Abstract
Background & aims: Oleanolic acid is abundantly distributed in Swertia mussotii Franch, a Chinese traditional herb for the treatment of jaundice. However, the hepatoprotective role of oleanolic acid in obstructive cholestasis and its underlying molecular mechanism are unclear. Methods: Normal rats and bile duct-ligated (BDL) rats were given oleanolic acid and serum biochemistry, bile salts, and pro-inflammatory factors were measured, as well as the expression levels of liver bile acid synthesis and detoxification enzymes, membrane transporters, nuclear receptors, and transcriptional factors. Results: Oral administration of oleanolic acid at 100 mg/kg did not cause rat liver injury. However, it significantly reduced the serum levels of alanine aminotransferase (ALT) on days 7 and 14, aspartate aminotransferase (AST) and TNF-α on day 14, and alkaline phosphatase (ALP) and IL-1β on days 3, 7, and 14 in the BDL rats. Furthermore, the serum levels of total bile acid (TBA) and bile acids, including CDCA, CA, DCA, and Tα/βMCA were significantly reduced by oleanolic acid on day 3 in the BDL rats. In addition, the expression levels of detoxification enzymes Cyp3a, Ugt2b, Sult2a1, Gsta1-2, and Gstm1-3, membrane transporters Mrp3, Mrp4, Ostβ, Mdr1, Mdr2, and Bsep, nuclear receptors Pxr, Vdr, Hnf4α, Rxrα, Rarα, Lxr, and Lrh-1, and transcriptional factors Nrf2, Hnf3β, and Ahr were significantly increased in oleanolic acid-treated rats. Conclusion: We demonstrated that the oral administration of oleanolic acid attenuates liver injury, inflammation, and cholestasis in BDL rats. The anti-cholestatic effect may be associated with the induction of hepatic detoxification enzymes and efflux transporters mediated by nuclear receptors and transcriptional factors.
Keywords: Oleanolic acid, obstructive cholestasis, synthetic enzyme, detoxification enzyme, hepatic efflux transporter, nuclear receptor, transcriptional factor
Introduction
Swertia mussotii Franch is a Chinese traditional herb for the treatment of jaundice resulted from hepatitis (e.g., HBV) and gallstone obstruction. Oleanolic acid is a triterpenoid that is abundantly distributed in Swertia mussotii Franch [1-3]. Previous studies reported that oleanolic acid derived from other plants has a hepatoprotective effect and low hepatotoxicity in rat primary hepatocytes [4]. Recent studies demonstrated that a high dose of oleanolic acid (≥ 90 mg/kg) by intraperitoneal (i.p.) injection and repeated gavages (> 135 mg/kg) produces cholestasis in mice [5,6]. In contrast, a low dose of oleanolic acid by i.p. (20 mg/kg) protects against lithocholic acid-induced cholestasis in mice [7]. However, whether oleanolic acid from Swertia mussotii Franch has a protective role in obstructive cholestasis remains unclear.
Chronic cholestasis resulted from biliary obstructions (e.g., gallstone and pancreas tumors obstructions), biliary atresia, hepatitis, and drug toxicity can lead to liver failure, fibrosis, cirrhosis, and even death [8-11]. It was reported that the expression levels of bile acid transporters and synthetic and detoxification enzymes were significantly altered in cholestasis [8-11]. Most of these changes, such as inhibiting bile acid synthesis (e.g., inhibiting CYP7A1), reducing hepatotoxicity (e.g., inducing Cyp3a11, Ugt2b, and Sult2a1) and enhancing efflux (e.g., inducing the expressions of MRP3, MRP4, and OSTα/β) are thought to be an adaptive response to cholestatic liver injury [8-11]. Furthermore, recent studies also indicated that the amplification of inflammation plays an important role in cholestatic liver injury [12]. The elevated serum pro-inflammatory cytokine levels of TNF-α, IL-1β, and IL-6 were observed in cholestatic rodents and some cholestatic patients [8-10,12,13]. Ursodeoxycholic acid (UDCA), the only drug approved so far for cholestasis, can stimulate the expression levels of efflux transporters (e.g., BSEP, MRP2, MRP3, and MRP4) and Phase I and II detoxification enzymes, and has a weak anti-inflammatory effect on cholestasis [8-11]. Recent studies showed that oleanolic acid significantly up-regulates the expression of efflux transporters, such as Mrp2, Mrp3, and Mrp4, and reduces liver injury in lithocholic acid-induced cholestatic mouse liver [7]. However, other studies found that oleanolic acid suppresses the expression of synthetic enzymes (e.g., Cyp7a1 and Cyp7b1) and hepatic uptake transporters (e.g., Ntcp and Oatp1b1), but induces the expression of efflux transporter Ostβ and hepatotoxicity in mouse liver [6]. Therefore, the effects of oleanolic acid on bile acid metabolism and inflammation are complicated and remain to be elucidated.
To explore the therapeutic effect and molecular mechanism of oleanolic acid in obstructive cholestasis, we determined the serum levels of pro-inflammatory cytokines and bile salts, and the expression levels of bile acid metabolic genes in rats treated with oleanolic acid from Swertia mussotii Franch. Our study provides insight into the molecular mechanism of oleanolic acid against cholestasis, and contributes to a basis for future investigations of oleanolic acid-mediated therapy for human cholestasis.
Materials and methods
Chemicals
Oleanolic acid, isolated from Swertia mussotii Franch, was provided from Chongqing Academy of Chinese Material Medical, with a purity of 98% as analyzed by HPLC. All other chemicals were of analytical grade and purchased from Sigma-Aldrich (Sigma-Aldrich Chemical Co., St Louis, MO, USA).
Animals and treatments
The animal use and experimental protocols were reviewed and approved by the Ethics Committee of Third Military Medical University, Chongqing, China. Male Sprague-Dawley (SD) rats, weighing 200-250 g, were purchased from the Center of Laboratory Animals of Third Military Medical University, Chongqing, China. The rats were housed in plastic cages individually in temperature–controlled (20-23°C) rooms with a 12 h light/dark cycle and free access to food and water. The animals were allowed 1 week to adapt to a new environment before starting the experiments. Two independent experiments were designed to detect the therapeutic effect of oleanolic acid on cholestasis and explore the molecular mechanism of its hepatoprotective effect in rats. To assess the therapeutic effect, the rats were randomized into three groups: sham operated group with saline, bile duct-ligated (BDL) group with saline, and the BDL group with oleanolic acid (7 rats per group). In the sham operated groups, rats were pre-treated orally with 1% Tween-20 saline for 1 day, and underwent a sham-operation followed by the saline treatment for 3, 7, and 14 days. In the BDL plus saline group, rats underwent BDL after the pre-treatment with the saline by for 1 day and then continued treatment with the saline for 3, 7 and 14 days. In the BDL plus oleanolic acid group, rats were pre-treated with oleanolic acid at 100 mg/kg dissolved in 1% Tween-20 by gavage for 1 day, and then underwent BDL followed by continued administration of oleanolic acid for 3, 7 and 14 days.
To explore the molecular mechanism of oleanolic acid on hepatoprotection, the rats were randomized into two groups (5 rats per group). One group was orally given oleanolic acid at 100 mg/kg daily for 7 days, while another group was used as a vehicle control. Because some significant changes in bile acid metabolic genes (e.g., Mrp3) have been observed in cholestatic rodents, the purpose of the current experimental design was to avoid interference of cholestatic factors [8]. After the treatments, animals were sacrificed in random order. Blood samples were collected and placed on ice for 1 h, followed by centrifugation at 8,000 g for 10 min. The serum was stored at -80°C for serum biochemistry and bile salt assays. The rat livers were removed and cut into small pieces, and then kept in liquid nitrogen until analysis.
Serum biochemistry, lipids, and bile salt analyses
The following analyses were performed using the corresponding ELISA Kits (BlueGene Biotechnology, Shanghai, China), according to the manufacturer’s instructions: serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bile salts (TBA), total bilirubin (TBIL), direct bilirubin (DBIL), chenodeoxycholic acid (CDCA), taurochenodeoxycholic acid (TCDCA), cholic acid (CA), taurocholic acid (TCA), deoxycholic acid (DCA), taurodeoxycholic acid (TDCA), tauroursodeoxycholic acid (TUDCA), tauro-alpha/-beta-muricholic acid (Tα/βMCA), alpha-muricholic acid (αMCA), and beta-muricholic acid (βMCA), and serum lipid triglyceride hydrolase (Tgh), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C).
RNA extraction and Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Liver samples (100 mg each) were stored in liquid nitrogen, and total RNA was extracted with Trizol reagent (Invitrogen, San Diego, CA, USA). The cDNA was prepared, and real-time qPCR was subsequently performed as described previously [13-14]. The primers used in this study are listed in Table 1.
Table 1.
Sense and Antisense Primers Used for Real-Time qPCR (SYBR Green)
| Gene | Accession No. | Sense Primer (5’→3’) | Antisense Primer (5’→3’) |
|---|---|---|---|
| Cyp7a1 | NM_012942.2 | GCAAAACCTCCAATCTGTCAT | GCTTCAAACATCACTCGGTAAC |
| Cyp7b1 | NM_019138.1 | GGTTCTGAGGTTGTGCTCCTAC | GACTTCTGGGTCATTGTGTATCA |
| Cyp8b1 | NM_031241.1 | AGGTTGGAAGCCGAGACAT | TGGCACAGAGACGAAGAGTC |
| Cyp27a1 | NM_178847.2 | CAGCAAGAAGGTGAGCCTAC | TGTCTATGGGAAGGGCAGAG |
| Cyp3a | AB084894.1 | CACAAAGACCCAAAGTGCTG | CCATTACCAAAGGGCAGGT |
| Ugt2b | NM_031533.5 | CCCACCACCGTAGATGAGAC | GCAAGGGTTTAGCAGGTTTG |
| Sult2a1 | NM_131903.1 | AGGAACGAACTGGCTGATTG | ATGGGAAGATGGGAGGTCAT |
| Gsta2 | NM_017013.4 | GCAGGAGTGGAGTTTGATGAG | TTTGGTGGCGATGTAGTTGA |
| Gsta3 | NM_031509.2 | GGCTAAGGAATGATGGGAGTT | TGGTGGCAATGTAGTTGAGAA |
| Gsta4 | NM_001106840.1 | GCTGGAGTGGAGTTTGAAGAA | GATGGCTCTGGTCTGTGTCA |
| Gstm1 | NM_017014.1 | CCCATCTCCTCAACCTCAC | GGGCAGACCTCAAATCACAG |
| Gstm2 | NM_177426.1 | TGCTCCCGACTATGACAGAA | CGTCCACACGAATCCTCTC |
| Gstm3 | NM_020540.1 | TGGACACTTTGGAGAACCAG | CTTGCCCAGGAACTCTGAAT |
| Gstm4 | NM_001024304.1 | TCACACTGGCTCTGGCTTCT | CCCTGCCTATCCAACTGAAAT |
| Mrp2 | NM_012833.1 | CCCAGTCTTCGCTATCATCA | GACAGAATCCAACCGTCTCAG |
| Bsep | NM_031760.1 | CGTGCTTGTGGAAGAAGTTG | GGGAGTAGATGGGTGTGACTG |
| Abcg2 | NM_181381.2 | CTTACTGGCTTCTGGGAAACTC | AGGGTTGTTGTAGGGCTCAC |
| Abcg5 | NM_053754.2 | TAAGATGGCAGGCAGGAAAG | AGCAAAGGACGGTGAGTTCT |
| Abcg8 | NM_130414.2 | CTCATCGTCATTGGCATCA | GTGGAAGCAAGGCTGAACAT |
| Mdr1 | NM_012623.2 | GAGCCCATCCTGTTTGACTG | TGTCTCCCACTCTGGTGTTG |
| Mdr2 | NM_012690.2 | TCAGCAACCAGAGCAGAGAA | GCCCAGGAGCATAAACAAT |
| Mrp3 | NM_080581.1 | TTCCGATTCACCACTTTCTACA | GGCAAGGATTTGTGTCAAGATT |
| Mrp4 | NM_133411.1 | GAAGGAAAATGAGGAAGCAGAG | GGATGACTGTTGAGACCAAATC |
| Ostα | NM_001107087.1 | AAGTCGGAAGGGTTGGGTAG | ATCCTCTGCTGTGCCATCTC |
| Ostβ | XM_001076555.1 | TTTGGTATTTCCGTTCAGAGG | GCATTCCGTTGTCTTGTGG |
| Ntcp | NM_017047.1 | CTGGCTACCTCCTCCCTGAT | ATGCTGATGGTGCGTCTG |
| Oatp1 | NM_017111.1 | TGTATGGAGAACCGAACACAGA | AAGGGCACAATAGGAGTTTCAC |
| Oct1 | NM_012697.1 | TGTGGCTTTGCCTGAGACTA | CTTGCCTGTTTGGACCTGAA |
| Fxr | NM_021745.1 | TGAGCGTCTACAGCGAAAGTG | GGGATGGTGGTCTTCAAATAAG |
| Shp | NM_057133.1 | ATCTCTTCTTCCGCCCTGTC | AGGTTTTGGGAGCCATCAAG |
| Pxr | NM_052980.2 | ACATCATCCCTCACCCTTCA | TCAGGTCTCATCTCCAGGTTTA |
| Car | NM_022941.4 | CCAAGGAACTGTGTGGTGTG | CTGGACAATGGCGTCTCTG |
| Vdr | NM_017058.1 | GCCGCCTGTCTGTGTTATTC | GGTCATCTTGGCAGTGAGTG |
| Pparα | NM_013196.1 | CGGTGTGTATGAAGCCATCTT | TCTTTAGGAACTCTCGGGTGAT |
| Hnf1α | NM_012669.1 | AGAGGGAAGCAGGGTGAAG | CACAGAAATCCAGGCAGTCA |
| Hnf4α | NM_022180.2 | TAGCAGAGATGAGCCGTGTG | GCTTTGAGGCAGGCGTATT |
| Rxrα | NM_012805.2 | TTCTCCCACCGCTCCATAG | CGTTAGCACCCTGTCAAAGA |
| Rarα | NM_031528.2 | CACCTGAGCAAGACACAATGA | GCGAAGGCAAAGACCAAGT |
| Lxr | NM_031626.1 | CGCTACAACCACGAGACAGA | GGCAATGAGCAAGGCATACT |
| Lrh-1 | NM_021742.1 | ATGGGAAGGAAGGGACAATC | CAAACTGAAGGGAACGGAGTC |
| Nrf2 | NM_031789.2 | CCTTCCTCTGCTGCCATTAG | GTGCCTTCAGTGTGCTTCTG |
| Hnf3β | NM_012743.1 | AACAAGATGCTGACGCTGAG | GAATGACGGATGGAGTTCTG |
| Ahr | NM_013149.2 | CAGGACCAGTGTAGAGCACAAG | CTGCCGTGACAACCAGAAC |
Western blot analysis
Western blotting was performed as described previously [13-14]. The dilutions of primary antibodies were as follows: CYP7A1 (Cytochrome P450s) (1:2000), CYP7B1 (1:2,000), CYP8B1 (1:2,000), CYP27A1 (1:2,000), CYP3A4 (1:4,000), UGT2B (UDP- glucuronosyltransferase) (1:4,000), SULT2A1 (soluble sulfotransferases) (1:2,000), GSTA1 (glutathione S-transferase) (1:1,000), GSTM2 (1:1,000), MDR1 (multidrug resistance transporter 1) (1:2,000), MDR2 (1:2,000), OSTα (organic solute transporter alpha) (1:4,000), ABCG5 (1:3,000), ABCG8 (1:2,000), BSEP (bile salt export pump) (1:1,000), NTCP (Na+/taurocholate cotransporter) (1:800), PXR (pregnane X receptor) (1:1,000), CAR (constitutive androstane receptor) (1:1,000), VDR (vitamin D receptor) (1:1,000), RXRα (retinoid X receptor) (1:1,600), RARα (retinoic acid receptor) (1:1,600), HNF1α (hepatocytes nuclear factor 1alpha) (1:2,000), HNF4α (1:2,000) and LXR (1:1,000) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA); GSTA2 (1:4,000) (GeneTex, Irvine, CA, USA), GSTM1 (1:1,000), PPARα (1:1,000), and AhR (1:1,000) (Proteintech Group, Chicago, IL, USA), OSTβ (1:500) (Sigma-Aldrich), MRP2 (1:2,000), ABCG2 (1:2,000), FXR (farnesoid X receptor/bile acid receptor) (1:10,000), SHP (short heterodimer partner) (1:1,000), LRH-1 (1:2,000), HNF3β (1:10,000), and NRF2 (1:10,000) (Abcam, Cambridge, MA, USA). GAPDH (1:40,000) (Abcam) was used as a loading control. The band intensities were analyzed by ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data are expressed as the means ± standard deviations (SD) and analyzed using the independent-samples Student’s t test (two-tailed) using the SPSS software (PASW Statistics 18, IBM; SPSS, Inc., Chicago, IL, USA). A value P < 0.05 was considered statistically significant.
Results
Oleanolic acid does not cause liver injury in normal rats
Repeated administration of a high dose of oleanolic acid (> 135 mg/kg) has been reported to induce mouse liver injury [5]. However, we found that oral administration of oleanolic acid from Swertia mussotii Franch at 100 mg/kg for continuous 7 days had no influences on the serum markers for liver injury, including ALT, AST, and ALP (Figure 1A). Furthermore, the serum TBA, TBIL, and DBIL were not affected by oleanolic acid (Figure 1B). However, the serum levels of TG and HDL-C were significantly decreased, while the levels of Tgh and LDL-C remained unchanged in rats with the treatment of oleanolic acid (Figure 1C). Our results indicated that oral administration of oleanolic acid at 100 mg/kg daily is not hepatotoxic to healthy rats.
Figure 1.
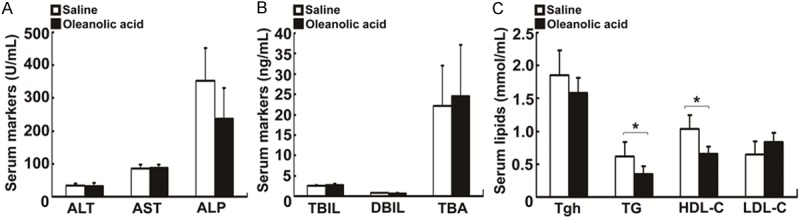
Changes in serum biochemistry and lipids in rats treated with oleanolic acid. SD rats were orally given oleanolic acid at 100 mg/kg per day for total 7 days. A. Serum levels of ALT, AST, and ALP. B. Serum levels of TBIL, DBIL, and TBA. C. Serum levels of lipids Tgh, TG, HDL-C, and LDL-C. Data are expressed as mean ± SD (n = 5 rats per group). *P < 0.01 vs the vehicle control.
Oleanolic acid attenuates liver injury and inflammation in the BDL rats
To determine whether oleanolic acid has a hepatoprotective effect on cholestasis, we measured the serum liver injury markers and proinflammatory cytokines in oleanolic acid-treated BDL rats. Figure 2A illustrated that serum ALP was markedly decreased (P < 0.05), but serum ALT and AST were increased (P < 0.01) after treatment with oleanolic acid for 3 days in the BDL rats. Furthermore, serum ALT and ALP at days 7 and 14, and AST at day 14 post-oleanolic acid treatment, were significantly decreased (Figure 2A). As shown in Figure 2B, the serum TNF-α level at day 14 and IL-6 level at all tested time points were significantly inhibited by oleanolic acid (P < 0.01, Figure 2B). These results indicate that oleanolic acid alleviates liver injury and inflammation in obstructive cholestasis.
Figure 2.
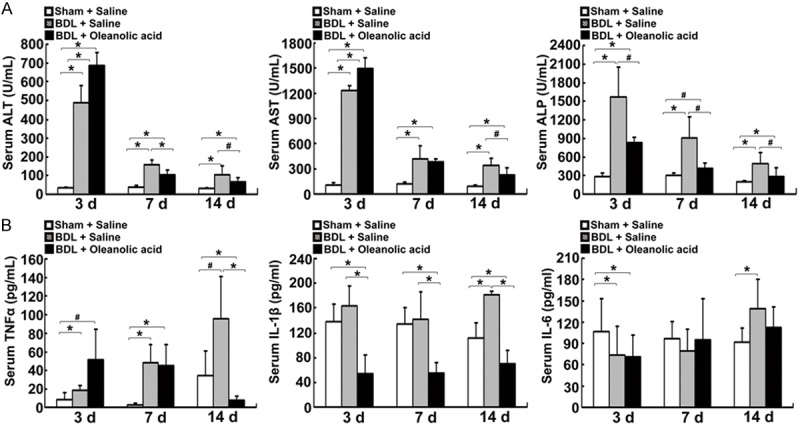
Changes in serum biochemistry and proinflammatory cytokine levels in the BDL rats-treated with oleanolic acid. The SD rats were pre-treated with oral administration of oleanolic acid at 100 mg/kg for 1 day, underwent BDL surgery, and continued to be treated with oleanolic acid daily for 3, 7, or 14 days. A. Serum levels of ALT, AST, and ALP. B. Serum levels of proinflammatory cytokines TNF-α, IL-1β, and IL-6. Data are expressed as mean ± SD (n = 7 rats per group). *P < 0.05 vs the sham operated normal rats-treated with saline, #P < 0.05 vs the BDL model rats treated with saline.
Oleanolic acid reduces serum total bile acid level and bile salt concentrations in the BDL rats
The accumulation of bile acids contributes to liver injury in cholestasis [8]. To address whether oleanolic acid exerts a protective role against cholestasis by altering bile acid pools, we measured the serum levels of total bile acids (TBA) and bile salts in the BDL rats. We found that the serum TBA level was significantly reduced at day 3 (P < 0.05) and further decreased at day 14 (P = 0.06) by oleanolic acid (Figure 3A-C). We then examined whether oleanolic acid could alter the serum bile acid concentrations in the BDL rats. Our results showed that of the tested biochemical parameters, the serum levels of CDCA, CA, DCA, and Tα/βMCA were significantly reduced at day 3 in the BDL rats-treated with oleanolic acid (Figure 3D). These results indicate that oleanolic acid reduces serum bile acid levels in the BDL rats.
Figure 3.
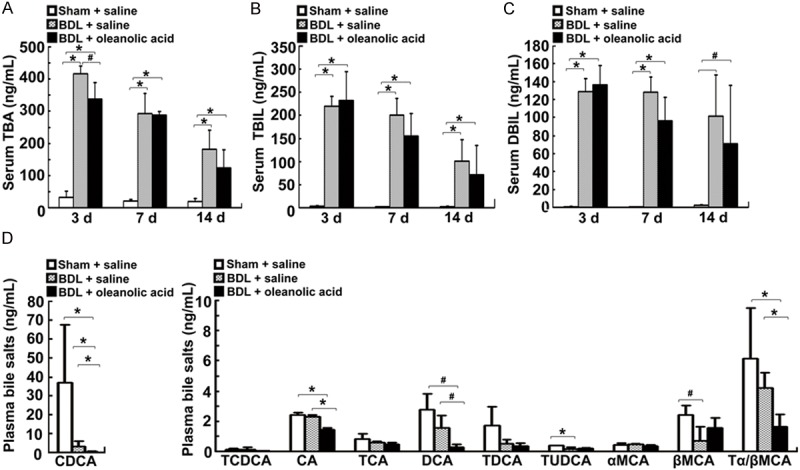
Changes in serum biochemistry and bile salt levels in the BDL rats -treated with oleanolic acid. The animals were treated as described in Figure 2. Levels of TBA (A), TBIL (B), and DBIL (C) on days 3, 7 and 14. (D) Serum levels of bile salts CDCA, TCDCA, CA, TCA, DCA, TDCA, TUDCA, Tα/βMCA, αMCA, and βMCA on day 3. Data are expressed as mean ± SD (n = 7 rats per group). *P < 0.01 vs sham operated rats or the BDL model rats-treated with saline, #P < 0.05 vs sham operated rats or the BDL model rats-treated with saline.
Oleanolic acid increases the expression of bile acid synthetic enzymes Cyp7b1 and Cyp8b1 in the rat liver
To understand the mechanism by which the oral administration of oleanolic acid reduce the serum bile acid levels in the BDL rats, we tested whether oleanolic acid represses hepatic bile acid synthetic enzymes expression in rat liver. The mRNA expression levels of Cyp7b1 and Cyp8b1 were significantly increased (1.8-fold and 2.1-fold, respectively), while the mRNA expression of Cyp7a1 and Cyp27a1 remained unchanged in the oleanolic acid-treated rats (Figure 4A). Furthermore, Western blotting results confirmed that oleanolic acid markedly up-regulated the protein expression of Cyp7b1 and Cyp8b1 (2.9-fold and 1.7-fold, respectively), but had no effects on the expression levels of Cyp7a1 and Cyp27a1 (Figure 4B, 4C). These results indicate that the alternative pathway of bile acid synthesis is activated by oleanolic acid in rats.
Figure 4.
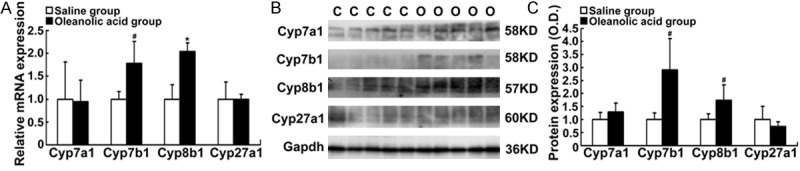
Alteration of bile acid synthetic enzymes Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1 in rats-treated with oleanolic acid. SD rats were orally given oleanolic acid at 100 mg/kg daily for 7 days. A. The mRNA levels of Cyp8b1, Cyp7a1, Cyp7b1, and Cyp27a1. B. Representative Western blotting for the protein levels of Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1. C. Western blot densitometry analyses (% of control group). Data are expressed as mean ± SD. *P < 0.01, #P < 0.05 vs the vehicle control. C. the control group; O. the oleanolic acid group.
Oleanolic acid stimulates the expression of detoxification enzymes in rats
Hepatic detoxification enzymes can increase the water solubility of hydrophobic bile acids and decrease their hepatotoxicity through hydroxylation (Cyp3a), glucuronidation (Ugt2b), sulfation (Sult2a1), and Gsts (glutathione conjugation) [8]. Figure 5A displays the increased mRNA expression levels of the detoxification enzymes Cyp3a, Ugt2b, Sult2a1, Gsta2, Gsta4, and Gstm1 (1.3-2.7-fold, P < 0.05) in the oleanolic acid-treated rats. Furthermore, we demonstrated that the protein levels of Cyp3a, Ugt2b, Sult2a1, Gsta1, Gsta2, Gstm1, Gstm2, and Gstm3 were significantly induced by oleanolic acid (1.4 to 3.3-fold, P < 0.05, Figure 5B, 5C). These results suggest that oral administration of oleanolic acid induces the expression of certain hepatic detoxification enzymes in rats.
Figure 5.
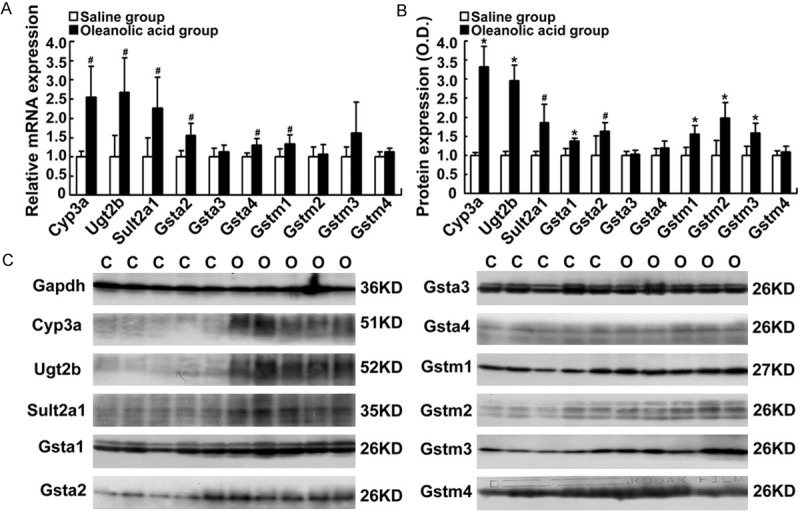
Changes in the expression of bile acid detoxification enzymes Cyp3a, Ugt2b, Sult2a1, Gsta1-4, Gstm1-4, Cyp8b1, and Cyp27a1 in rats-treated with oleanolic acid. SD rats were given 100 mg/kg of oleanolic acid by gavage daily for 7 days. A. The mRNA expression of Cyp3a, Ugt2b, Sult2a1, Gsta2-a4, and Gstm1-4. B. The protein levels of Cyp3a, Ugt2b, Sult2a1, Gsta1-4, and Gstm1-4 analyzed by densitometry of the Western blotting (% of control group). C. Representative Western blots. *P < 0.01; #P < 0.05 vs the vehicle control; n = 5 rats per group. C, the control group; O, the oleanolic acid group.
Oleanolic acid alters canalicular and basolateral membrane transporters in rats
Hepatic canalicular membrane and basolateral membrane transporters exert a crucial role in eliminating the accumulation of toxic bile acids in cholestatic hepatocytes [8]. Next, we determined whether oleanolic acid affects mRNA and protein levels of these membrane transporters. Among the canalicular membrane transporters, Bsep and Mdr2 mRNA expression levels were increased by oleanolic acid (1.4- and 1.6-fold, respectively, P < 0.005) when compared with the vehicle control (Figure 6A). Western blotting demonstrated the protein expression levels of Bsep and Mdr2 as well as Mrp2 and Mdr1were markedly increased in rats-treated with oleanolic acid (2.9 to 5.7-fold, P < 0.01 Figure 6B, 6C). Among the basolateral membrane transporters, we found that oleanolic acid up-regulated the gene expression of Mrp3, Mrp4, Ntcp, Oatp1b1, and Oct1 (1.3 to 2.0-fold, P < 0.05) when compared to the vehicle control (Figure 6D). Furthermore, oleanolic acid increased the protein levels of Mrp3, Mrp4, Ostβ, Ntcp, and Oatp1b1 (2.0 to 6.4-fold, P < 0.01) when compared to with the vehicle control (Figure 6E, 6F). In contrast, Ostα protein expression was decreased (over 25%) in rats-treated with oleanolic acid when compared to the control animals (P < 0.01, Figure 6E, 6F). These results indicate that oleanolic acid up-regulates the efflux transporter expression in rat liver.
Figure 6.
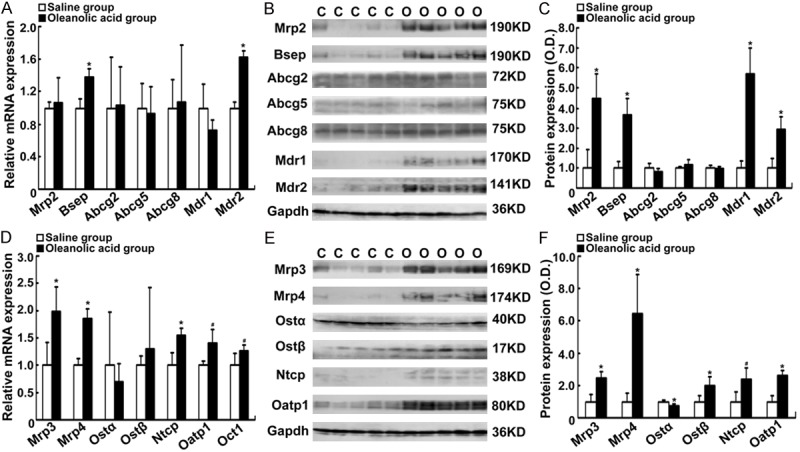
Changes in the expression levels of canalicular and basolateral membrane transporters in rats-treated with oleanolic acid. SD rats were given oleanolic acid at 100 mg/kg/d daily for 7 days. A. The mRNA expression of Mrp2, Bsep, Abcg2, Abcg5/8, Mdr1, and Mdr2. B. Representative Western blots for Mrp2, Bsep, Abcg2, Abcg5/8, Mdr1, and Mdr2. C. Densitometry analyses of Western blots (% of control group). D. The mRNA expressions of Mrp3, Mrp4, Ostα/β, Ntcp, Oatp1b1, and Oct1. E. Representative Western blotting for Mrp3, Mrp4, Ostα/β, Ntcp, and Oatp1b1. F. Densitometry analysis of Western blots (% of control group). *P < 0.01; #P < 0.05 vs the vehicle control; n = 5 rats per group. C, the control group; O, the oleanolic acid group.
Oleanolic acid increases the expression of nuclear receptors and transcriptional factors in rats
To explore whether nuclear receptors and transcriptional factors are involved in the up-regulation of hepatic detoxification enzymes and efflux transporters by oleanolic acid, we determined their mRNA and protein levels in rats with oleanolic acid treatment. The qPCR results showed that oleanolic acid significantly increased the gene expression levels of nuclear receptors Rarα, Lxr, and Lrh-1 (1.4 to 2.6-fold, P < 0.005) (Figure 7A). Furthermore, oleanolic acid increased the gene expression levels of hepatic transcriptional factors Nrf2, Hnf3β, and Ahr (1.4 to 1.9-fold, P < 0.005, Figure 7A). Western blotting results demonstrated that nuclear receptors Pxr, Vdr, Hnf4α, Rxrα, Rarα, Lxr, and Lrh-1 (1.7 to 5.3-fold) and transcriptional factors Nrf2, Hnf3β, and Ahr (1.3 to 3.3-fold) were significantly increased by oleanolic acid at the protein levels (Figure 7B, 7C). These results suggest that oleanolic acid can significantly increase the expression of nuclear receptors and transcriptional factors related to bile acid metabolism.
Figure 7.
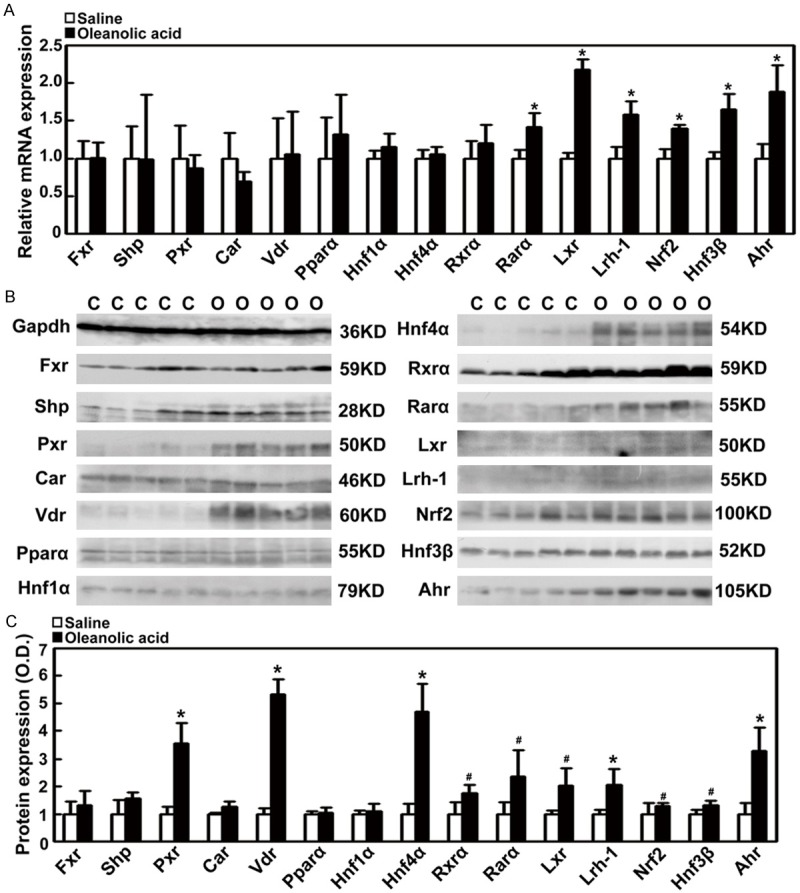
Changes in the expression of nuclear receptors and transcriptional factors in rats-treated with oleanolic acid. SD rats were treated with oleanolic acid via gastric gavage at 100 mg/kg daily for 7 days. A. The mRNA expression of nuclear receptors Fxr, Shp, Pxr, Car, Vdr, Pparα, Hnf1α, Hnf4α, Rxrα, Rarα, Lxr, and Lrh-1, and transcriptional factors Nrf2, Hnf3β, and Ahr. B. Representative Western blots for Fxr, Shp, Pxr, Car, Vdr, Pparα, Hnf1α, Hnf4α, Rxrα, Rarα, Lxr, and Lrh-1and transcriptional factors, Nrf2, Hnf3β, and Ahr. C. Densitometry analysis of Western blots (% of control group). *P < 0.01; #P < 0.05 vs the vehicle control; n = 5 rats per group; C, the control group; O, the oleanolic acid group.
Discussion
We demonstrated that oral administration of oleanolic acid at 100 mg/kg/d does not induce liver damage in rats. Furthermore, we observed that oleanolic acid attenuates liver injury and inflammation in the BDL rats. The serum TBA and bile salt concentrations were also decreased in oleanolic acid-treated BDL rats. These findings may result from significant changes in (1) the induced expression of synthetic enzymes Cyp7b1 and Cyp8b1, (2) the up-regulated expression of detoxifying enzymes Cyp3a, Ugt2b, Sult2a1, Gsta2, and Gstm1-3, and (3) the stimulated expression of canalicular membrane efflux transporters Bsep, Mrp2, Mdr1, and Mdr2 and basolateral membrane transporters Mrp3, Mrp4, and Ostβ, and the reduced expression of basolateral membrane transporter Ostα. These results indicate that oleanolic acid from Swertia mussotii Franch attenuates liver injury, inflammation, and cholestasis in the BDL rats.
Previous studies demonstrated that oleanolic acid isolated from other plants has weak hepatotoxic effects on rat primary hepatocytes [4]. Recent studies reported that a dose of oleanolic acid over 90 mg/kg/d (i.p.) produces cholestasis, but a low dose below 20 mg/kg/d (i.p.) has anti-cholestasis effect in mice [6,7]. Furthermore, repeated oral administration of a high dose of oleanolic acid at > 135 mg/kg causes liver injury in mice [5]. These data indicate that the tolerance of oleanolic acid and its benefits vary depending on the administration routes and dosages in rodents, implying that oral administration may be the best way to deliver oleanolic acid for its hepatoprotective effect. In the present study, we demonstrated that no liver injury was observed in rats treated with oleanolic acid orally (isolated from Swertia mussotii Franch) at 100 mg/kg daily for 7 days. Although slight increases of serum ALT and AST levels at day 3 were observed, the serum ALT level at days 7 and 14 and the AST level at day 14 were significantly decreased by oleanolic acid in the BDL animals. Interestingly, serum ALP levels were markedly decreased by oleanolic acid at several tested time points in the BDL rats. Moreover, similar to the effect of UDCA, oleanolic acid reduces the serum levels of TNF-α at day 14 and IL-1β at the test time points in the BDL rats. These indicate that oleanolic acid from Swertia mussotii Franch can reduce liver injury and inflammation in rats with obstructive cholestasis.
The accumulation of toxic bile acids exerts a key role in hepatocellular injury under cholestasis [8-11]. The induced mRNA and protein expressions of hepatic alternative synthetic enzymes Cyp7b1 and Cyp8b1 by oleanolic acid may drive the bile acid synthesis pathways towards CA, but not CDCA, considering that the production of CDCA over CA is seen in Cyp8b1 deficiency mice and CDCA is more hepatotoxic than CA [15,16]. This can explain why serum CDCA, the major bile acid, was significantly repressed by oleanolic acid in BDL rats. Furthermore, the significant reduction of serum TG may be also associated with the up-regulation of hepatic Cyp8b1 by oleanolic acid, because Cyp8b1 deficiency and the product of 12α-hydroxylated bile acids interfere with the TG-lowering effects of Fxr [17,18]. Moreover, recent reports demonstrated that nuclear receptor Lxr up-regulates the expression of Cyp7b1 and Cyp8b1 in hepatoma cells and rodents [19], indicating that oleanolic acid induces the expression of Cyp7b1 and Cyp8b1 genes via nuclear receptors Lxr in rats.
We also demonstrated that hepatic detoxification enzymes, such as Cyp3a, Ugt2b, Sult2a1, Gsta1-2, and Gstm1-3, were dramatically increased in rats after oleanolic acid treatment. These up-regulated detoxification enzymes can increase the water solubility of hydrophobic bile acids and decrease its hepatotoxicity through hydroxylation (Cyp3a), glucuronidation (Ugt2b), sulfation (Sult2a1), and glutathione conjugation (Gsta1-2 and Gstm1-3) [8-11]. The detoxificated bile acids in rat liver following the treatment of oleanolic acid were then eliminated from hepatocytes by increased hepatic basolateral membrane efflux transporters (e.g., Mrp3, Mrp4, and Ostβ) and canalicular membrane transporters (e.g., Mrp2 and Bsep) [8-11]. Moreover, the induction of Mdr1 and Mdr2 expression by oleanolic acid may also have a protective role in the BDL rats, because Mrd1 may function as an intracellular transporter of cholesterol and secondary bile salt export pump, and Mdr2 mutation causes the cholestasis in rodents [9,20,21]. A recent study showed that mouse Ostα deficiency attenuates cholestatic liver injury [22], indicating that the down-regulation of Ostα expression by oleanolic acid plays a role in reducing liver injury in BDL rats. Furthermore, significant increase in the expression of detoxification enzyme and efflux transporter may contribute to the up-regulation of nuclear receptors (e.g., Vdr, Pxr, and Lrh-1) and transcriptional factors (e.g., Ahr, Nrf2, and Hnf3β) in rats following oleanolic acid treatment. Additionally, the activation of these nuclear receptors and transcriptional factors not only induces the expression of detoxification enzymes, including Cyp3a, Ugt2b, Sult2a1, Gsta1, and Gstm1, but also involves the up-regulation of efflux transporters, including Mrp3 and Mrp4 in hepatoma cells and rodent liver [8-11,19,23-29]. However, the molecular mechanism of the up-regulated Bsep and Mdr1 expression remains unclear, because the modulators of Fxr and Pparα were not affected by oleanolic acid in the rat liver. In addition, the up-regulation of Mrp2, Mdr1, Ostβ, and Gstm2-3 expressions, and the down-regulation of Ostα expression may be a post-transcriptionally regulated, since the mRNA levels of these genes remained in rats-treated with oleanolic acid. Contrary to the lowering bile acid intake transporters Ntcp and Oatp1b1 in mouse liver with oleanolic acid treatment by i.p [6], our results demonstrated that oral administration of oleanolic acid could induce the expression of Ntcp and Oatp1b1 in rat liver. This up-regulation may be associated with the induction of nuclear receptors Rxrα, Rarα, and Hnf4α by oleanolic acid. However, whether the up-regulation of Ntcp and Oatp1b1 by oleanolic acid contributes the liver injury in the BDL rats at day 3 warrants further study. Nevertheless, our results demonstrated that oleanolic acid could reduce the bile acid accumulation in rat hepatocytes through the alterations of bile acid synthetic pathway and the enhancement of its detoxification and excretion abilities.
In summary, we have demonstrated that oleanolic acid from Swertia mussotii Franch significantly increased alternative bile acid synthetic enzymes, detoxification enzymes, and efflux transporters, which may reduce the hepatotoxicity of bile acids and enhance the elimination of bile acids in hepatocytes. Our results may contribute to the understanding of the protective role of oleanolic acid against liver injury in obstructive cholestasis. Our study suggests that oral oleanolic acid may be a potential agent for the treatment of cholestatic patients.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81070320, 81100280, and 81470880), Scholarship Foundation of China Scholarship Council (CSC No.2 01307610015), and Scholarship Foundation of Third Military Medical University (2013).
Disclosure of conflict of interest
None.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ALP
alkaline phosphatase
- TBA
total bile acid
- Mrp
multidrug resistance-associated protein
- Bsep
bile salt export pump
- Mdr
multidrug resistance transporter
- Ntcp
Na+/taurocholate cotransporter
- Oatp
organic anion transporter
- Ostα/β
organic solute transporter alpha and beta
- CAR (NR1I3)
constitutive androstane receptor
- Pxr (Nr1i2)
pregnane X receptor
- Fxr (Nr1h4)
farnesoid X receptor/bile acid receptor
- Shp (Nr0b2)
short heterodimer partner
- Rara (Nr1b1)
retinoic acid receptor
- Rxra (Nr2b1)
retinoid X receptor
- Vdr
vitamin D receptor
- Nrf2
NF-E2-related factor
- Hnf
hepatocyte nuclear factors
- Cyp
Cytochrome P450 s
- Ugt
UDP- glucuronosyltransferase
- Sult
soluble sulfotransferases
- Gst
glutathione S-transferase
- UDCA
ursodeoxycholic acid
References
- 1.Fan G, Luo WZ, Luo SH, Li Y, Meng XL, Zhou XD, Zhang Y. Metabolic discrimination of Swertia mussotii and Swertia chirayita known as “Zangyinchen” in traditional Tibetan medicine by HNMR-based metabolomics. J Pharm Biomed Anal. 2014;98:364–370. doi: 10.1016/j.jpba.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Gao R, Wang L, Yang Y, Ni J, Zhao L, Dong S, Guo M. Simultaneous determination of oleanolic acid, ursolic acid, quercetin and apigenin in Swertia mussotii Franch by capillary zone electrophoresis with running buffer modifier. Biomed Chromatogr. 2014;29:402–9. doi: 10.1002/bmc.3290. [DOI] [PubMed] [Google Scholar]
- 3.Tian C, Zhang T, Wang L, Shan Q, Jiang L. The hepatoprotective effect and chemical constituents of total iridoids and xanthones extracted from Swertia mussotii Franch. J Ethnopharmacol. 2014;154:259–266. doi: 10.1016/j.jep.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Kinjo J, Okawa M, Udayama M, Sohno Y, Hirakawa T, Shii Y, Hirakawa T, Shii Y, Nohara T. Hepatoprotective and hepatotoxic actions of oleanolic acid-type triterpenoidal glucuronides on rat primary hepatocyte cultures. Chem Pharm Bull (Tokyo) 1999;47:290–292. doi: 10.1248/cpb.47.290. [DOI] [PubMed] [Google Scholar]
- 5.Lu YF, Wan XL, Xu Y, Liu J. Repeated oral administration of oleanolic acid produces cholestatic liver injury in mice. Molecules. 2013;18:3060–3071. doi: 10.3390/molecules18033060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Lu YF, Zhang Y, Wu KC, Fan F, Klaassen CD. Oleanolic acid alters bile acid metabolism and produces cholestatic liver injury in mice. Toxicol Appl Pharmacol. 2013;272:816–824. doi: 10.1016/j.taap.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P, Zeng H, Wang Y, Fan X, Xu C, Deng R, Zhou X, Bi H, Huang M. Low dose of oleanolic acid protects against lithocholic acid-induced cholestasis in mice: potential involvement of nuclear factor-E2-related factor 2-mediated upregulation of multidrug resistance-associated proteins. Drug Metab Dispos. 2014;42:844–852. doi: 10.1124/dmd.113.056549. [DOI] [PubMed] [Google Scholar]
- 8.Trauner M, Meier PJ, Boyer JL. Molecular patho-genesis of cholestasis. N Engl J Med. 1998;339:1217–27. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 9.Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–51. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 11.Trauner M, Wagner M, Fickert P, Zollner G. Molecular regulation of hepatobiliary transport systems: clinical implications for understanding and treating cholestasis. J Clin Gastroenterol. 2005;39:S111–S124. doi: 10.1097/01.mcg.0000155551.37266.26. [DOI] [PubMed] [Google Scholar]
- 12.Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chai J, He Y, Cai SY, Jiang Z, Wang H, Li Q, Chen L, Peng Z, He X, Wu X, Xiao T, Wang R, Boyer JL, Chen W. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette Subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and c-Jun NH2-terminal kinase/stress-activated protein kinase-signaling pathway. Hepatology. 2012;55:1485–94. doi: 10.1002/hep.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai J, Luo D, Wu X, Wang H, He Y, Li Q, Zhang Y, Chen L, Peng ZH, Xiao T, Wang R, Chen W. Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis. J Gastrointest Surg. 2011;15:996–1004. doi: 10.1007/s11605-011-1473-2. [DOI] [PubMed] [Google Scholar]
- 15.Li-Hawkins J, Gafvels M, Olin M, Lund EG, An-dersson U, Schuster G, Björkhem I, Russell DW, Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monte MJ, Marin JJ, Antelo A, Vazquez-Tato J. Bile acids: chemistry, physiology, and pathophysiology. World J Gastroenterol. 2009;15:804–816. doi: 10.3748/wjg.15.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell Metab. 2012;15:65–74. doi: 10.1016/j.cmet.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SD, Thornton SJ, Sachs-Barrable K, Kim JH, Wasan KM. Evaluation of the contribution of the ATP binding cassette transporter, P-glycoprotein, to in vivo cholesterol homeostasis. Mol Pharm. 2013;10:3203–12. doi: 10.1021/mp4002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer JL. Nuclear receptor ligands: rational and effective therapy for chronic cholestatic liver disease? Gastroenterology. 2005;129:735–740. doi: 10.1016/j.gastro.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 20.Connelly-Smith L, Pattinson J, Grundy M, Shang S, Seedhouse C, Russell N. P-glycoprotein is downregulated in KG1a-primitive leukemia cells by LDL cholesterol deprivation and by HMG-CoA reductase inhibitors. Exp Hematol. 2007;35:1793–800. doi: 10.1016/j.exphem.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs M, Stange EF. Cholesterol and cholestasis: a lesson from the Mdr2 (-/-) mouse. J Hepatol. 2001;34:339–341. doi: 10.1016/s0168-8278(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 22.Soroka CJ, Velazquez H, Mennone A, Ballatori N, Boyer JL. Ostalpha depletion protects liver from oral bile acid load. Am J Physiol Gastrointest Liver Physiol. 2011;301:G574–579. doi: 10.1152/ajpgi.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleksunes LM, Klaassen CD. Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice. Drug Metab Dispos. 2012;40:1366–79. doi: 10.1124/dmd.112.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, Denk H, Trauner M. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 25.Bohan A, Chen WS, Denson LA, Held MA, Boyer JL. Tumor necrosis factor alpha-dependent up-regulation of Lrh-1 and Mrp3(Abcc3) reduces liver injury in obstructive cholestasis. J Biol Chem. 2003;278:36688–36698. doi: 10.1074/jbc.M304011200. [DOI] [PubMed] [Google Scholar]
- 26.Zollner G, Fickert P, Silbert D, Fuchsbichler A, Marschall HU, Zatloukal K, Denk H, Trauner M. Adaptive changes in hepatobiliary transporter expression in primary biliary cirrhosis. J Hepatol. 2003;38:717–727. doi: 10.1016/s0168-8278(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 27.Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Upregulation of a basolateral FXR-dependent bile acid efflux transporter OSTalpha-OSTbeta in cholestasis in humans and rodents. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1124–1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- 28.Jonker JW, Liddle C, Downes M. FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol. 2012;130:147–158. doi: 10.1016/j.jsbmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin Liver Dis. 2010;30:160–177. doi: 10.1055/s-0030-1253225. [DOI] [PubMed] [Google Scholar]


