Abstract
A brain drug delivery system for glioma chemotherapy based on transferrin and cell-penetrating peptide dual-functioned liposome, Tf/TAT-lip, was made and evaluated with doxorubicin (DOX) as a model drug. TAT conjugated liposome (TAT-lip) loaded with doxorubicin (DOX) were prepared by the thin film hydration methods (lip-DOX) and then conjugated with transferrin (Tf) to yield Tf/TAT-lip-DOX which was characterized for their various physicochemical and pharmaceutical properties. Cellular uptakes were explored in both brain capillary endothelial cells (BCECs) of rats and U87 cells. The blood brain barrier model in vitro was established to evaluate the trans-endothelial ability crossing the BBB. The biodistribution of each formulation was further identified. The Tf/TAT-lip-DOX presents the best anti-proliferative activity against U87 cells. The orthotropic glioma model was established for the evaluation of anti-glioma effect. In conclusion, the experimental data in vitro and in vivo indicated that the Tf/TAT-lip was a promising brain drug delivery system due to its high delivery efficiency across the BBB.
Keywords: Dual-ligand, cell penetrating peptide, transferrin receptor, liposome, blood-brain barrier
Introduction
Central nervous system (CNS) diseases such as brain tumor, pilepsy, and neurodegenerative disorders have become one of the most dangerous threatens to the health of the human [1]. Delivery of drugs to the brain is a major challenge due to the presence the blood-brain barrier (BBB). In oncology, there has been little breakthrough since the past few decades in terms of increasing the average life span of patients suffering from brain cancers [2]. According to National Cancer Data Base (NCDB), prognosis has been poor with overall 5-year survival rate of only 30% for patients with glioma [3]. Therefore, novel strategies are urgently required to deliver drugs across BBB and increase the survival rate of glioma patients. Thus, researchers utilized various methods to conquer these barriers described above and achieved efficient glioma treatment. Since brain capillary endothelial cells express numerous different receptors, receptor-mediated transcytosis (RMT) is considered as one of the most common strategies among all the methods [4,5]. RMT provides a selective means for active BBB transporting and has been extensively studied for brain targeting [6-8]. Specific ligands only have high affinity for targeted receptors and are usually not efficient enough to enhance endocytosis or solid tumor penetration [9,10]. On the other hand, cell penetrating peptides (CPP), a class of diverse short peptides widely used for siRNA, proteins and small molecular drugs delivery, also have the ability to carry drugs to penetrate BBB efficiently [11,12]. Nevertheless, because of their non-specific affinity to different cells, CPP-mediated brain delivery systems showed high drug distribution in the whole brain in vivo after systemic administration [1,11], and this property would lead to unwanted toxicity to normal brain tissues.
Transferrin (Tf) is specific ligand for TfR, which is over-expressed on both BBB and tumor cells [13]. There is documented that Tf targeting liposome could increase the BBB penetration of encapsulating drug and thereby improve the therapeutic efficacy of brain glioma in vivo [14-16]. The cationic cell-penetrating peptide CPP (TAT) derived from the HIV-1 protein TAT could facilitate the intracellular delivery of cargoes with various sizes and physicochemical properties. Liposome modified with TAT could deliver the cargoes into cells with high efficiency via an unsaturated and receptor/transporter independent pathway [17-19]. To overcome the impediments of both CPP and specific ligands, we developed Transferrin and cell-penetrating peptide (TAT) dual-functioned liposome. The stability of Tf/TAT-lip-DOX was measured in the phosphate buffered saline (PBS) containing 50% (v/v) fetal bovine serum (FBS). The cell uptake efficacy and mechanism, the tumor growth inhibitory ability and the targeting specificity toward receptors presenting on BCEC cells and glioma cancer cells in vitro was also investigated. In addition, using DOX as a model chemotherapy drug, Tf/TAT-lip-DOX showed superior cytotoxicity against cancer cells in comparison with the other liposome formulations.
Materials and methods
U87 cells and BCEC cells were from American Type Culture Collection. Soybean lecithin consisting of 90-95% phosphatidylcholine and mPEG2000-DSPE and Mal-PEG2000-DSPE were purchased from Avanti lipid (USA). Coumarin-6, coumarin-7 and Transferrin were purchased from sigma (USA). Cholesterol (CHO) was purchased from Chengdu Kelong Chemical Company (Chengdu, China). Rhodamine-PE was purchased from Avanti lipid (USA). TAT peptide with terminal cysteine (Cys-AYGRKKRRQRRR) was synthesized according to the standard solid phase peptide synthesis by Shanghai Jier Bio-pharmaceutical Co., Ltd. (Shanghai, China). Cell culture plates were purchased from Wuxi NEST Biotechnology Co. Ltd. (Wuxi, China). Other chemicals and reagents were of analytical grade and obtained commercially.
BALB/c male athymic nude mice (about 20 g) were purchased from the Experiment Animal Center of Sichuan University (P. R. China). All of the animal experiments adhered to the principles of care and use of laboratory animals and were approved by the Experiment Animal Administrative committee of Sichuan University.
Synthesis of DSPE-PEG2000-TAT
The DSPE-PEG2000-TAT was synthesized according to literature with a modest modification [20]. TAT was conjugated to DSPE-PEG2000-Mal in 0.01 M isotonic HEPES buffer (pH 7.4) under the conditions of reaction (4 h at 4°C, gentle stirring and the molar ratio of peptides to DSPE-PEG2000-Mal was 1:2). The reaction was traced by TLC until the peptide was completely consumed. The mixture was then dialyzed against water, and lyophilized. The resulting conjugate DSPE-PEG2000-TAT was analyzed by TOF MS ES+ (Waters Q-TOF Premier, Milford, USA), then used for preparing TAT modified liposome without further purification.
Preparation of the liposomal formulations
TAT modified DOX loaded liposome (TAT-lip-DOX) were prepared by thin film hydration methods [21,22]. Briefly, SPC, cholesterol, DSPE-PEG2000, DSPE-PEG2000-TAT were dissolved in chloroform (total molar ratio of phospholipid and cholesterol derivatives was 2:1, molar ratio of DSPE-PEG2000 and DSPE-PEG2000-TAT was 9.4:0.1). Chloroform was then evaporated by rotary evaporation and residual organic solvent was removed in vacuum overnight. Then the thin film was hydrated in 0.25 M ammonium sulfate at 60°C, The obtained large unilamellar vesicles were subjected to probe sonication and dialyzed against 9% sucrose solution for 24 h using a dialysis bag (MWCO, 7000). The resultant liposome was mixed with DOX sucrose solution and incubated at 60°C for 2 h. Untrapped drug was removed using a Sephadex G-50 column.
The Tf modified liposomes (Tf-lip) and Tf/TAT-lip were prepared through the post-insertion method [23]. Firstly, the Tf was reacted with Traut’s reagent at a molar ratio of 1:5 to yield TF-SH. Secondly; the Tf-SH was reacted with the micelles of DSPE-PEG2000-Mal at a molar ratio of 1:10, and then incubated with lip or TAT-lip for 1h at 37°C. The ratio of Tf-PEG2000-DSPE to lipid was 1:50. DOX loaded Tf/TAT-lip was prepared by an ammonium sulfate gradient method.
Characterization of liposomes
The size and zeta potential of the liposomes were measured by a dynamic light scattering detector (Zetasizer Nano-ZS90, Malvern, UK). The amount of trapped DOX within the liposomal formulation was determined by HPLC (Agilent 1100, USA).
In order to demonstrate the serum stability of liposomes, turbidity variations were monitored in the presence of fetal bovine serum (FBS). Briefly, liposomes were mixed with equal volume of FBS under 37°C with gentle shaking at 30 rpm. At predetermined time points (1 h, 2 h, 4 h, 8 h and 24 h); 200 mL of the sample was pipetted out and onto a 96-well plate to measure the transmittance at 750 nm by a microplate reader (Thermo Scientific Varioskan Flash, USA).
Cellular uptake
Both U87 cells and BCEC cells were grown in RPMI-1640 medium (GIBCO) contains 10% FBS, 100 μg/mL streptomycin, and 100 U/mL penicillin. The cells were maintained at 37°C in a humidified incubator with 5% CO2.
For quantitative study, Both U87 cells and BCEC cells were harvested with 0.125% trypsin-EDTA solution and seeded into 24 well assay plates at 105-viable cells/well. After the cells reached confluence, the cells were incubated with 100 μl DOX-loaded liposomes (all three types) in culture medium supplemented with 10% FBS and 1% penicillin-streptomycinat 37°C for 4 h. At designated time period, the suspension was removed and the wells were washed 3 times with cold PBS. After that, 50 μl of 0.5% Triton X-100 was introduced into each well for cell lysis. The fluorescence intensity of each well was measured by Tecanmicroplate reader (GENios) with excitation wavelength at 560 nm and emission wavelength at 578 nm.
For the qualitative study, Both U87 cells and BCEC cells were harvested with 0.125% trypsin-EDTA solution and seeded in LABTEK cover glass chambers at a concentration of 5 × 103 viable cells/chamber. The cells were incubated overnight and were subsequently incubated with DOX-loaded liposome at 37°C. After 4 h, the cells were washed 3 times with cold PBS and fixed by 4% paraformaldehyde for 20 min. Then the cells were washed twice with cold PBS. The nuclei were stained by incubating with DAPI for another 10 min. The cell monolayer was washed 3 times with PBS and observed by confocal laser scanning microscopy (CLSM) (TCS SP5 AOBS confocal microscopy system, Leica, Germany).
To study the effect of different inhibitors on the cellular uptake of Tf/TAT-lip, the cells were pre-incubated with different inhibitors for 30 min at 37°C. Poly-lysine (800 mg/mL), amiloride (1.48 mg/mL), chlorpromazine (20 mg/mL), filipin (5 mg/mL), free Tf (5 mg/mL) and free TAT peptide (5 mg/mL) were added respectively. To study the effect of temperature on the cellular uptake, the cells were incubated under both 37°C and 4°C. Then the inhibitor-containing culture media was discarded and DOX-loaded liposome - containing culture media was applied for 4 h incubation. Then the cells were treated as the quantitative study described, and the fluorescence intensity was determined.
BBB model study
Millicell Hanging Cell Culture Inserts were used to establish BBB modelin vitro. In brief, BCEC cells were plated into the 6-well cell culture inserts at a density of 106 cells per well, and cultured for about one week. Then the transendothelial electric resistance (TEER) of the BBB model was measured by Millicell ERS (Millipore, USA). Only the BCEC monolayers with TEER over 200 Ω were used for further studies. On the other hand, U87 cells were plated in another 6-well plate. Then the cell culture inserts with BCEC monolayers were transferred to the plates containing U87 cells and co-cultured for another day. DOX-loaded liposomes were added in the cell culture inserts (donor chamber). After 6 h incubation, BCEC cells on the cell culture inserts and U87 cells on the plates ofthe substratum (acceptor chamber) were washed with cold PBS for three times, trypsinized, and finally resuspended in 0.5 ml PBS, respectively. Then the fluorescent intensity of both cells was measured by a flow cytometer (Cytomics FC500, Beckman Coulter, USA).
Anti-proliferative assay
Comparison of in vitro cytotoxicity and tumor cell proliferation assay of various formulations was performed on U87 cells using SRB colorimetric assay. In brief, U87 cells (4000 cells) were seeded into 96-well plates and incubated overnight. Then the cells were exposed to serial concentrations of different DOX formulations in the culture medium for 48 hours at 37°C. Afterward, cells were fixed with trichloroacetic acid, washed, and stained with SRB. Absorbance was measured at 540 nm using a 96-well plate reader (Bio-Rad Laboratories, Hercules, California). Dose-response curves were generated, and the concentrations of drug resulting in 50% cell killing (IC50) were calculated by Origin7.0 (OriginLab, Northampton, Massachusetts).
Ex vivo bio-distribution of liposome
72 mice were randomly assigned to four groups; each was then divided into 6 time point groups. Lip, Tf-lip, TAT-lip, and Tf/TAT-lip were given to the mice via the tail veins; each was equivalent to the administration dose of coumarin-6 of 0.1 mg/kg. At 30 min, 1 h, 2 h, 4 h, 8 h, and 12 h after injection, the animals were sacrificed by cervical dislocation, and the organs (heart, liver, spleen, lung, kidney, and brain) were removed and flushed with water for three times to remove the blood remained. All the tissues were homogenized with triple amount of water.10 μl of internal standard (coumarin-7) was added into 200 μl organ homogenate, and extracted with 1 ml N-hexane. The mixture was vortexed for 5 min, and centrifuged at 10,000 rpm for 5 min. The supernatant was transferred to another centrifuge tube, anddried under air stream at room temperature. The dry residue was reconstituted with 50 μl of methanol. The solution was centrifuged at 12,000 rpm for 10 min, and then 20 μl of the supernatant was injected into the HPLC system for analysis. A reversed-phase HPLCwas employed for the determination of coumarin-6 concentrationsin the tissue samples. A HPLC (Agilent 1100, USA) system with a Diamonsil C18 column (200 mm × 4.6 mm, 5 μm), and a mixture of methanol-water (92:8) as mobile phase was employed for thein vivo studies. The concentrations of coumarin-6 were analyzedbyfluorescence detector with Ex 465 nm and Em 502 nm.
In vivo anti-GBM effect
5 μl of U87 cells at a density of 108 cells/mL were slowly injected into the right corpus striata of nude mice with the help of a stereotaxic apparatus. Eight days following the cell injection, the rats were randomly divided into 6 groups (10 rats per group): a PBS group, free DOX solution group, lip-DOX group, Tf-lip-DOX group, TAT-lip-DOX group, and Tf/TAT-lip-DOX group; each mouse received DOX-loaded preparations at a dose of 2.5 mg/kg. The mice were dosed by tail vein injection every 3 days for 3 times. Survival time was recorded and analyzed by SPSS 21.0 (IBM, USA).
Statistical analysis
Analysis of variance (ANOVA) was used to check the variance of the whole values in each group. Statistical significance was evaluated by using Student’s t-test for the comparisons of experimental groups.
Results and discussion
Physicochemical properties of liposomal formulations
As showed in Figure 1, the chemical structure of DSPE-PEG2000-TAT was verified via TOF MS ES+. The relative molecular mass of DSPE-PEG2000-TAT observed is 4697Da and the relative molecular mass of DSPE-PEG2000-TAT calculated from theory is 4675Da.
Figure 1.
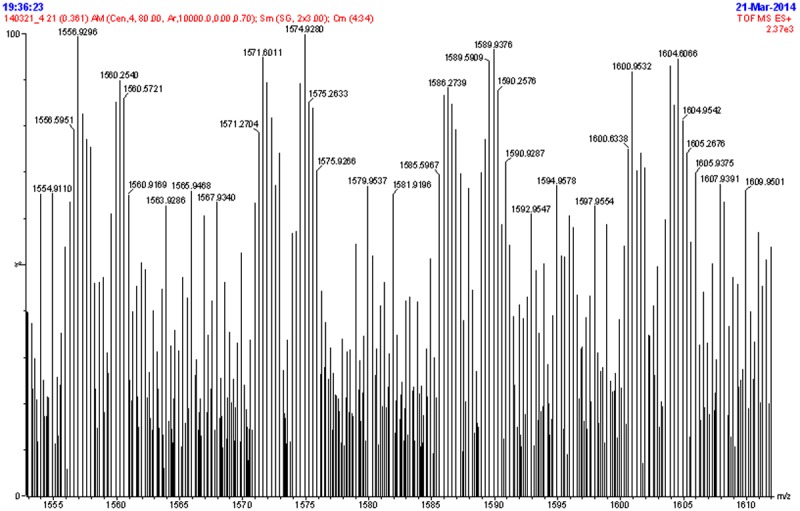
M.S+ spectrum of DSPE-PEG2000-TAT.
The particle size and polydispersity index (PDI) of liposomal samples were measured via a nanoparticle analyzer at a fixed 90° angle at 20°C with results given in Table 1. Tf/TAT-lip-DOX had a mean particle size about 130 nm. All of the liposomal samples had a narrow PDI value, indicating homogeneity in their dispersion state. The encapsulation efficiency (EE) of DOX in the liposome determined by HPLC was 95%.
Table 1.
Characteristics of liposomal formulations (n = 3)
| Group | Particle Size (nm) | Polydispersity | Zeta-potential (mV) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| lip-DOX | 113.8 ± 9.8 | 0.127 | 2.57 ± 1.31 | 95.15 ± 2.85 |
| TAT-lip-DOX | 128.3 ± 10.4 | 0.130 | 22.86 ± 3.88 | 94.59 ± 1.77 |
| Tf-lip-DOX | 131.5 ± 11.5 | 0.162 | -2.32 ± 1.21 | 94.48 ± 2.97 |
| Tf/TAT-lip-DOX | 134.9 ± 12.1 | 0.152 | 19.86 ± 4.81 | 95.45 ± 3.81 |
Particle stability against physiological condition is prerequisite for the further application in vivo, so 50% FBS was employed to mimic the in vivo situation. Transmittance variations as important parameters were monitored inour study to explore the serum stability of liposome. As can be seen in Figure 2, the transmittance of all liposomal formulationsare above 90% over 24 h, indicating thatthere was no aggregation in the presence of serum.
Figure 2.
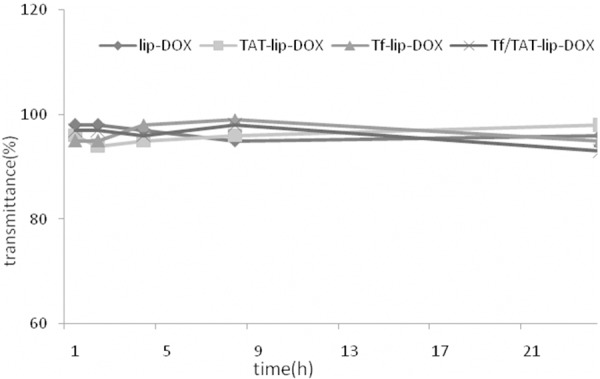
The variation in transmittancy versus the different incubation time ofliposomes at the wavelength of 750 nm when incubated with phosphatebufferedsaline containing 50% (v/v) fetal bovine serum for 24 hours at 37°C (n = 3).
Cellular uptake
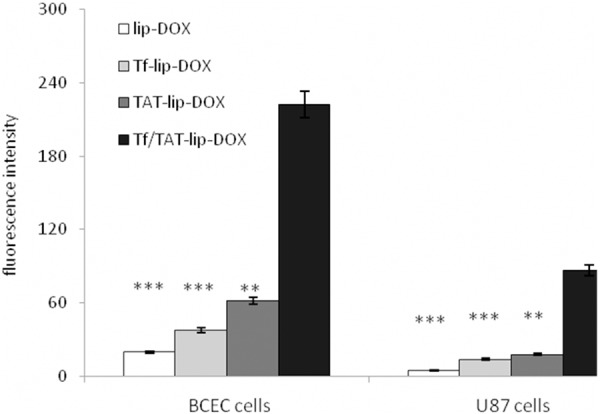
The U87 cells and BCEC cells could take uptake lip-DOX, TAT-lip-DOX, Tf-lip-DOX and Tf/TAT-lip-DOX at different capacities (Figure 3). The BCEC cells were murine brain endothelial cells exhibiting endothelial properties. Thus this cell line was widely used as amodel for mimicking brain capillary endothelial cells and evaluating the BBB penetrating capability in vitro [24,25]. As the transferrin receptors were certified to be expressed on U87 cells and BCEC cells, the cellular uptake of different modified liposomes was studied. Unmodified liposomes were used as negative control. Liposomes modified with Tf increased the cellular uptake on both cells compared to the negative control. However, the fluorescence intensity of Tf-lip-DOX treated cells was far lower than TAT-lip-DOX group and Tf/TAT-lip-DOX group. This was because specific receptor targeting ligand like Tf had high affinity to their homologous receptors but relatively lower internalization properties. Therefore specific ligandsmainly delivered their cargo to the cell surface instead of penetrating the cell membranes. TAT modified liposomes unsurprisingly displayed higher uptake level than negative control group, as CPP had alreadyproved their ability to deliver cargo into cells in many reports [26,27]. And Tf/TAT-lip-DOX showed the highest cellular uptake level on BCEC cells and U87 cells, In BCEC cells, Tf/TAT-lip-DOX was about 2.6 times higher than TAT-lip-DOX and nearly 5.3 times higher than Tf-lip-DOX. In U87 cells, Tf/TAT-lip-DOX was about 2.2 times higher than TAT-lip-DOX and nearly 4.7 times higher than Tf-lip-DOX. The quantitative results indicated almost the same results as the fluorescence imaging (Figure 4). The uptake of Tf-lip-DOX by BCEC cells and U87 cells was greater than that of the lip-DOX, which confirmed the transferrin receptors were over expressed on U87 cells and BCEC cells. After conjugation with TAT, the uptake of liposomeby bothU87 cells and BCEC cells significantly increased, demonstrating that TAT effectively mediated liposome uptake by cells. The cellular uptake demonstrated that Tf was good bind to the BCEC cells and TAT help to enhance deliver drug across BBB.
Figure 3.
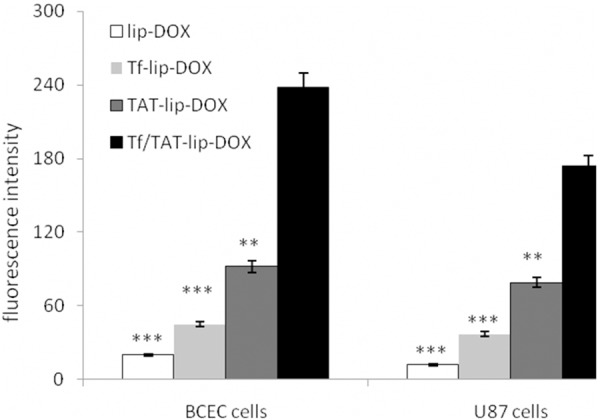
Measurement of in vitro uptake of lip-DOX, TAT-lip-DOX, Tf-lip-DOX and Tf/TAT-lip-DOX by U87 cells and BCEC cells. Data represented the mean ± SD, n = 3. **: P < 0.01; ***: P < 0.001.
Figure 4.
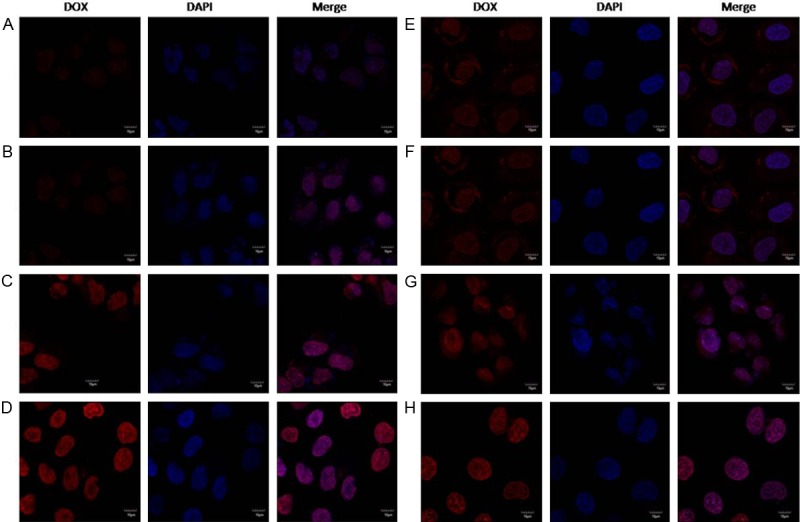
Confocal laser scanning microscopy (CLSM) images show the internalization of fluorescent liposomes in BCEC cells (A-D) and U87 cells (E-H) (4 h incubation). (A and E) represent lip-DOX; (B and F) represent Tf-lip-DOX; (C and G) represent TAT-lip-DOX; (D and H) represent Tf/TAT-lip-DOX.
In order to study the uptake mechanism of Tf/TAT-lip-DOX, a series of endocytosis inhibitors were pre-incubated with BCEC cells and the inhibition rate was calculated to analyze the uptake mechanism [28]. As shown in Figure 5, the cellular uptake of Tf/TAT-lip-DOX was significantly decreased in the presence of free Tf. When targeting sites were competitively bound by free Tf, the cellular uptake of Tf/TAT-lip-DOX decreased until 56.6%. In addition, other inhibitorswere used to study the uptake mechanism. Poly-lysine was used aspositive charge inhibitor; 4°C and sodium amide were selected to study the effect of energy; amiloride, chlorpromazine and filipin were chosen to block macropinocytosis, clathrin-mediated and caveolin-mediated endocytosis respectively. As the results showed, Poly-lysine showed a significant inhibition of liposomalcellular uptake down to 18.7%, indicating that Tf/TAT-lip-DOX enhanced cellular internalizing through positive charged mediated adsorption. 4°C and sodium amide alsodisplayed strong impact on cellular uptake (down to 13.6% and 34.6% respectively), showing energy-dependent properties of Tf receptor mediated endocytosis. Meanwhile, amiloride, chlorpromazine and filipin inhibited the cellular uptake of Tf/TAT-lip-DOX down to 52.2%, 25.5% and 44.1%, respectively. Specific internalization is commonly considered to be clathrin-mediated endocytosis pathway [29], but our system had a specific ligand and aCPP at the same time. Therefore the Tf/TAT-lip-DOX might enhance the cellular uptake through a comprehensive pathway.
Figure 5.
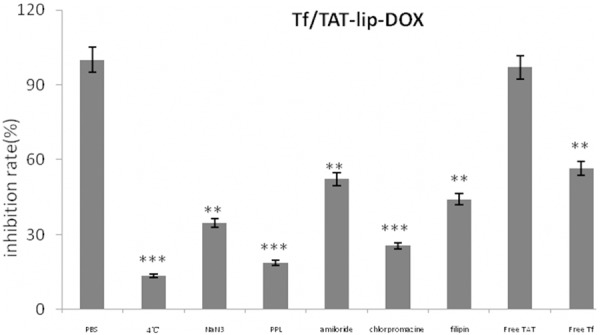
Effects of endoeytosis inhibitors on the cellular uptake of liposomes in BCEC cells. The data are presented as the mean ± SD (n = 3), *P < 0.05, **: P < 0.01; ***: P < 0.001.
BBB model study
The BCEC cells were used to establish a BBB model in vitro. In this study, BCEC cells were plated in the Millicell Hanging Cell Culture Inserts to construct a monolayer. As shown in Figure 6, the cellular uptakeresults on BCEC monolayer were similar to the uptake data on BCEC cellsin vitro. The Tf/TAT-lip-DOX uptake by BCEC monolayer was 3.58-fold, 5.84-fold and 11.1-fold than TAT-lip-DOX, Tf-lip-DOX and lip-DOX. Forthe liposomal uptake on U87 cells after penetrating BCEC monolayer, the amount of liposome uptaken by U87 cells decrease significantly. Also, the Tf/TAT-lip-DOX uptake by U87 cells was 4.83-fold, 6.21-fold and more than 1000-fold than TAT-lip-DOX, Tf-lip-DOX and lip-DOX. To sum up, TAT peptide and Tf co-modified liposome showed superior BBB transporting and tumor penetrating capabilities in the BBB model study.
Figure 6.
The BBB model study. Uptake of liposomes on U87 cells and uptake on BCEC cells on the transwell membrane. The data are presented as the mean ± SD (n = 3), *: P < 0.05; **: P < 0.01; ***: P < 0.001.
In vitro cytotoxicity and anti-proliferation assay
The cytotoxicity effect against U87 cells of various DOX formulations are summarized in Table 2. Modification with Tf and TAT resulted in improved efficacy for DOX loaded liposomes. Specifically, Tf/TAT-lip-DOX led to 68.7%, 54.1% and 40.7% decreases in the IC50 values compared to lip-DOX, Tf-lip-DOX and TAT-lip-DOX after 48 hours’ incubation with U87 cells, respectively.
Table 2.
Cytotoxicity against U87 cells of various DOX formulations in vitro after 48 hours’ incubation
| Formulations | IC50 on U87 cells, μg/mL |
|---|---|
| DOX | 0.0810 |
| lip-DOX | 0.00895 |
| Tf-lip-DOX | 0.00610 |
| TAT-lip-DOX | 0.00472 |
| Tf/TAT-lip-DOX | 0.00280 |
Ex vivo biodistribution of liposomes
Biodistribution of the coumarin-6 loaded liposomes was investigated ex vivo after i.v. administration of the liposomes. The biodistribution analysis was performed by quantifyingthe amount of coumarin-6 in the various organs. The quantitative study of biodistribution could reveal the characterof liposomesin vivo more precisely and literally. The biodistribution of coumarin-6 in each tissue at each time point was shown in Figure 7. The data had showed that the Tf/TAT-lip accumulated the most in the brain than other lipsomes. The concentration of Tf/TAT-lip in the liver and kidney was less than other liposomes. The amount of Tf/TAT-lip accumulated in lung for a long time, that may because the positive of TAT, which could help to accumulate in lung.
Figure 7.
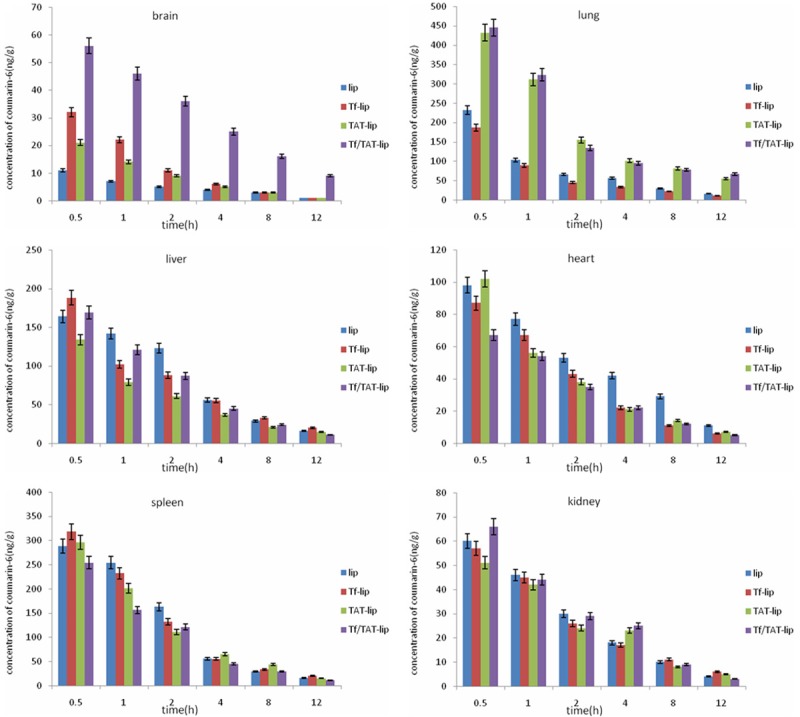
Biodistribution of liposomes in mice at different time points, expressed as the concentration of coumarin-6 in different tissues. Data represented the mean ± SD (n = 3).
In vivo anti-GBM effect
For defining the efficacy of the functionalized liposomes in animals, brain GBM-bearing rat models were applied. The anti-GBM effect of DOX-loaded Tf/TAT-lip was also demonstrated by the survival time of intracranial U87 GBM-bearing rats. As shown in Figure 8, the median survival time of lip-DOX group, Tf-lip-DOX group, TAT-lip-DOX group and Tf/TAT-lip-DOX group, free DOX group were prolonged to 26, 42, 50, 59 and 17 days compared to physiological saline group (10 days), respectively. Obvious, all liposomal groups significantly prolonged the survival time compared to physiological saline and free DOX group, such superiority of the liposomes in vivo compared to free DOX may be attributed to prolonged blood circulation time and good ability to across the BBB.
Figure 8.
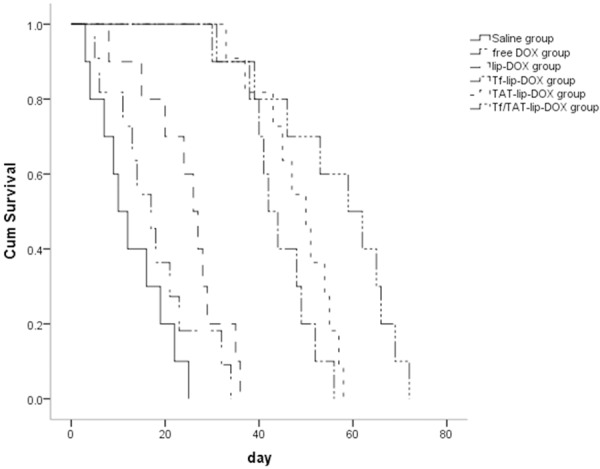
Cumulative survival study of GBM-bearing mice treated with 10 mg/kg DOX, DOX-loaded liposomes or saline, n = 6.
Discussion
The blood-brain barrier (BBB) is a metabolic cellular structure in the central nervous system (CNS), which restricts the passage of various small molecular chemical agents and micro-objects between the bloodstream and the neutral tissues; overcoming the difficulty of delivering anticancer agents to brain tumors presents a major challenge. The ability of carriers to specifically targeting delivery cargoes to tumors is important to effective cancer therapy. The active tumor targeted liposomes, which were modified with some specific ligands such as transferrin, folic acid, peptides or antibodies, could selectively recognize and bind to the specific receptor over-expressed on tumor cells, then result in increased targeting efficiency and less toxicity. It was reported transferrin receptor is over-expressed on both BBB and tumor cells, so transferrin could be used to link to the cargos and transport the drugs to brain and tumor. To generate further therapeutic efficacy, TAT which could promote carriers to enter the BBB and tumor cells effectively via an unsaturated and receptor/transporter independent pathway. In the present study, we proposed a novel type of dual-targeting liposome with the purposes of delivering anticancer agent through the BBB and then targeting the inside brain tumor cells. To construct the dual-targeting liposomes, PEGylated liposomes loaded with DOX were included for consideration of reducing the cytotoxicity to the normal tissues, which were associated with sterical stability and with the bulky polyethylene glycol (PEG) headgroup which inhibits the rapid uptake of reticuloendothelial system (RES).
MTT assay demonstrated that DOX resulted in obviously inhibitory effects to U87 glioma cells, thus proving the anticancer effects on such brain tumors. In addition, there was significant difference in the inhibitory effect between liposomal DOX and liposomes modified with Tf. This could be explained by the high affinity of Tf to U87 glioma cells, while common PEGylated liposomes would hinder the contact of DOX with tumor cells in vitro. liposomes modified with TAT exhibited a stronger toxicity to U87 glioma cells than liposomes modified with Tf, demonstrating that TAT could enhance the celluar uptake of TAT modified liposomes by U87 glioma cells, leading to more DOX stays in the cells. More significantly, liposomes modified with Tf and TAT exhibited the strongest inhibitory effects, suggesting that Tf plus TAT contributes to a stronger drug delivering effect into glioma cells.
To build a BBB in vitro model and to closely mimic the situation in vivo, BCEC cells were cultured. This model had been characterized by displaying BBB characteristics and linking the barrier junction with its transendothelial electrical resistance (TEER) value. Drug transport across the BBB model showed that the transport of DOX liposomes modified with TAT and Tf, or both was evidently higher than that of PEGylated liposomes, demonstrating that TAT and Tf contributed to the transfer of DOX across the BBB.
In the brain tumor-bearing rats, the dual-targeting effects of DOX liposomes modified with TAT and Tf could be evidently observed as this combination leads to a significantly improved chemotherapy in the overall survival of the brain tumor-bearing rats, compared with free DOX or with PEGylated liposomes. Results from an extended treatment group indicated that the survival could be further significantly enhanced, indicating that an extended chemotherapy with DOX liposomes modified with TAT and Tf would be beneficial for the treatment. The dual-targeting effects in vivo of DOX liposomes modified with TAT and Tf could be relevant to the following aspects: an enhanced effect with Tf via transferrin receptors in the BBB and the brain tumor; an enhanced effect with TAT via the positive charge through BBB and tumor cells.
In conclusions, this study developed a liposome system for targeted drug delivery across the blood-brain barrier (BBB), which consists of the DSPE-PEG2000 with transferrin and TAT conjugated on their surface as targeting ligands. Theliposomes were prepared by the film hydrationmethod and characterized for their size and size distribution; drug encapsulation efficiency and in vitro stability in FBS. Cellular uptake, mechanism of cell uptake and cytotoxicityof the DOX loaded Tf and TAT co-modified liposomes were investigated in comparison with Tf or TAT modified liposomes. The Tf and TAT co-modified liposomes demonstrated greatest advantage over the other liposomal formulations. IC50 data showed that Tf/TAT-lip-DOX led to 68.7%, 54.1% and 40.7% decreases in the IC50 values compared to lip-DOX, Tf-lip-DOX and TAT-lip-DOX after 48 h treatment, respectively. The BBB model in vitro demonstrated Tf and TAT co-modified liposomes could deliver drugs across the BBB effectively. Moreover, our preliminary Ex Vivo biodistribution investigation demonstrated that the Tf/TAT-lip could be able to delivery imaging/therapeutic agents across the BBB more effectively.
Acknowledgements
The work was funded by the National Natural Science Foundation of China (81260371).
Disclosure of conflict of interest
None.
References
- 1.Qin Y, Chen H, Yuan W, Kuai R, Zhang Q, Xie F, Zhang L, Zhang Z, Liu J, He Q. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm. 2011;419:85–95. doi: 10.1016/j.ijpharm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumors in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 3.Gan CW, Feng SS. Transferrin-conjugated nanoparticles of Poly (lactide)-D-a-Tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials. 2010;31:7748–7757. doi: 10.1016/j.biomaterials.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Gao H, Yang Z, Cao S, Xiong Y, Zhang S, Pang Z, Jiang X. Tumor cells and neo-vasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35:2374–2382. doi: 10.1016/j.biomaterials.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 6.Lu W, Xiong C, Zhang R, Shi L, Huang M, Zhang G, Song S, Huang Q, Liu GY, Li C. Receptor-mediated transcytosis: a mechanism for active extravascular transport of nanoparticles in solid tumors. J Control Release. 2012;161:959–966. doi: 10.1016/j.jconrel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Yang Z, Zhang S, Cao S, Pang Z, Yang X, Jiang X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J Control Release. 2013;172:921–928. doi: 10.1016/j.jconrel.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Gao H, Qian J, Cao S, Yang Z, Pang Z, Pan S, Fan L, Xi Z, Jiang X, Zhang Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials. 2012;33:5115–5123. doi: 10.1016/j.biomaterials.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 9.Beduneau A, Saulnier P, Benoit JP. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Huile G, Shuaiqi P, Zhi Y, Shijie C, Chen C, Xinguo J, Shun S, Zhiqing P, Yu H. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials. 2011;32:8669–8675. doi: 10.1016/j.biomaterials.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 11.Qin Y, Chen H, Zhang Q, Wang X, Yuan W, Kuai R, Tang J, Zhang L, Zhang Z, Zhang Q, Liu J, He Q. Liposome formulated with TAT-modified cholesterol for improving brain delivery and therapeutic efficacy on brain glioma in animals. Int J Pharm. 2011;420:304–312. doi: 10.1016/j.ijpharm.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Kuai R, Yuan W, Qin Y, Chen H, Tang J, Yuan M, Zhang Z, He Q. Efficient delivery of payload into tumor cells in a controlled manner by TAT and thiolytic cleavable PEG co-modified liposomes. Mol Pharm. 2010;7:1816–1826. doi: 10.1021/mp100171c. [DOI] [PubMed] [Google Scholar]
- 13.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;3:539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL, Han M. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34:5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 15.Miyata S, Kawabata S, Hiramatsu R, Doi A, Ikeda N, Yamashita T, Kuroiwa T, Kasaoka S, Maruyama K, Miyatake S. Computed tomography imaging of transferrin targeting liposomes encapsulating both boron and iodine contrast agents by convection-enhanced delivery to F98 rat glioma for boron neutron capture therapy. Neurosurgery. 2011;68:1380–1387. doi: 10.1227/NEU.0b013e31820b52aa. [DOI] [PubMed] [Google Scholar]
- 16.Ying X, Wen H, Lu WL, Du J, Guo J, Tian W, Men Y, Zhang Y, Li RJ, Yang TY, Shang DW, Lou JN, Zhang LR, Zhang Q. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 TAT. Exp Neurol. 2005;193:218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Brooks H, Lebleu B, Vivès E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuai R, Yuan W, Li W, Qin Y, Tang J, Yuan M, Fu L, Ran R, Zhang Z, He Q. Targeted Delivery of Cargoes into a Murine Solid Tumor by a Cell-Penetrating Peptide and Cleavable Poly (ethylene glycol) Comodified Liposomal Delivery System via Systemic Administration. Mol Pharm. 2011;8:2151–2161. doi: 10.1021/mp200100f. [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Fujimoto K. A reduction-triggered delivery by a liposomal carrier possessing membrane-permeable ligands and a detachable coating. Colloids Surf B Biointerfaces. 2006;49:15–21. doi: 10.1016/j.colsurfb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Song S, Liu D, Peng J, Sun Y, Li Z, Gu JR, Xu Y. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int J Pharm. 2008;363:155–161. doi: 10.1016/j.ijpharm.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Chiu SJ, Liu S, Perrotti D, Marcucci G, Lee RJ. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J Control Release. 2006;112:199–207. doi: 10.1016/j.jconrel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Brown RC, Morris AP, O’Neil RG. Tight junction protein expression and barrier properties of immortalized mouse brain microvessel endothelial cells. Brain Res. 2007;1130:17–30. doi: 10.1016/j.brainres.2006.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon MJ, Kang WH, Gao S, Banta S, Morrison B 3rd. Increased delivery of TAT across an endothelial monolayer following ischemic injury. Neurosci Lett. 2010;486:1–4. doi: 10.1016/j.neulet.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas S, Deshpande PP, Perche F, Dodwadkar NS, Sane SD, Torchilin VP. Octaarginine-modified pegylated liposomal doxorubicin: an effective treatment strategy for non-small cell lung cancer. Cancer Lett. 2013;335:191–200. doi: 10.1016/j.canlet.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Y, Wu X, Yang Z, Zhao J, Wang X, Zhang Q, Yuan M, Xie L, Liu H, He Q. The potential efficacy of R8-modified paclitaxel-loaded liposomes on pulmonary arterial hypertension. Pharm Res. 2013;30:2050–2062. doi: 10.1007/s11095-013-1058-8. [DOI] [PubMed] [Google Scholar]
- 28.Du W, Fan Y, Zheng N, He B, Yuan L, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. Transferrin receptor specific nanocarriers conjugated with functional 7peptide for oral drug delivery. Biomaterials. 2013;34:794–806. doi: 10.1016/j.biomaterials.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv Drug Deliv Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


