Abstract
Background: Whether oral antiseptics could reduce the risk of ventilator associated pneumonia (VAP) in patients receiving mechanical ventilation remains controversial. We performed a meta-analysis to assess the effect of oral care with antiseptics on the prevalence of ventilator associated pneumonia in adult critically ill patients. Methods: A comprehensive search of PubMed, Embase and Web of Science were performed to identity relevant studies. Eligible studies were randomized controlled trials of mechanically ventilated adult patients receiving oral care with antiseptics. The quality of included studies was assessed by the Jadad score. Relative risks (RRs), weighted mean differences (WMDs), and 95% confidence intervals (CIs) were calculated and pooled using a fixed-effects model or random-effects model. Heterogeneity among the studies was assessed with I 2 test. Results: 17 studies with a total number of 4249 met the inclusion criteria. Of the 17 studies, 14 assessed the effect of chlorhexidine, and 3 investigated the effect of povidone-iodine. Overall, oral care with antiseptics significantly reduced the prevalence of VAP (RR=0.72, 95% CI: 0.57, 0.92; P=0.008). The use of chlorhexidine was shown to be effective (RR=0.73, 95% CI: 0.57, 0.93; P=0.012), whereas this effect was not observed in povidone-iodine (RR=0.51, 95% CI: 0.09, 2.82; P=0.438). Subgroup analyses showed that oral antiseptics were most marked in cardiac surgery patients (RR=0.54, 95% CI: 0.39, 0.74; P=0.00). Patients with oral antiseptics did not have a reduction in intensive care unit (ICU) mortality (RR=1.11, 95% CI: 0.95, 1.29; P=0.201), length of ICU stay (WMD=-0.10 days, 95% CI: -0.25, 0.05; P=0.188), or duration of mechanical ventilation (WMD=-0.05 days, 95% CI: -0.14, 0.04; P=0.260). Conclusion: Oral care with antiseptics significantly reduced the prevalence of VAP. Chlorhexidine application prevented the occurrence of VAP in mechanically ventilated patients but povidone-iodine did not. Further large-scale, well-designed randomized controlled trials are needed to identify the findings and determine the effect of povidone-iodine application.
Keywords: Oral care, antiseptics, ventilator associated pneumonia, meta-analysis
Introduction
Ventilator associated pneumonia (VAP) is defined as the occurrence of pneumonia in patients receiving mechanical ventilation for more than 48 hours after endotracheal intubation [1]. VAP remains the leading cause for nosocomial infection in intensive care units (ICU), affecting 10-30% patients receiving mechanical ventilation [2,3]. Moreover, VAP is associated with prolonged hospital stay [4-6], longer duration of mechanical ventilation [3], higher health-care cost [3,4,7], and a two-fold risk of mortality [3]. Thus, considering the consequences attributable to VAP, prevention of VAP is a priority in ICU care [8,9], and many new efforts have been taken to assess the various preventive measures [10-12].
The most important mechanism of the development of VAP is aspiration of oropharyngeal organisms into the lower respiratory tract, followed by bacterial proliferation and parenchymal invasion [13-15]. The oropharynx and upper gastrointestinal tract are the potential reservoirs for bacteria associated with VAP [16,17], so the reduction of oral bacteria might have a potential for prevention of VAP [27].
Oral care with antiseptics, such as chlorhexidine and povidone-iodine, has been proven to be effect in the prevention of VAP. However, potential factors, including type of antiseptics, frequency of use and targeted patients, still have remained inconclusive. Thus, we conducted this meta-analysis to assess the effects of oral care with antiseptics on the prevalence of VAP and other clinical outcomes in critically ill patients undergoing mechanical ventilation.
Material and methods
Literature search
A comprehensive literature search was conducted to search for relevant randomized controlled trials. PubMed, Embase and Web of Science were searched before 6 April 2014, using the searching terms: (“mouth” [MeSH Terms] OR “mouth” [All Fields] OR “oral” [All Fields]) AND care [All Fields] AND (“pneumonia, ventilator-associated” [MeSH Terms] OR (“pneumonia” [All Fields] AND “ventilator-associated” [All Fields]) OR “ventilator-associated pneumonia” [All Fields] OR (“ventilator” [All Fields] AND “associated” [All Fields] AND “pneumonia” [All Fields]) OR “ventilator associated pneumonia” [All Fields]). Other websites, including Cochrane Central Register of Controlled Trials, Google Scholar, and hettp://ClinicalTrials.gov, were also searched. No language restriction was limited. We also manually screened the reference lists of the retrieved articles to find the potentially eligible trials. This process was performed iteratively until no potential articles could be identified. For studies without complete data, we contacted the authors for detail information if needed.
Review strategy and data extraction
Endnote bibliographic software was used to establish an electronic library of citations identified in the literature searches. PubMed, Embase and Web of Science were performed, and duplicate records were deleted. Two authors (LONGTI LI and ZHIBING AI) independently trained to screen the abstract review and then the full text review. Disagreements were resolved by consensus and discussion between the authors.
A structured questionnaire was used for data extraction. The following data were extracted independently by the two authors (LONGTI LI and ZHIBING AI): first author, year of publication, baseline characteristics of the population (age, gender), sample size, inclusion and exclusion criteria, definitions and diagnosis of VAP, intervention group (oral care with antiseptics), control group (oral care without antiseptics), the prevalence of VAP, and other important clinical outcome data.
Study inclusion and exclusion criteria
We include studies in all languages when they met the following criteria: (1) randomized controlled trials; (2) adults patients receiving mechanical ventilation; (3) randomized allocation to intervention group (oral care with antiseptics) or control group (standard oral care without antiseptics); (4) reporting data on the prevalence of VAP; (5) sample size more than 50.
Quality assessment
The quality of the included studies was assessed by using the Jada scale [18]. The scale consists of the following three items to define the quality of a randomized controlled trial: (1) randomization (0-2 points); (2) masking (0-2 points); (3) dropouts and withdrawals (0-1 points). A score of 1 is given for each of the item described. A further point is obtained when the method of randomization or double-blinding is given. The scale ranges from 0 to 5 points. The RCTs are considered to be of high quality if the Jadad score is ≥ 3 [18].
Statistical analysis
We assessed the effect of the oral antiseptics on the prevention of VAP based on the data from 17 randomized trials. The prevalence of VAP and ICU mortality were treated as dichotomous variables and were expressed as relative risk (RR) with 95% confidence intervals (CI). Length of ICU and duration of mechanical ventilation were treated as continuous variables, thus they were expressed as mean difference (WMD) with 95% CI. Heterogeneity among the studies was tested using the I2 statistic, a quantitative method measuring inconsistency across the studies. Studies with an I2 of 25% to 50%, 50% to 75%, and greater than 75% are considered to have low, moderate and high heterogeneity, respectively [19]. Pooled estimates were calculated using fixed-effects model (Mantel-Haenszel method) [20] or randomized-effects model (DerSimonian-Laird method) [21]. A randomized-effects model was used to summarize the pool data if substantial heterogeneity was found (I2 > 10%). A subgroup analysis was performed based on type of antiseptics and type of surgery patients. Publication bias was evaluated using the Begg tests [22]. P < 0.05 was considered statistical significant. All analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX, USA).
Results
Study identification and selection
The initial search yielded 795 relevant publications, of which 32 were excluded for duplicate studies and 642 were excluded based on the title and abstracts screening (Figure 1). The remaining 121 were then retrieved for the full text review, and five were excluded because they did not report the outcomes of interest, one was excluded because antiseptics were used in both groups [23], and two were excluded because the sample size was less than 50 [24,25]. Thus, 17 RCTs included in the meta-analysis [26-42].
Figure 1.
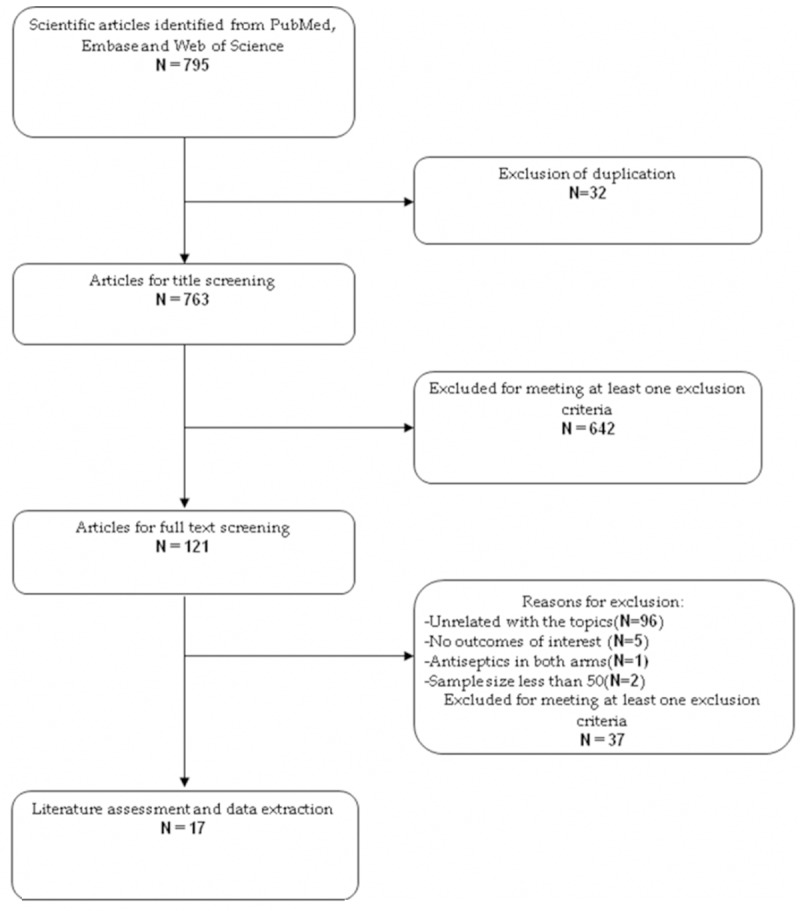
Search strategy and flow diagram for this meta-analysis.
Description of the included studies
The main characteristics of the 17 RCTs included in the meta-analysis are presented in Table 1. These studies were published from 1996 to 2014. The sample size ranged from 52 to 954. All the 17 studies were randomized trials, including a total number of 4249 patients. Of the 4249 patients, 2161 were randomized allocation to the intervention group (oral care with antiseptics), and 2088 were allocated to the control group (standard oral care, or use of placebo). 15 studies including 4032 patients assessed the effect of chlorhexidine [26,28-41], whereas 2 studies involving 217 patients evaluated the effect of povidone-iodine [27,42]. Most of the studies included patients in non-cardiac surgery ICU, comprising surgical, medical-surgical or mixed (medical, surgical/trauma) ICU [26,27,29,31-37,39-42]. However, 3 studies [28,30,38], exclusively included cardiac surgical patients, accounting for 51.3% of all the patients forming the total population for this meta-analysis.
Table 1.
Baseline characteristics of the trials included in the meta-analysis
| Study | Number of patients (I/C) | Setting | Diagnosis VAP | Intervention group | Control group | Jadad score |
|---|---|---|---|---|---|---|
| F.Fourrier [26] | 60 (30/30) | Medical surgical ICU | Temperature > 38°C or < 36°C, presence of infiltrates on chest radiography, leukocytosis or leukopenia, positive culture from tracheal aspirate (106 CFU/mL) and/or BAL (104 CFU/mL) | CHX 0.2% gel TID | Bicarbonate isotonic serum, gentle oropharyngeal sterile aspiration, QID | 3 |
| Philippe Seguin [27] | 67 (36/31) | Surgical ICU | New clinical findings, with new pulmonary infiltrates plus two of the following items: 1) fever of > 38°3C or hypothermia of < 36°C, 2) purulent endotracheal aspirate; and 3) a peripheral white blood cell count of > 10,000 cells/mm3 or < 5,000 cells/mm3 | CHX 2% paste QID | placebo | 5 |
| Anthony J [28] | 353 (173/180) | Cardiac surgery ICU | New or progressing pulmonary infiltrate, fever, leucocytosis, and purulent tracheobronchial secretions | CHX 0.12% oral rinse BID | placebo | 4 |
| Francois Fourrier [29] | 228 (114/114) | Medical-surgical ICU | Temperature > 38°C or < 36°C, new infiltrates on chest radiographs, leucocytosis (> 10,000 cells per μL)or leucopenia (< 3,000 cells per μL),positive quantitative culture from tracheal aspirate or BAL, or both | CHX 0.2% gel TID, without toothbrushing | placebo | 4 |
| Susan Houston [30] | 561 (270/291) | Cardiac surgery ICU | New or progressing pulmonary infiltrate, fever, leucocytosis, positive microbial culture results | CHX 0.12% oral rinse BID, | Listerine 15 ml oral rinse BID | 3 |
| Mirelle Koeman [31] | 385 (128/130) | Mixed and surgical ICUs | New, persistent or progressive infiltrate on chest radiograph and at least three of four criteria: fever > 38°C or < 35.5°C, leucocytosis (> 10,000 cells per μL) or leucopenia (< 3,000 cells per μL), purulent aspect of tracheal aspirate, positive semiquantitative culture from tracheal aspirate | CHX 2% paste QID | placebo | 5 |
| Macnaughton P D [32] | 179 (91/88) | Mixed surgical ICU | Leukocytosis, fever > 38°C, deterioration in oxygenation or chest signs, new consolidation on chest radiograph, substantial bacterial growth on BAL, CPIS > 6 | CHX 0.2% BID | placebo | 3 |
| Hutsaya Tantipong [33] | 207 (102/105) | Mixed surgical ICU | New, persistant, or progressive infiltrate on chest radiograph and at least three of four criteria: fever > 38°C or < 35.5°C, leucocytosis (> 10×103 cells per μL) or leucopenia (< 3×103 cells per μL), purulent tracheal aspirate, positive semiquantitative culture from tracheal aspirate | CHX 2% 15 ml solution QID with toothbrushing | Saline | 2 |
| Bellissimo-Rodrigues [34] | 194(98/96) | Mixed ICU | Defined based on CDC criteriab | CHX 0.12% 15 ml TID | Placebo 15 ml TID | 4 |
| Tanmay S [35] | 471(250/262) | Mixed surgical ICU | development of new persistent alveolar infiltrates on chest radiograph; > 38°C; leucocytosis (> 12×103 WBCs per μL), and purulent sputum developing > 48 h after ICU admission with worsening of hypoxaemia on arterial blood gas analysis; semiquantitative cultures obtained by the protected non-bronchoscopic mini-BAL technique were considered positive with > 103 CFU per mL. | CHX 0.2% 10 ml BID | potassium permanganate 10 mL 0.01% BID | 3 |
| Frank A Scannapieco [36] | 99(50/49) | Trauma ICU | Upon suspicion of pneumonia, lung secretions analysis by bqBAL by use of a mini-BAL technique with > 104 CFU/mL of a target PRP in bqBAL fluid or a positive pleuralfluid culture in the absence of previous pleural instrumentation regarded as positive evidence for diagnosis of pneumonia | CHX 0.12% BID | Placebo BID | 4 |
| Suzana M L [37] | 52 (28/24) | Mixed surgical ICU | the presence of a new radiological pulmonary infiltrate on chest X-rays after 48 hours in the ICU, plus 2 or more of the following clinical or laboratory signs: axillary temperature ≥ 38°C or ≤ 36°C, leukocytes > 11×103/mm3 or leucopenia < 4×103/mm3, or the presence of purulent tracheal secretions. | CHX gel 2% | Placebo | 4 |
| Patrique Segers [38] | 954 (485/469) | Cardiac surgery ICU | Defined based on CDC criteriac,d | CHX gluconate solution 0.12% | Placebo | 4 |
| A.M. Berry [40] | 148 (71/77) | Mixed surgical ICU | Not described | CHX 0.2% solution BID | Sterile water | 4 |
| Oz,caka [41] | 61 (29/32) | Respiratory ICU | Using a mini-BAL technique collecting sample; The presence of ≥ 104 colony-forming units/mL of a target PRP in mini-BAL fluid, or a positive pleural fluid culture in the absence of previous pleural instrumentation | CHX 0.2% QID | Saline QID | 4 |
| Jafari Sa [39] | 80 (40/40) | Mixed surgical ICU | Not described | CHX 0.2% solution BID | Normal saline | None |
| Philippe Seguin [42] | 150 (78/72) | Brain-Injured or Cerebral Hemorrhage ICU | new and persistent pulmonary infiltrates on chest radiograph, occurring after 48 hours of mechanical ventilation, combined with at least two of the following criteria: purulent tracheal secretions and/or body core temperature > 38°C and/or leukocytosis > 10,000/mm3 or leukopenia < 3,000/mm3 and microbiological confirmation with quantitative culture from bronchoalveolar lavage or endotracheal aspirate, growing ≥ 104 cfu/mL or ≥ 106 cfu/mL, respectively | Povidone-iodine 10% 20 ml | Placebo (sterile water) | 4 |
Abbreviations: CHX, chlorhexidine; BID, twice daily; MRN, medical record number; QID, 4 times daily; TID, 3 times daily.
only abstracts were available for review;
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36: 309-332.
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999; 20: 250-278.
Kluytmans JA, Wertheim HF. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005; 33: 3-8.
With respect to interventions, Seguin et al [27] randomly assigned the patients into three arms: povidone-iodine group, saline group and control group. In this meta-analysis, we set the povidone-iodine group as the intervention group, and compared it with the control group. In another RCT [31], there were two intervention groups: 2% chlorhexidine with colistin, and 2% chlorhexidine. We preferred the former as the experimental group. In another study conducted by Scannapieco et al [36], patients were randomly allocated to three arms: (1) a control arm with oral placebo application twice daily; (2) an experimental arm with 0.12% chlorhexidine once daily plus placebo oral application once daily; (3) an additional experimental arm with 0.12% chlorhexidine twice daily. For this meta-analysis, we chose the third group as intervention group. The median Jadad score of the included studies was 4 (range from 3 to 5).
Prevalence of ventilator-associated pneumonia
All 17 RCTs reported the VAP in patients. Meta-analysis from the included studies showed that oral care with antiseptics significantly reduced the prevalence of VAP (RR=0.72, 95% CI: 0.57, 0.92; P=0.008) (Figure 2). This analysis showed a moderate statistical heterogeneity (I2=54.8%, P=0.004). To explore the reasons for the heterogeneity, we performed subgroup analysis based on type of antiseptics. The pooled results showed that the use of chlorhexidine was associated with a relative risk of 0.73 (95CI: 0.57, 0.93; P=0.012), indicating a 27% relative risk reduction for VAP for that patient population. In contrast, the use of povidone-iodine did not show any effect (RR=0.51, 95% CI: 0.09, 2.82; P=0.438) (Figure 2). The Begg’s test (P=0.296) revealed no publication bias (Figure 3).
Figure 2.
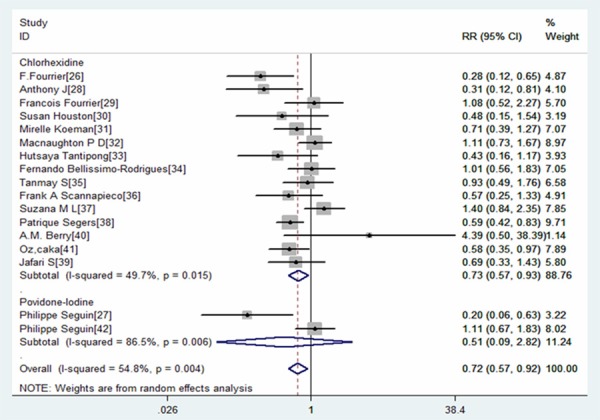
Forest plot showing the effect of oral care with chlorhexidine or povidone-iodine on the prevalence of ventilator associated pneumonia (VAP).
Figure 3.
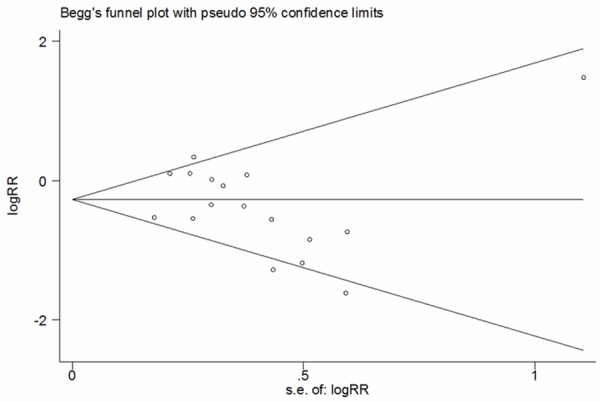
Forest plot assessing publication bias for ventilator associated pneumonia.
Subgroup analysis was performed on different type of surgery patients: cardiac surgery patients and non-cardiac surgery patients. The results revealed that the use of oral care with antiseptics significantly reduced the prevalence of VAP in the cardiac surgery patients (RR=0.54, 95% CI: 0.39, 0.74; P=0.00), but not in non-cardiac surgery patients (RR=0.78, 95% CI: 0.60, 1.02; P=0.072) (Figure 4).
Figure 4.
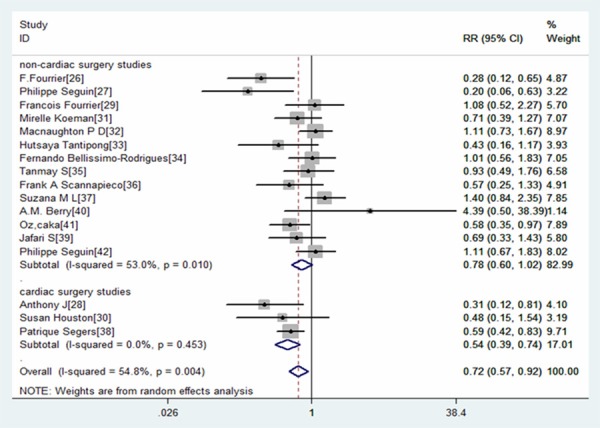
Forest plot showing the effect of oral care with antiseptics on the prevalence of VAP in different surgery patients.
ICU mortality
Eleven RCTs reported the data of ICU mortality [26-28,32,34-38,41,42]. The pooled results of these studies suggested that oral antiseptics did not decrease the ICU mortality (RR=1.11, 95% CI: 0.95, 1.29; P=0.201) (Figure 5). No heterogeneity was observed among the studies (I2=0.0%, P=0.540).
Figure 5.
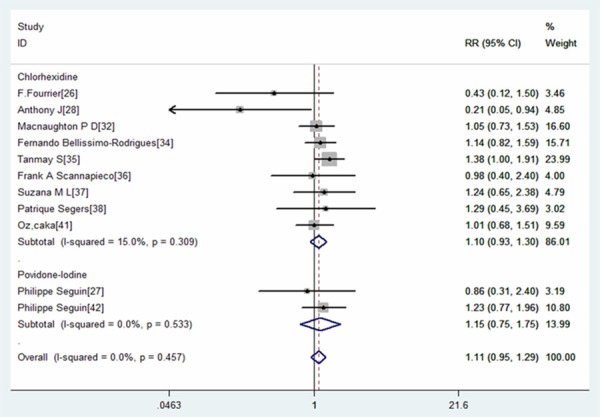
Forest plot showing the effect of oral care with chlorhexidine or povidone-iodine on intensive care unit (ICU) mortality.
Subgroup analysis based on type of antiseptics showed that, neither oral care with chlorhexidine or povidone-iodine would significantly reduce the ICU mortality (for chlorhexidine, RR=1.10, 95% CI: 0.93, 1.30; P=0.26; for povidone-iodine, RR=1.15, 95% CI: 0.75, 1.75; P=0.528).
Subgroup analysis base on different type of surgery patients resulted in similar results: oral antiseptic did not decrease the ICU mortality for cardiac surgery patients (RR=1.00, 95% CI: 0.79, 1.28; P=0.968) or non-cardiac surgery patients (RR=1.17, 95% CI: 0.96, 1.44; P=0.117) (Figure 6).
Figure 6.
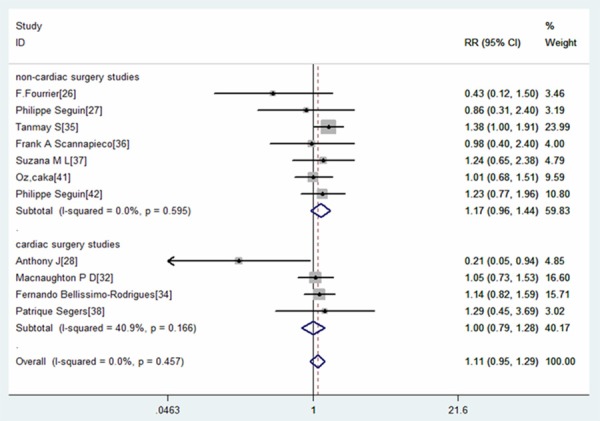
Forest plot showing the effect of oral care with antiseptics on intensive care unit (ICU) mortality in different surgery patients.
Length of ICU stay
Eight RCTs reported the data of length of ICU stay [26,27,29,31,36,38,41,42]. The pooled results revealed that oral antiseptics were not associated with a decrease in the length of ICU stay (WMD=-0.10 days, 95% CI: -0.25, 0.05; P=0.188). No heterogeneity was found among the studies (I2=0.0%, P=0.692). Among six of the eight studies, chlorhexidine was used in the intervention group, while the remaining two were povidone-iodine. Base on the type of antiseptics, subgroup analysis was performed. The results showed that these two types of oral care regimens could not reduce the length of ICU stay (for chlorhexidine, WMD=-0.10 days, 95% CI: -0.25, 0.05; P=0.202; for povidone-iodine, WMD=-1.83 days, 95% CI: -5.52, 1.85; P=0.329) (Figure 7). Due to the inadequate data, we did not perform the subgroup analysis to explore the effect of antiseptic on different surgery patients.
Figure 7.
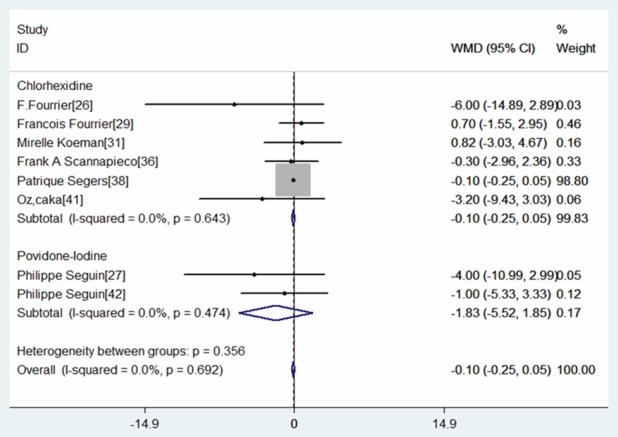
Forest plot showing the effect of oral care with chlorhexidine or povidone-iodine on length of intensive unit stay (ICU).
Duration of mechanical
Seven RCTs provided the data of duration of mechanical ventilation [26,27,29,31,36,38,41]. The aggregated results of these studies suggest that oral care with antiseptic was not associated with a significantly decrease in the duration of mechanical ventilation (WMD=-0.05 days, 95% CI: -0.14, 0.04; P=0.260) (Figure 8). The test for heterogeneity was not significant (I2=33.4%, P=0.173). Owing to the limited number of studies included in this analysis, the subgroup analysis based on type of antiseptics, or different survey patients were not performed.
Figure 8.
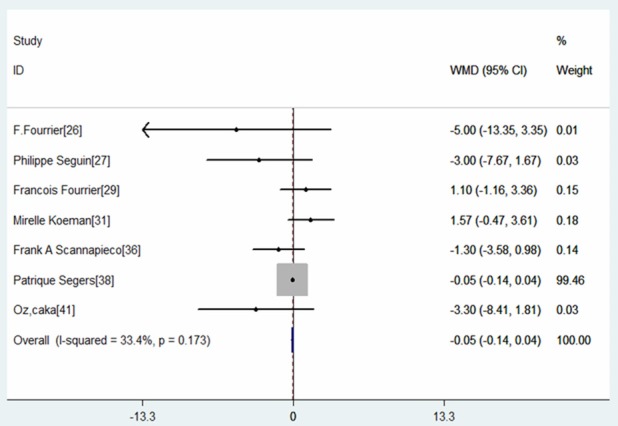
Forest plot showing the effect of oral care with antiseptics on duration of mechanical ventilation.
Discussion
The major purpose of this meta-analysis was to update and evaluate the effect of antiseptics on the prevention of VAP in adult critically ill patients undergoing mechanical ventilation. Our results indicate that oral care with antiseptics significantly reduces the prevalence of VAP. However, oral care with antiseptics is not effective at reducing ICU mortality, length of ICU stay or duration of mechanical ventilation.
According to this meta-analysis, oral antiseptic is associated with a 28% relative reduction in the risk of VAP. This beneficial effect is more remarkable in cardiac surgery patients, and no significant benefit is found in non-cardiac surgery patients. Possible explanations for these discrepant findings between the two categories of patients include the different characteristics of the populations and oral care protocols. Moreover, cardiac surgery is an elective procedure, and cardiac surgery patients usually have better physical condition than those admitted to the intensive care units. Also, for cardiac surgery patients, the intubation is performed under optimum and controlled conditions, whereas the intubation is more often emergently performed under less optimum condition for the critically ill patients. Thirdly, the cardiac surgery patients usually have a shorter period of mechanical ventilation (less than 1 day), whereas the non-cardiac surgery patients are usually required prolonged period of ventilation (1 to 2 weeks). Considering these differences above, it is not surprising that oral care with antiseptics show beneficial effect in cardiac surgery patients rather than non-cardiac surgery patients in the terms of prevalence of VAP.
Additionally, it is assumed that oral care with antiseptic would be more effective for the prevention of early onset rather than later onset VAP. However, in the two studies included in this meta-analysis [26,29], this difference was not observed between the oral antiseptics and control group in the time to develop VAP. In other words, the beneficial effect from oral care with antiseptics on preventing the development of VAP is limited to the patients who received cardiac surgery and who required shorted period of ventilation. It is possible that oral antiseptics just delays rather than prevents the occurrence of VAP. Thus, for patients intubated a short period of ventilation, oral antiseptic would reduce the risk of VAP, whereas for critically ill patients requiring prolonged period of ventilation, this benefit may not be observed. Owning to a lack of available data, we did not address this issue in this meta-analysis.
The subgroup analyses based on type of antiseptics showed that oral care with chlorhexidine significantly reduced the prevalent of VAP, whereas the povidone-iodine did not. Notably, the subgroup analyses for povidone-iodine application was based on only two RCTs with high statistically heterogeneity. Of the two studies, one [27] found a beneficial effect of povidone-iodine for prevention of VAP, whereas the other not. However, in the study with positive outcomes, the baseline characteristics of the patients were significantly different between the two groups. Thus, based on the current evidence, it is difficult to tell whether povidone-iodine is effective for prevention of VAP. Much more prospective RCTs are needed to verify the effect of povidone-iodine in oral care.
Another factor that may affect the results of the trials is the concentration of chlorhexidine solutions. Chlorhexidine 0.12%, which is the recommended dosage by the CDC for cardiac surgery patients [43], significantly reduced the rate of VAP in cardiac surgery patients [28,30]. However, among the non-cardiac surgery patients, a higher concentration may be necessary. In the trial conducted by Koeman et al. [31], a 2% chlorhexidine solution was used for mixed ICU patients, which is a higher concentration than that (0.12%, or 0.2%) in other studies. This may partially explain why the use of chlorhexidine significantly reduced the prevalence of VAP in this trial.
Our meta-analysis showed that oral care with antiseptic was not associated with a reduced ICU mortality. One possible explanation is that some patients aspirate small amounts of oral antiseptic, leading to acute respiratory lung injury [44-46]. This association was observed in one of RCTs included in our study [42]. Seguin and colleagues [42] found that, among the patients with oral povidone-iodine solution, a 6% rate of respiratory distress syndrome occurred, whereas 0% occurred in those with placebo. Another possible explanation is that the use of antiseptic may interfere with VAP diagnosis by inhibiting pathogen culture in the laboratory. The false-negative diagnoses would lead to withholding antibiotics. Moreover, some researchers have found that patients with culture-negative VAP have a higher mortality rates than those with culture-positive VAP [47].
This meta-analysis involving 4249 patients provides a comprehensive and updated assessment of the benefit profile of oral care with antiseptics in the prevention of ventilator-associated pneumonia. It differs from previous meta-analysis [48-51] in several ways: it specifically addresses the effects of oral care with antiseptics on different type of surgery patients: cardiac and non-cardiac surgery patients; it provides a quantitative assessment of different type of antiseptics on the prevalence of VAP; it includes recently published studies [37,40-42] that had not been included in the prior meta-analysis; it excludes studies with sample size less than 50 [24,25]; it systematically assesses the effect of oral care with antiseptics in terms of clinical endpoints, including ICU mortality, duration of mechanical ventilation, and length of ICU stay.
Although this meta-analysis is based on high quality RCTs, there are some limitations of our study. First, although studies with small size were excluded in this meta-analysis, some of the included studies had a modest sample size, which would overestimate the treatment effect when compared with larger trials. In addition, some of the subgroup analyses are based only on 2 to 3 studies; thus caution should be taken when applying these conclusions into the clinical practice. Second, there was considerable heterogeneity among the studies, including different patient population, the definition of VAP, concentrations of antiseptic (0.12%, 0.2%, 2%), forms of agent (oral rinse, gel), the frequencies of administration. These factors would have a potential impact on the results. Finally, it is possible that the existence of some unpublished studies could lead to bias in effect size. In general, considering the limitations mentioned above, we should interpret our results with adequate caution.
In conclusion, this meta-analysis suggests that oral care with antiseptics significantly reduces the prevalence of VAP, especially in cardiac surgery patients, but does not have effect on other important clinical outcomes, such as ICU mortality, length of ICU stay, duration of mechanical ventilation. However, given the relatively small studies and heterogeneity among studies, further large-scale, well-designed RCTs are needed to indentify the current findings and investigate the effects of povidone-iodine in patients with mechanical ventilation.
Disclosure of conflict of interest
None.
References
- 1.American Thoracic Society. Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 4.Rello J, Ollendorf DA, Oster G. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 5.Bercault N, Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: A prospective case-control study. Crit Care Med. 2001;29:2303–2309. doi: 10.1097/00003246-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249–1256. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 7.Amin A. Clinical and economic consequences of ventilator-associated pneumonia. Clin Infect Dis. 2009;49(Suppl 1):S36–43. doi: 10.1086/599814. [DOI] [PubMed] [Google Scholar]
- 8.Rello J, Lode H, Cornaglia G, Masterton R The VAP Care Bundle Contributors. A European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010;36:773–80. doi: 10.1007/s00134-010-1841-5. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH. Prevention of nosocomial pneumonia in the intensive care unit: beyond the use of bundles. Surg Infect (Larchmt) 2011;12:211–20. doi: 10.1089/sur.2010.060. [DOI] [PubMed] [Google Scholar]
- 10.Lorente L, Blot S, Rello J. Evidence on measures for the prevention of ventilator-associated pneumonia. Eur Respir J. 2007;30:1193–207. doi: 10.1183/09031936.00048507. [DOI] [PubMed] [Google Scholar]
- 11.Blot S. Limiting the attributable mortality of nosocomial infection and multidrug resistance in intensive care units. Clin Microbiol Infect. 2008;14:5–13. doi: 10.1111/j.1469-0691.2007.01835.x. [DOI] [PubMed] [Google Scholar]
- 12.Lorente L, Blot S, Rello J. New issues and controversies in the prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2010;182:870–76. doi: 10.1164/rccm.201001-0081CI. [DOI] [PubMed] [Google Scholar]
- 13.Safdar N, Crnich CJ, Maki DG. The pathogenesis of ventilator-associated pneumonia: Its relevance to developing effective strategies for prevention. Respir Care. 2005;50:725–739. [PubMed] [Google Scholar]
- 14.Ewig S, Torres A, El-Ebiary M. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med. 1999;159:188–198. doi: 10.1164/ajrccm.159.1.9803097. [DOI] [PubMed] [Google Scholar]
- 15.George DL, Falk PS, Wunderink RG. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:1839–1847. doi: 10.1164/ajrccm.158.6.9610069. [DOI] [PubMed] [Google Scholar]
- 16.Chan EY. Oral decontamination for ventilator-associated pneumonia prevention. Aust Crit Care. 2009;22:3–4. doi: 10.1016/j.aucc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Munro CL, Grap MJ. Oral health and care in the intensive care unit: State of the science. Am J Crit Care. 2004;13:25–33. discussion 34. [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 23.Angel Pobo, Thiago Lisboa. A randomized trial of dental brushing for preventing ventilator-associated pneumonia. Chest. 2009;136:433–439. doi: 10.1378/chest.09-0706. [DOI] [PubMed] [Google Scholar]
- 24.Michelle Bopp, Michele Darby. Effects of daily oral care with 0.12% chlorhexidine gluconate and a standard oral care protocol on the development of nosocomial pneumonia in intubated patients: a pilot study. J Dental Hygiene. 2006;80:1–13. [PubMed] [Google Scholar]
- 25.Grap MJ, Munro CL, Elswick RK Jr, Sessler CN, Ward KR. Duration of action of a single, early oral application of chlorhexidine on oral microbial flora in mechanically ventilated patients: A pilot study. Heart Lung. 2004;33:83–91. doi: 10.1016/j.hrtlng.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Fourrier F, Cau-Pottier E, Boutigny H, Roussel-Delvallez M, Jourdain M, Chopin C. Effects of dental plaque antiseptic decontamination on bacterial colonization and nosocomial infections in critically ill patients. Intensive Care Med. 2000;26:1239–47. doi: 10.1007/s001340000585. [DOI] [PubMed] [Google Scholar]
- 27.Seguin P, Tanguy M, Laviolle B, Tirel O, Malledant Y. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit Care Med. 2006;34:1514–19. doi: 10.1097/01.CCM.0000214516.73076.82. [DOI] [PubMed] [Google Scholar]
- 28.DeRiso AJ 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–61. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 29.Fourrier F, Dubois D, Pronnier P. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter study. Crit Care Med. 2005;33:1728–35. doi: 10.1097/01.ccm.0000171537.03493.b0. [DOI] [PubMed] [Google Scholar]
- 30.Houston S, Hougland P, Anderson JJ, LaRocco M, Kennedy V, Gentry LO. Effectiveness of 0.12% chlorhexidine gluconate oral rinse in reducing prevalence of nosocomial pneumonia in patients undergoing heart surgery. Am J Crit Care. 2002;11:567–70. [PubMed] [Google Scholar]
- 31.Koeman M, vander Ven AJ, Hak E. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173:1348–55. doi: 10.1164/rccm.200505-820OC. [DOI] [PubMed] [Google Scholar]
- 32.Macnaughton PD, Bailey J, Donlin N, Branfield P, Williams A, Rowswell H. A randomised controlled trial assessing the efficacy of oral chlorhexidine in ventilated patients. Intensive Care Med. 2004;30:S12. [Google Scholar]
- 33.Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2008;29:131–36. doi: 10.1086/526438. [DOI] [PubMed] [Google Scholar]
- 34.Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT, Viana JM. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect Control Hosp Epidemiol. 2009;30:952–58. doi: 10.1086/605722. [DOI] [PubMed] [Google Scholar]
- 35.Panchabhai T, Dangayach N, Krishnan A, Kothari V, Karnad D. Oropharyngeal cleansing with 0.2% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: an open-label randomized trial with 0.01% potassium permanganate as control. Chest. 2009;135:1150–56. doi: 10.1378/chest.08-1321. [DOI] [PubMed] [Google Scholar]
- 36.Scannapieco F, Yu J, Raghavendran K. A randomized trial of chlorhexidine gluconate on oral bacterial pathogens in mechanically ventilated patients. Crit Care. 2009;13:R117. doi: 10.1186/cc7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ataee RA. To: The use of 2% chlorhexidine gel and toothbrushing for oral hygiene of patients receiving mechanical ventilation: effects on ventilator-associated pneumonia. Rev Bras Ter Intensiva. 2012;24:369–374. doi: 10.1590/S0103-507X2012000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296:2460–2466. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 39.Jafari S. Ranjbar H. Effects of Chlorhexidine and normal saline on dental plaque formation in ICU patients: A comparative study [in Persian] . J Nurse Midwifery. 2007;17:36–43. [Google Scholar]
- 40.Berry AM, Davidson PM. Effects of three approaches to standardized oral hygiene to reduce bacterial colonization and ventilator associated pneumonia in mechanically ventilated patients: A randomised control trial. Int J Nurs Stud. 2011;48:681–688. doi: 10.1016/j.ijnurstu.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Ozcaka O, Başoğlu OK, Buduneli N, Taşbakan MS, Bacakoğlu F, Kinane DF. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res. 2012;47:584–592. doi: 10.1111/j.1600-0765.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 42.Seguin P, Laviolle B, Dahyot-Fizelier C Study of povidone iodine to reduce pulmonary infection in head trauma and cerebral hemorrhage patients (SPIRIT) ICU Study Group; AtlanRea Group. Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: a multicenter randomized controlled trial. Crit Care Med. 2014;42:1–8. doi: 10.1097/CCM.0b013e3182a2770f. [DOI] [PubMed] [Google Scholar]
- 43.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R CDC; Healthcare Infection Control Practices Advisory Committee. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 44.Hirata K, Kurokawa A. Chlorhexidine gluconate ingestion resulting in fatal respiratory distress syndrome. Vet Hum Toxicol. 2002;44:89–91. [PubMed] [Google Scholar]
- 45.Orito K, Hashida M, Hirata K, Kurokawa A, Shirai M, Akahori F. Effects of single intratracheal exposure to chlorhexidine gluconate on the rat lung. Drug Chem Toxicol. 2006;29:1–9. doi: 10.1080/01480540500408416. [DOI] [PubMed] [Google Scholar]
- 46.Xue Y, Zhang S, Yang Y. Acute pulmonary toxic effects of chlorhexidine (CHX) following an intratracheal instillation in rats. Hum Exp Toxicol. 2011;30:1795–1803. doi: 10.1177/0960327111400104. [DOI] [PubMed] [Google Scholar]
- 47.Muscedere JG, McColl C, Shorr A, Jiang X, Marshall J, Heyland DK Canadian Critical Care Trials Group. Determinants of outcome in patients with a clinical suspicion of ventilator-associated pneumonia. J Crit Care. 2008;23:41–49. doi: 10.1016/j.jcrc.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care. 2006;10:R35. doi: 10.1186/cc4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labeau SO, de Vyver KV. Prevention of ventilator-associated pneumonia with oral antiseptics: a systematic review and meta-analysis. Lancet Infect Dis. 2011;11:845–54. doi: 10.1016/S1473-3099(11)70127-X. [DOI] [PubMed] [Google Scholar]
- 50.Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: A meta-analysis. Crit Care Med. 2007;35:595–602. doi: 10.1097/01.CCM.0000253395.70708.AC. [DOI] [PubMed] [Google Scholar]
- 51.Chan EY, Ruest A. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ. 2007;334:889–93. doi: 10.1136/bmj.39136.528160.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]


