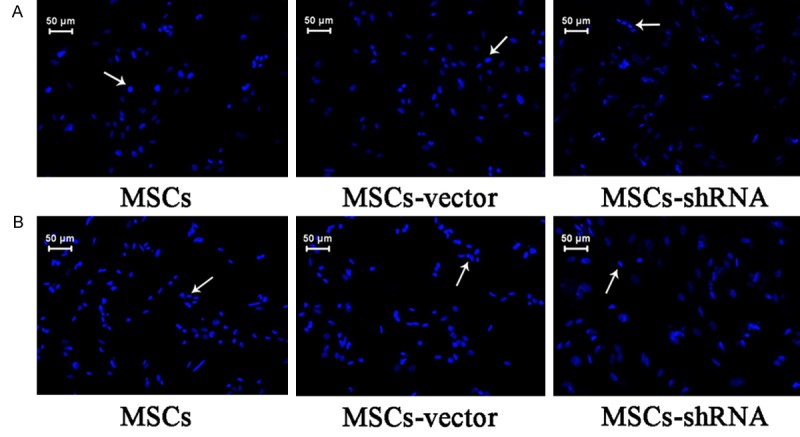
Apoptosis of MSCs. Chromatin condensed, nuclei shrunk, and there was a decrease in the size of apoptotic cells.A: Normal condition; B: Hypoxia and serum deprivation for 48 h (×400).
mRNA expression of caspase-3, Bcl-2, and bax
Under normoxic conditions, the expression of caspase-3 mRNA in transfection, empty vector, and blank control groups was 0.39 ± 0.04, 1.66 ± 0.18, and 1.92 ± 0.18, respectively. Expression of Bcl-2 mRNA in transfection, empty vector, and blank control groups was 2.57 ± 0.12, 2.25 ± 0.13 and 2.16 ± 0.21, respectively. Bax mRNA expression in transfection, empty vector, and blank control groups was 1.33 ± 0.17, 1.66 ± 0.14, and 1.68 ± 0.13, respectively. The ratio of Bcl-2/Bax mRNA levels in transfection, empty vector, and blank control groups was 1.83 ± 0.24, 1.35 ± 0.18 and 1.29 ± 0.17, respectively. After caspase-3 silencing, mRNA expression of caspase-3 was down-regulated (F = 93.585, P < 0.01), Bcl-2 expression was up-regulated (F = 5.560, P = 0.043), and Bax expression was down-regulated (F = 5.415, P = 0.045), which increased the Bcl-2/Bax ratio (F = 6.322, P = 0.03).
Under hypoxic conditions for 48 h, caspase-3 mRNA expression in transfection, empty vector, and blank control groups was 1.31 ± 0.12, 7.98 ± 0.22, and 7.46 ± 0.53, respectively; Bcl-2 mRNA expression was 6.74 ± 0.10, 6.28 ± 0.10, and 6.37 ± 0.27 respectively; Bax mRNA expression was 1.08 ± 0.20, 3.44 ± 0.13, and 3.72 ± 0.25, respectively; Bcl-2/Bax mRNA level ratios were 5.83 ± 0.38, 1.97 ± 0.18, and 1.74 ± 0.17, respectively. After caspase-3 silencing, mRNA expression of caspase-3 was down-regulated (F = 362.496, P < 0.01), Bcl-2 mRNA expression was up-regulated (F = 5.993, P = 0.037), Bax expression was down-regulated (F = 163.297, P < 0.01), and there was an increase of the Bcl-2/Bax ratio (F = 229.777, P < 0.01). The results are shown in Figure 11.
Figure 11.
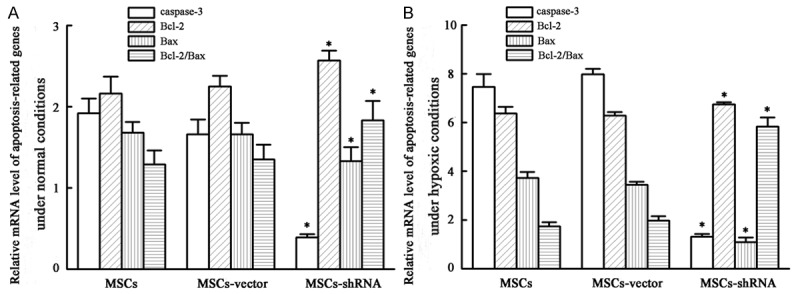
mRNA expression level of apoptosis-related genes. A: Normal condition; B: Hypoxia and serum deprivation for 48 h. The results are expressed as the means ± SD (n = 3). *P < 0.05 compared with MSCs or MSCs-vector.
Discussion
Although clinical evidence indicates that injection of autologous MSCs improves the clinical signs of myocardial infarction, increases myocardial perfusion, enhances left ventricle function, and decreases the incidence rate of heart failure [12,13], the current therapeutic effects are unsatisfactory. The myocardial microenvironment affects the survival probability of transplanted MSCs. The extracellular matrix and bioactive molecules not only provide support and protection for MSCs, but also play important roles in modulation of MSC proliferation, differentiation and migration. After myocardial infarction, local hypoxic ischemia, inflammatory reactions, and reperfusion injury present transplanted MSCs with a complex and severe microenvironment [14,15]. The lack of survival signals in the extracellular matrix and production of cytotoxic factors results in a limited proliferative capacity and massive apoptosis of transplanted MSCs [16,17]. Simultaneously, myocardial mechanical stimulation also has certain effects on the proliferation and survival of MSCs [18].
Cell apoptosis involves complicated signaling pathways. Caspase-3 is downstream of the ordered cascade reaction of apoptosis, and is activated at the end of endogenous and exogenous pathways [19]. Bcl-2 family genes are also involved in apoptosis, including pro-apoptotic genes (Bax and Bag) and anti-apoptotic genes (Bcl-xl and Bcl-2) [20]. Any factor that disrupts the balance of expression between pro-apoptotic and anti-apoptotic genes or their related proteins will increase the sensitivity of cells to external apoptotic stimuli [21]. Bcl-2 and Bax are the most widely studied genes related to apoptosis, and the ratio of Bcl-2/Bax determines the fate of cells [22].
In the present study, we constructed a shRNA expression vector against caspase-3, the key executor of apoptosis, and transfected MSCs through a lentivirus to effectively interfere with the expression of target genes at mRNA and protein levels. The lentiviral expression system infects dividing and non-dividing cells, has low immunogenicity, can hold large exogenous genes, and is therefore one of the most commonly used viral vectors [23,24]. Here, we found that the proliferation rate of MSCs was significantly increased by caspase-3 silencing, probably because of cell cycle modulation, resulting in more cells entering S-phase and less apoptotic cells. Some studies have induced apoptosis of target cells by decreasing the oxygen concentration in culture conditions, and found that hypoxia can induce obvious apoptosis but only after long-term exposure to hypoxia. However, a lack of serum is the major cause of MSC apoptosis under hypoxic conditions [25,26]. We applied serum-free and hypoxic culture of MSCs, which better mimics the microenvironment of myocardial infarction [27,28], and found agglomerated nuclear chromatins and condensed nuclei, a reduction of the cellular volume, and loss of membrane integrity. These observations are consistent with previous studies [27,29]. The apoptosis rate in the transfection group was lower than that in the control group and decreased further over time, suggesting that caspase-3 silencing increases the survival of MSCs in a severe microenvironment. The reason for this observation may be related to the up-regulation of Bcl-2, down-regulation of Bax, and subsequent increase of the Bcl-2/Bax ratio, except for the down-regulation of target genes.
The present study examined apoptosis at 48 h and did not explore the long-term survival of MSCs. The microenvironment of myocardial infarctions is complex and modeling hypoxic ischemia in vitro cannot effectively illustrate an increase of survival of transplanted MSCs in the infarction area. Based on the present study, we will transplant MSCs into rats to further investigate the survival rate of MSCs with caspase-3 silencing in myocardial infarction areas and the effects on paracrine, ventricle remodeling, and heart functions.
Acknowledgements
This work was supported by the Social Development Science and Technology Program of Guangdong Province (2012B03180025); the Fundamental Research Funds for the Central Universities(11ykpy30); the National Natural Science Foundation of China (81000508) (http://www.nsfc.gov.cn/) and the Pearl River Science and Technology Star Fund (2012J2200090).
Disclosure of conflict of interest
None.
References
- 1.Choudhery MS, Khan M, Mahmood R, Mehmood A, Khan SN, Riazuddin S. Bone marrow derived mesenchymal stem cells from aged mice have reduced wound healing, angiogenesis, proliferation and ani-apoptosis capabilities. Cell Biol Int. 2012;36:747–53. doi: 10.1042/CBI20110183. [DOI] [PubMed] [Google Scholar]
- 2.Khan WS, Hardingham TE. Mesenchymal stem cells, sources of cells and differentiation potential. J Stem Cells. 2012;7:75–85. [PubMed] [Google Scholar]
- 3.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuri MM, Hafeez S. Autologous bone marrow stem cell transplant in acute myocardial infarction. J Pak Med Assoc. 2012;62:2–6. [PubMed] [Google Scholar]
- 9.Wang S, Qin X, Sun D, Wang Y, Xie X, Fan W, Wang Y, Liang D, Pei X, Cao F. Effects of hepatocyte growth factor overexpressed bone marrow-derived mesenchymal stem cells on prevention from left ventricular remodelling and functional improvement in infarcted rat hearts. Cell Biochem Funct. 2012;30:574–581. doi: 10.1002/cbf.2836. [DOI] [PubMed] [Google Scholar]
- 10.Beitnes JO, Hopp E, Lunde K, Solheim S, Arnesen H, Brinchmann JE, Forfang K, Aakhus S. Long-term results after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: the ASTAMI randomised, controlled study. Heart. 2009;95:1983–1989. doi: 10.1136/hrt.2009.178913. [DOI] [PubMed] [Google Scholar]
- 11.Scatena R, Bottoni P, Botta G, Martorana GE, Giardina B. The role of mitochondria in pharmacotoxicology: a reevaluation of an old, newly emerging topic. Am J Physiol Cell Physiol. 2007;293:C12–C21. doi: 10.1152/ajpcell.00314.2006. [DOI] [PubMed] [Google Scholar]
- 12.Beitnes JO, Lunde K, Brinchmann JE, Aakhus S. Stem cells for cardiac repair in acute myocardial infarction. Expert Rev Cardiovasc Ther. 2011;9:1015–1025. doi: 10.1586/erc.11.108. [DOI] [PubMed] [Google Scholar]
- 13.Mardikar HM, Deshpande NV, Admane P. Recent advances in the management of acute myocardial infarction. J Assoc Physicians India. 2011;59(Suppl):31–36. [PubMed] [Google Scholar]
- 14.Lu HH, Li YF, Sheng ZQ, Wang Y. Preconditioning of stem cells for the treatment of myocardial infarction. Chin Med J (Engl) 2012;125:378–384. [PubMed] [Google Scholar]
- 15.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Hou JF, Shen Y, Wang W, Wei YJ, Hu S. Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. J Cell Mol Med. 2010;14:1975–1987. doi: 10.1111/j.1582-4934.2009.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 18.Pereira MJ, Carvalho IF, Karp JM, Ferreira LS. Sensing the cardiac environment: exploiting cues for regeneration. J Cardiovasc Transl Res. 2011;4:616–630. doi: 10.1007/s12265-011-9299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 20.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–1424. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165–175. [PMC free article] [PubMed] [Google Scholar]
- 22.Boumela I, Assou S, Aouacheria A, Haouzi D, Dechaud H, De Vos J, Handyside A, Hamamah S. Involvement of BCL2 family members in the regulation of human oocyte and early embryo survival and death: gene expression and beyond. Reproduction. 2011;141:549–561. doi: 10.1530/REP-10-0504. [DOI] [PubMed] [Google Scholar]
- 23.Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- 24.Picanco-Castro V, de Sousa RE, Tadeu CD. Advances in lentiviral vectors: a patent review. Recent Pat DNA Gene Seq. 2012;6:82–90. doi: 10.2174/187221512801327433. [DOI] [PubMed] [Google Scholar]
- 25.Das R, Jahr H, van Osch GJ, Farrell E. The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev. 2010;16:159–168. doi: 10.1089/ten.TEB.2009.0296. [DOI] [PubMed] [Google Scholar]
- 26.Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007;13:1325–1331. doi: 10.1089/ten.2006.0325. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24:416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X, Yu SP, Taylor T, Ogle M, Wei L. Protective effect of apelin on cultured rat bone marrow mesenchymal stem cells against apoptosis. Stem Cell Res. 2012;8:357–367. doi: 10.1016/j.scr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]


