Abstract
Background and purpose: Data on the association between PDE4D SNP 87 and the risk of ischemic stroke are contentious and debatable. The present meta-analysis was undertaken to systematically summarize the possible association. Methods: Based on comprehensive search of PubMed, Embase, and CNKI databases, we identified 18 eligible articles examining the relationship between PDE4D SNP 87 and ischemic stroke risk. We evaluated the strength of relationship using odds ratios (ORs) with 95% confidence intervals (CIs). Results: In the overall analysis, PDE4D SNP 87 was not found to have effects on the risk of ischemic stroke. The null association persisted in the subgroup analyses according to ethnicity and sample size. Conclusions: Our meta-analysis suggests that PDE4D SNP 87 may not represent an independent risk factor for ischemic stroke development.
Keywords: PDE4D, SNP, ischemic stroke, risk
Introduction
Stroke is a leading cause of death and disability with a high prevalence among old and very old people worldwide [1,2]. There are two subtypes of stroke: hemorrhagic stroke and ischemic stroke [3]. Ischemic stroke characterized by a sudden decrease in blood flow to one or more central nervous system areas constitutes 80% of all strokes. Ischemic stroke is a highly complex disease composed of various heterogeneous disorders attributable to both genetic and environmental factors [4,5]. Experimental evidence demonstrates that genetics should be responsible for a large part of stroke risk [6]. However, the investigations fail to identify candidate genetic factors for the aggressive disease.
Several previous studies of an Icelandic population used a genome-wide approach highlighted the implication of single nucleotide polymorphisms (SNPs) and haplotypes in the 5-lipoxygenase-activating protein (ALOX5AP or FLAP) and phosphodiesterase 4D (PDE4D) genes in ischemic stroke [7,8]. PDE4D located on chromosomal region 5q12 is a very large gene that spans 1.5 Mb and consists of 8 splice variants, 22 exons, and several hundred SNPs [9]. PDE4D belongs to the large superfamily of cyclic nucleotide phosphodiesterases and has been reported to be involved in inflammation [10], cell proliferation [11], and migration [12] that could consequently result in ischemic stroke. A growing body of replicated reports has sought to challenge the preconceived findings of the Icelandic study in diverse populations, showing conflicting results [13-17]. The relatively small studies using participants of different ethnic backgrounds and different analytical methods may be the major sources of the controversy.
Among the numerous SNPs (SNP 26, 45, 56, 83, 87, and 89) of PDE4D gene, SNP 87 (rs2910829) has been extensively investigated in ischemic stroke community [18-21]. However, the efforts did not confirm the susceptibility role of SNP 87 in ischemic stroke. In this study, we hypothetized that SNP 87 was associated with the onset of ischemic stroke. To test this hypothesis, we performed a meta-analysis to re-evaluate the association between SNP 87 and ischemic stroke.
Materials and methods
Identification and eligibility of relevant studies
The genetic association studies concerning the association of SNP 87 and ischemic stroke risk published before March 2014 were identified by comprehensively searching PubMed, Embase and CNKI (China National Knowledge Infrastructure) databases. The following search terms were used: (polymorphism) OR (polymorphisms) AND (phosphodiesterase 4D) OR (PDE4D) OR (SNP 87) OR (rs2910829) AND (ischemic stroke). We reviewed the abstracts of the retrieved studies to examine their appropriateness for inclusion in the meta-analysis. Then, the full texts of the articles were screened in order to check their eligibility for the present study. Finally, all the reference lists of the eligible articles and the journals known to publish articles relevant to the current topic were systematically reviewed to identify additional published articles. The case-control studies provided the genotype distribution of SNP 87 in ischemic stroke risk were eligible for inclusion in the meta-analysis. For studies used the same series of cases, the latest or the largest study was considered. Review articles, comment letters, case reports were excluded from this meta-analysis.
Data extraction
Two reviewers independently gathered the data from each eligible study and reached a consensus on all items. The information required to be collected was: first author, journal, year of publication, study country, ethnicity, gender and mean age of the cases, sample sizes of the cases and controls, genotyping methods and genotype frequencies of SNP87.
Statistical analysis
STATA software (version 12.0) was used for all statistical analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the association of SNP 87 and ischemic stroke risk.
A chi-square-based Q test was used to measure between-study heterogeneity. Statistical significance was defined at P < 0.10. In addition, I2 statistic was calculated to quantify the proportion of the total variation across studies due to heterogeneity (I2 = 0%-25%: no heterogeneity; I2 = 25-50%: moderate heterogeneity; I2 = 50-75%: large heterogeneity; I2 = 75-100%: extreme heterogeneity) [22]. The ORs were pooled using the fixed effects model (the Mantel-Haenszel method) [23] when the P value is above 0.10, and the random effects model (the DerSimonian and Laird method) [24] if P < 0.10. Subgroup analyses by ethnicity (Asian or Caucasian) and sample size (500-1000, < 500, > 1000) were performed to further identify heterogeneity.
Hardy-Weinberg equilibrium (HWE) of the control groups were checked by a Chi-square test. A P-value < 0.10 was considered significant. Sensitivity analysis was carried out to identify the study modifying the summary ORs. Begg’s funnel plots and Egger’s test [25] were used to determine publication bias among the studies included in this meta-analysis. A two-sided P value < 0.10 was considered significant.
Results
Characteristics of studies
A flow diagram of the study selection process for the meta-analysis of SNP 87 and ischemic stroke is described in Figure 1. The original search provided 223 records. After eliminating duplications, 187 records remained. Of these, 161 were discarded after reviewing the abstracts. The full texts of the remaining 26 studies were examined in detail and 8 articles were further excluded according to the criteria for inclusion. Therefore, we identified 18 records [13-21,26-34] with 8,363 cases and 12,223 control subjects for the final meta-analysis, including 7 records for Caucasian descent, and 11 records for Asian descent. All 18 articles were based on a case-control design. The main information and genotype frequencies for SNP 87 for ischemic stroke cases and controls in each of the studies included are summarized in Table 1.
Figure 1.
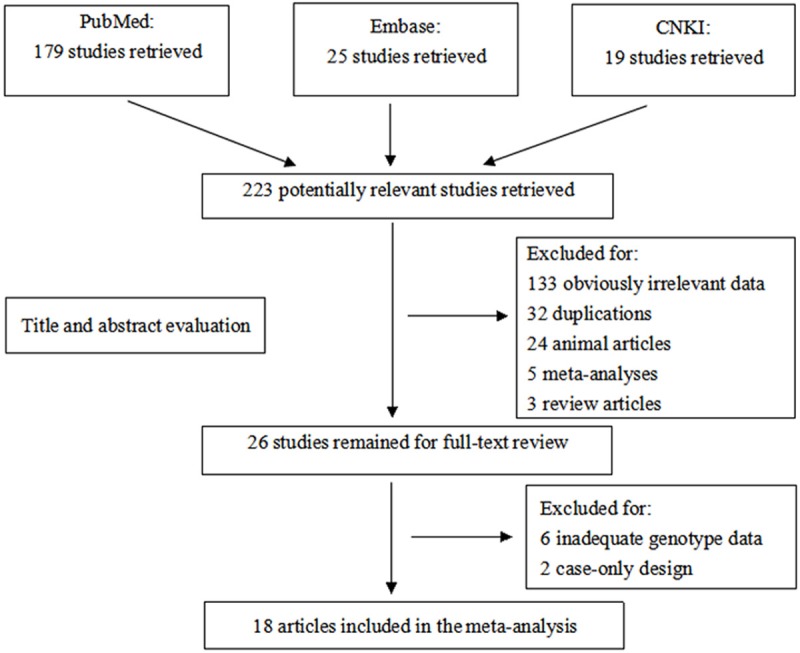
Flow chart for primary selection in this meta-analysis.
Table 1.
Main characteristics of all studies included in the meta-analysis
| First author | Year | Population | Gender | Mean age | Genotyping method | Sample size | Cases | Controls | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Cases | Controls | CC | CT | TT | CC | CT | TT | |||||||
| Gretarsdottir | 2003 | Caucasian | NR | NR | RT-PCR | 642 | 583 | 148 | 315 | 179 | 156 | 290 | 137 | 0.921 |
| Bevan | 2005 | Caucasian | F/M | 65 ± 12.5 | PCR | 726 | 923 | 154 | 360 | 212 | 214 | 464 | 245 | 0.842 |
| Lohmussaar | 2005 | Caucasian | F/M | 65 ± 18.2 | MALDI-TOF | 598 | 728 | 128 | 296 | 174 | 146 | 366 | 216 | 0.688 |
| Saleheen | 2005 | Asian | F/M | 62.4 ± 12.4 | PCR | 170 | 203 | 76 | 57 | 37 | 86 | 78 | 39 | 0.007 |
| Woo | 2006 | Caucasian | F/M | 69 | TaqMan | 352 | 268 | 80 | 175 | 97 | 58 | 134 | 76 | 0.941 |
| Kuhlenbaumer | 2006 | Caucasian | F/M | 66.9 ± 14.6 | RT-PCR | 1014 | 1564 | 216 | 505 | 293 | 353 | 759 | 452 | 0.313 |
| Staton | 2006 | Caucasian | F/M | 67.3 ± 11.7 | NR | 151 | 164 | 45 | 72 | 34 | 36 | 72 | 56 | 0.164 |
| Lin | 2007 | Asian | NR | NR | NR | 180 | 210 | 120 | 52 | 8 | 149 | 54 | 7 | 0.447 |
| Lovkvist | 2008 | Caucasian | F/M | 73.6 | RT-PCR | 929 | 394 | 187 | 473 | 269 | 72 | 208 | 114 | 0.177 |
| Xue | 2009 | Asian | F/M | 60.8 ± 9.2 | PCR-RFLP | 424 | 887 | 12 | 119 | 293 | 26 | 257 | 604 | 0.832 |
| Matsushita | 2009 | Asian | F/M | 69.6 | NR | 1092 | 3847 | 826 | 248 | 18 | 2840 | 950 | 57 | 0.025 |
| Sun | 2009 | Asian | F/M | 73.2 ± 9.4 | RT-PCR | 646 | 761 | 439 | 182 | 25 | 539 | 202 | 20 | 0.837 |
| Hsieh | 2009 | Asian | F/M | 70 ± 11 | DS | 108 | 280 | 71 | 31 | 6 | 187 | 81 | 12 | 0.398 |
| Li | 2010 | Asian | F/M | 63.88 ± 7.36 | PCR-RFLP | 371 | 371 | 170 | 117 | 84 | 160 | 141 | 70 | < 0.10 |
| Kalita | 2011 | Asian | F/M | 61 | PCR | 148 | 188 | 51 | 77 | 20 | 72 | 92 | 24 | 0.520 |
| Zhang | 2012 | Asian | F/M | 59.9 | PCR | 226 | 220 | 157 | 58 | 11 | 129 | 78 | 13 | 0.791 |
| He | 2012 | Asian | F/M | 61 ± 10 | PCR-RFLP | 400 | 400 | 276 | 108 | 16 | 286 | 103 | 11 | 0.640 |
| He | 2013 | Asian | F/M | 36.5 ± 6.4 | PCR-RFLP | 186 | 232 | 84 | 82 | 20 | 168 | 58 | 6 | 0.712 |
F: female; M: male; PCR: polymerase chain reaction; PCR-RFLP: PCR-restriction fragment length polymorphism; RT-PCR: real-time PCR; MALDI-TOF: matrix-assisted laser desorption/ionization time-of-flight; DS: direct sequencing; HWE: Hardy-Weinberg equilibrium.
Quantitative synthesis
Table 2 summarizes for each of the studies the P value for heterogeneity and ORs with 95% CIs for the association between SNP 87 and ischemic stroke risk assuming the homozygote and heterozygote genotypes, the dominant, recessive and allele genetic models. The pooled effect estimates among all studies showed that none of the genetic models were significantly associated with an increased or decreased risk of ischemic stroke. However, a tend to increase ischemic stroke risk was indicated under all of the contrast models, and the tendency was more pronounced under the homozygote genotypes (TT vs.CC, OR, 1.05, 95% CI, 0.97-1.14, fixed-effects), the recessive model (TT vs. CT + CC, OR, 1.05, 95% CI, 0.98-1.13, fixed-effects), and the allele model (T vs. C, OR, 1.03, 95% CI, 0.97-1.09, random-effects). Meanwhile, neither the stratified analyses according to ethnicity nor according to sample size did we find the association of SNP 87 and ischemic stroke was significant (Table 2; Figures 2, 3, 4, 5 and 6).
Table 2.
Meta-analysis results for PDE4D SNP 87 and ischemic stroke risk
| Variables (studies) | TT vs. CC | TT + CT vs. CC | TT vs. CT + CC | T vs. C | CT vs. CC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| OR (95% CI) | P h | I2 | OR (95% CI) | P h | I2 | OR (95% CI) | P h | I2 | OR (95% CI) | P h | I2 | OR (95% CI) | P h | I2 | |
| Total (18) | 1.05 (0.97, 1.14) | 0.292 | 13.5% | 1.01 (0.96, 1.06) | 0.293 | 13.5% | 1.05 (0.98, 1.13) | 0.417 | 3.2% | 1.03 (0.97, 1.09) | 0.015 | 47.1% | 1.00 (0.95, 1.06) | 0.425 | 2.5% |
| Ethnicity | |||||||||||||||
| Caucasian (7) | 1.02 (0.92, 1.12) | 0.732 | 0.0% | 1.01 (0.94, 1.07) | 0.987 | 0.0% | 1.02 (0.93, 1.12) | 0.498 | 0.0% | 1.01 (0.96, 1.06) | 0.695 | 0.0% | 1.01 (0.93, 1.09) | 0.995 | 0.0% |
| Asian (11) | 1.11 (0.97, 1.28) | 0.125 | 34.2% | 1.02 (0.94, 1.10) | 0.045 | 46.3% | 1.13 (0.99, 1.28) | 0.366 | 8.2% | 1.07 (0.95, 1.21) | 0.002 | 64.0% | 1.00 (0.91, 1.09) | 0.080 | 40.3% |
| Sample size | |||||||||||||||
| 500-1000 (5) | 1.06 (0.94, 1.20) | 0.582 | 0.0% | 1.02 (0.95, 1.10) | 0.911 | 0.0% | 1.08 (0.96, 1.21) | 0.616 | 0.0% | 1.03 (0.97, 1.10) | 0.669 | 0.0% | 1.02 (0.93, 1.11) | 0.952 | 0.0% |
| < 500 (11) | 1.05 (0.92, 1.20) | 0.081 | 40.1% | 1.02 (0.94, 1.11) | 0.067 | 42.4% | 1.05 (0.93, 1.19) | 0.158 | 30.3% | 1.05 (0.93, 1.19) | 0.002 | 64.5% | 1.01 (0.91, 1.13) | 0.118 | 35.1% |
| > 1000 (2) | 1.03 (0.87, 1.23) | 0.847 | 0.0% | 0.98 (0.89, 1.08) | 0.369 | 0.0% | 1.01 (0.86, 1.18) | 0.708 | 0.0% | 0.99 (0.91, 1.07) | 0.393 | 0.0% | 0.97 (0.88, 1.08) | 0.322 | 0.0% |
Ph: p value of heterogeneity test; I2: heterogeneity (%); CI: confidence interval; OR, odds ratio.
Figure 2.
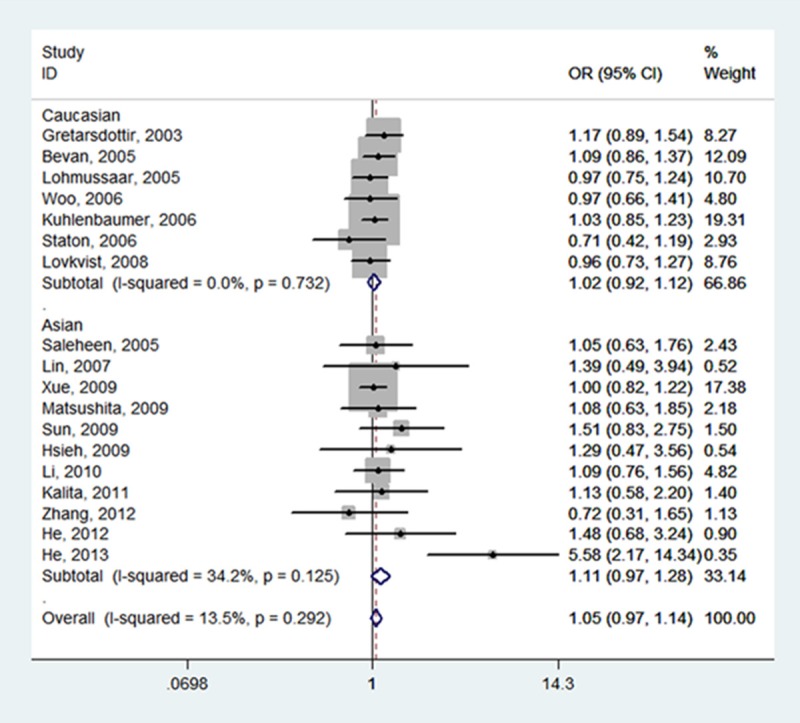
Forest plot of ischemic stroke risk associated with PDE4D SNP 87 stratified by ethnicity under TT vs. CC model. The boxes and horizontal lines represent the OR and the corresponding 95% CI. The area of the boxes indicates the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
Figure 3.
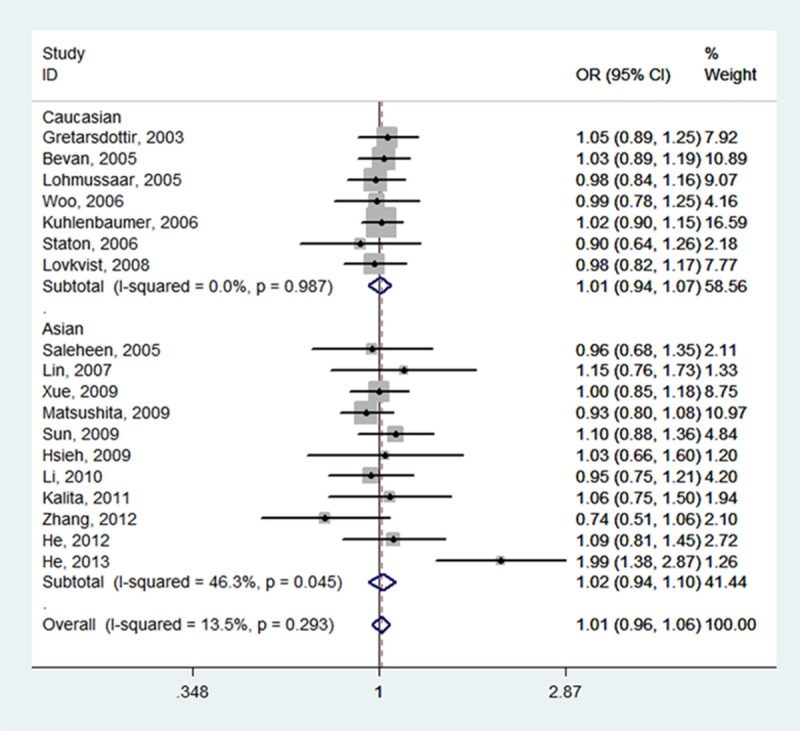
Forest plot of ischemic stroke risk associated with PDE4D SNP 87 stratified by ethnicity under TT + CT vs. CC model. The boxes and horizontal lines represent the OR and the corresponding 95% CI. The area of the boxes indicates the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
Figure 4.
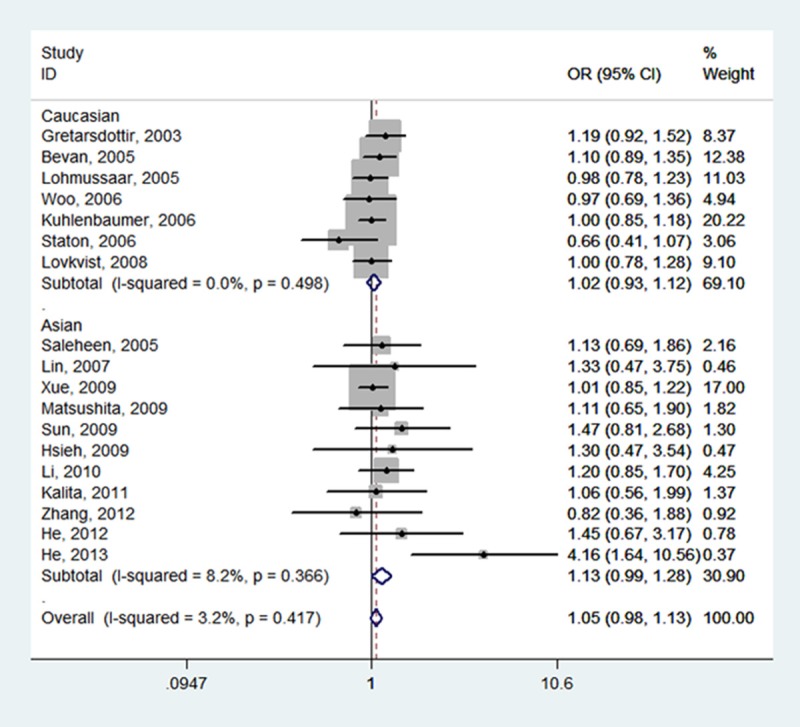
Forest plot of ischemic stroke risk associated with PDE4D SNP 87 stratified by ethnicity under TT vs. CT + CC model. The boxes and horizontal lines represent the OR and the corresponding 95% CI. The area of the boxes indicates the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
Figure 5.
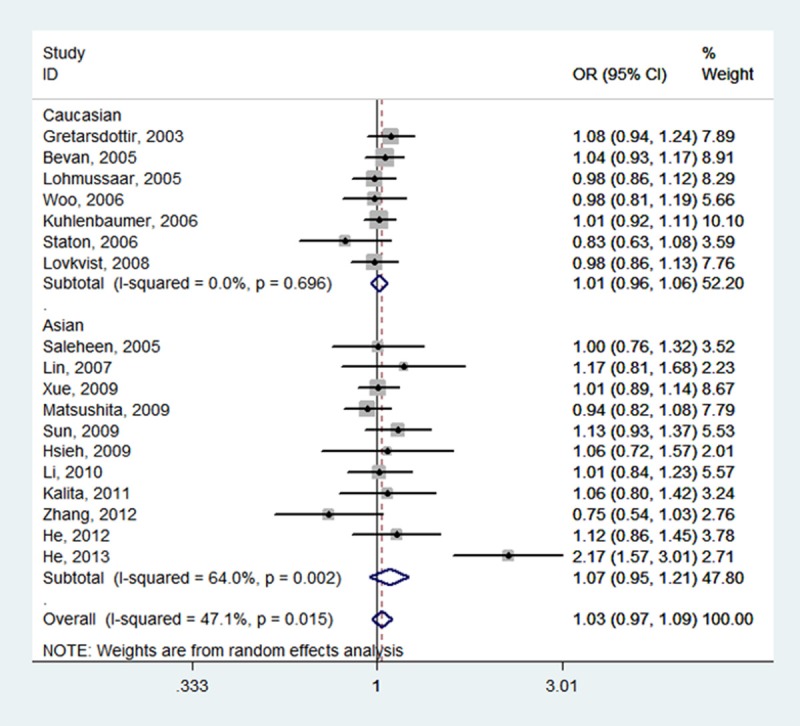
Forest plot of ischemic stroke risk associated with PDE4D SNP 87 stratified by ethnicity under T vs. C model. The boxes and horizontal lines represent the OR and the corresponding 95% CI. The area of the boxes indicates the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
Figure 6.
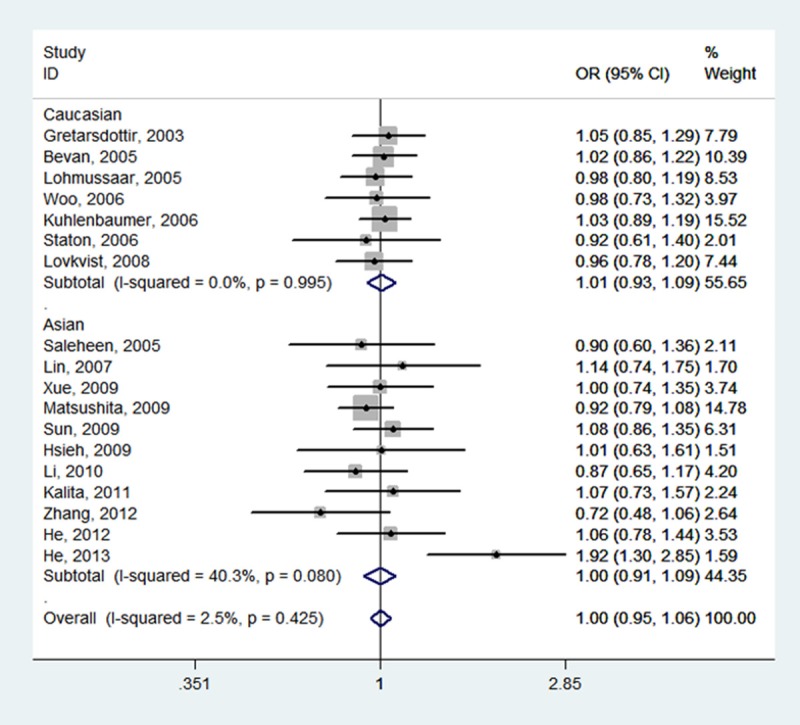
Forest plot of ischemic stroke risk associated with PDE4D SNP 87 stratified by ethnicity under CT vs. CC model. The boxes and horizontal lines represent the OR and the corresponding 95% CI. The area of the boxes indicates the weight (inverse of the variance). The diamond corresponds to the summary OR and 95% CI.
Test of heterogeneity and sensitivity analysis
No significant heterogeneity was detected under all genetic models except for the allele model (P = 0.015, I2 = 47.1) (Table 2). Subgroup analyses and sensitivity analyses together identified the study by He et al. [34] was the source of the moderate heterogeneity. Excluding this study obviously diminished the heterogeneity and increased the homogeneity among the remaining studies (P = 0.816, I2 = 0.0%). Nonetheless, the corresponding pooled ORs were not quantitatively altered by removing any single study.
Publication bias
Both Begg’s funnel plots and Egger’s test were performed to determine the publication bias of the studies included in this meta-analysis. The shapes of the funnel plot did not indicate obvious asymmetry under any genetic model (Figure 7), and the statistical evidence of Egger’s test suggested no significant publication bias in the meta-analysis (TT vs. CC: P = 0.905).
Figure 7.
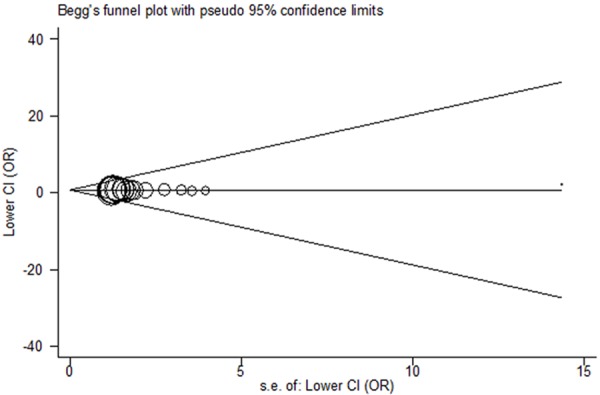
Begg’s funnel plot for PDE4D SNP 87. Log OR is plotted versus standard error of Log OR for each included study. Each circle dot represents a separate study for the indicated association between PDE4D SNP 87 and ischemic stroke risk under TT vs. CC model.
Discussion
PDE4D selectively degrading cyclic AMP that has effects on the vasculature and nervous system has been implicated to play a pivotal role in the etiology of stroke [18,35]. Since the first study addressing the associations of PDE4D SNPs and ischemic stroke risk was reported [18], an increasing body of research has been subsequently published to assess how the PDE4D gene SNPs, especially SNP 87, act in the progress of ischemic stroke [19-21,31-34]. The investigations failed to reach a consensus on the association between PDE4D SNP 87 and the risk of ischemic stroke due to the small sample sizes. This promoted us to conduct the current meta-analysis, in an attempt to assess the controversial association through pooling the data supplied by the eligible studies.
This meta-analysis examined all the available data on the association between PDE4D SNP 87 and ischemic stroke risk, including a total of 8,363 cases and 12,223 control subjects. Although the pooled results showed that SNP 87 was not associated with the risk of ischemic stroke, the trend to an increased risk of developing ischemic stroke was observed. The stratified analyses based on ethnicity and sample size did not suggest any statistical evidence for a significant association.
By comparing the results of the meta-analyses published before and the present study, we found an implication of great interest. The initial meta-analysis published in 2008 investigating six SNPs (SNP 26, 45, 56, 83, 87, and 89) of PDE4D gene suggested that no SNPs examined in PDE4D showed a robust and reproducible association to ischemic stroke [36]. Similar results were observed in a following meta-analysis by Domingues-Montanari and the co-authors [37]. Later, two studies based on 7 datasets and 13 datasets respectively revealed a significant association between PDE4D SNP 83 and ischemic stroke, but not SNP 87 [38,39]. Taken together, a uniformly non-significant association was indicated in the four meta-analyses. However, we could draw an interesting conclusion from the five meta-analyses that the more studies were included, the higher risk of developing ischemic stroke was indicated in the results. This implies that the modification effects of SNP 87 are necessary to be further validated in future larger studies.
Heterogeneity is an important index to evaluate the quality of a meta-analysis. Despite some differences in certain aspects such as study designs, inclusion criteria for participants, sample sizes, and ethnic backgrounds, we only observed moderate between-study heterogeneity for the allele model, but not for the rest of four genetic models. In addition, when we deleted the study resulting in the heterogeneity, no significant alternation occurred in the corresponding ORs with 95% CIs, suggesting our results were statistically reliable.
Some limitations need to be considered when interpreting the results. To start with, although we have included all available data on the association of SNP 87 and ischemic stroke, the sample size does not appear to be sufficiently large enough to detect the potential relationship. Moreover, ischemic stroke is a multifactorial disease that is caused by the interplay of genetic and environmental factors, but we are unable to assess the effects of gene-environment interaction on ischemic stroke on account of lacking related data. Finally, only the published data were considered in this meta-analysis, and the unpublished or the ongoing studies were not included, which may have introduced selection bias.
In conclusion, we found from this meta-analysis that SNP 87 of PDE4D gene was not an independent risk factor for ischemic stroke risk. Further larger rigorous genetic association studies that take gene-gene and gene-environment interaction into consideration are needed to provide conclusive evidence for the association between PDE4D SNP 87 and ischemic stroke.
Disclosure of conflict of interest
None.
References
- 1.Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–23. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- 2.Gandolfo C, Conti M. Stroke in young adults: epidemiology. Neurol Sci. 2003;24(Suppl 1):S1–3. doi: 10.1007/s100720300024. [DOI] [PubMed] [Google Scholar]
- 3.Caplan LR. Diagnosis and treatment of ischemic stroke. JAMA. 1991;266:2413–8. [PubMed] [Google Scholar]
- 4.Hassan A, Markus HS. Genetics and ischaemic stroke. Brain. 2000;123:1784–812. doi: 10.1093/brain/123.9.1784. [DOI] [PubMed] [Google Scholar]
- 5.Dichgans M. Genetics of ischaemic stroke. Lancet Neurol. 2007;6:149–61. doi: 10.1016/S1474-4422(07)70028-5. [DOI] [PubMed] [Google Scholar]
- 6.Dichgans M, Markus HS. Genetic association studies in stroke: methodological issues and proposed standard criteria. Stroke. 2005;36:2027–31. doi: 10.1161/01.STR.0000177498.21594.9e. [DOI] [PubMed] [Google Scholar]
- 7.Gretarsdottir S, Sveinbjörnsdottir S, Jonsson HH, Jakobsson F, Einarsdottir E, Agnarsson U, Shkolny D, Einarsson G, Gudjonsdottir HM, Valdimarsson EM, Einarsson OB, Thorgeirsson G, Hadzic R, Jonsdottir S, Reynisdottir ST, Bjarnadottir SM, Gudmundsdottir T, Gudlaugsdottir GJ, Gill R, Lindpaintner K, Sainz J, Hannesson HH, Sigurdsson GT, Frigge ML, Kong A, Gudnason V, Stefansson K, Gulcher JR. Localization of a susceptibility gene for common forms of stroke to 5q12. Am J Hum Genet. 2002;70:593–603. doi: 10.1086/339252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–9. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Deng C, Bugaj-Gaweda B, Kwan M, Gunwaldsen C, Leonard C, Xin X, Hu Y, Unterbeck A, De Vivo M. Cloning and characterization of novel PDE4D isoforms PDE4D6 and PDE4D7. Cell Signal. 2003;15:883–91. doi: 10.1016/s0898-6568(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 10.Ariga M, Neitzert B, Nakae S, Mottin G, Bertrand C, Pruniaux MP, Jin SL, Conti M. Nonredundant function of phosphodiesterases 4D and 4B in neutrophil recruitment to the site of inflammation. J Immunol. 2004;173:7531–8. doi: 10.4049/jimmunol.173.12.7531. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, Arauz E, Krzanowski JJ, Fitzpatrick DF, Polson JB. Synergistic interactions between selective pharmacological inhibitors of phosphodiesterase isozyme families PDE III and PDE IV to attenuate proliferation of rat vascular smooth muscle cells. Biochem Pharmacol. 1994;48:827–35. doi: 10.1016/0006-2952(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 12.Palmer D, Tsoi K, Maurice DH. Synergistic inhibition of vascular smooth muscle cell migration by phosphodiesterase 3 and phosphodiesterase 4 inhibitors. Circ Res. 1998;82:852–61. doi: 10.1161/01.res.82.8.852. [DOI] [PubMed] [Google Scholar]
- 13.Woo D, Kaushal R, Kissela B, Sekar P, Wolujewicz M, Pal P, Alwell K, Haverbusch M, Ewing I, Miller R, Kleindorfer D, Flaherty M, Chakraborty R, Deka R, Broderick J. Association of Phosphodiesterase 4D with ischemic stroke: a population-based case-control study. Stroke. 2006;37:371–6. doi: 10.1161/01.STR.0000198843.72824.0a. [DOI] [PubMed] [Google Scholar]
- 14.Staton JM, Sayer MS, Hankey GJ, Attia J, Thakkinstian A, Yi Q, Cole VJ, Baker R, Eikelboom JW. Association between phosphodiesterase 4D gene and ischaemic stroke. J Neurol Neurosurg Psychiatry. 2006;77:1067–9. doi: 10.1136/jnnp.2006.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleheen D, Bukhari S, Haider SR, Nazir A, Khanum S, Shafqat S, Anis MK, Frossard P. Association of phosphodiesterase 4D gene with ischemic stroke in a Pakistani population. Stroke. 2005;36:2275–7. doi: 10.1161/01.STR.0000182242.59466.ee. [DOI] [PubMed] [Google Scholar]
- 16.Lohmussaar E, Gschwendtner A, Mueller JC, Org T, Wichmann E, Hamann G, Meitinger T, Dichgans M. ALOX5AP gene and the PDE4D gene in a central European population of stroke patients. Stroke. 2005;36:731–6. doi: 10.1161/01.STR.0000157587.59821.87. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlenbaumer G, Berger K, Huge A, Lange E, Kessler C, John U, Funke H, Nabavi DG, Stögbauer F, Ringelstein EB, Stoll M. Evaluation of single nucleotide polymorphisms in the phosphodiesterase 4D gene (PDE4D) and their association with ischaemic stroke in a large German cohort. J Neurol Neurosurg Psychiatry. 2006;77:521–4. doi: 10.1136/jnnp.2005.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, Jonsdottir T, Gudmundsdottir T, Bjarnadottir SM, Einarsson OB, Gudjonsdottir HM, Hawkins M, Gudmundsson G, Gudmundsdottir H, Andrason H, Gudmundsdottir AS, Sigurdardottir M, Chou TT, Nahmias J, Goss S, Sveinbjörnsdottir S, Valdimarsson EM, Jakobsson F, Agnarsson U, Gudnason V, Thorgeirsson G, Fingerle J, Gurney M, Gudbjartsson D, Frigge ML, Kong A, Stefansson K, Gulcher JR. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat Genet. 2003;35:131–8. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 19.Bevan S, Porteous L, Sitzer M, Markus HS. Phosphodiesterase 4D gene, ischemic stroke, and asymptomatic carotid atherosclerosis. Stroke. 2005;36:949–53. doi: 10.1161/01.STR.0000162713.06519.41. [DOI] [PubMed] [Google Scholar]
- 20.Lovkvist H, Smith JG, Luthman H, Höglund P, Norrving B, Kristoffersson U, Jönsson AC, Lindgren AG. Ischaemic stroke in hypertensive patients is associated with variations in the PDE4D genome region. Eur J Hum Genet. 2008;16:1117–25. doi: 10.1038/ejhg.2008.62. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita T, Kubo M, Yonemoto K, Ninomiya T, Ashikawa K, Liang B, Hata J, Doi Y, Kitazono T, Ibayashi S, Iida M, Kiyohara Y, Nakamura Y. Lack of association between variations of PDE4D and ischemic stroke in the Japanese population. Stroke. 2009;40:1245–51. doi: 10.1161/STROKEAHA.108.527408. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin HF, Liao YC, Liou CW, Liu CK, Juo SH. The phosphodiesterase 4D gene for early onset ischemic stroke among normotensive patients. J Thromb Haemost. 2007;5:436–8. doi: 10.1111/j.1538-7836.2007.02350.x. [DOI] [PubMed] [Google Scholar]
- 27.Xue H, Wang H, Song X, Li W, Sun K, Zhang W, Wang X, Wang Y, Hui R. Phosphodiesterase 4D gene polymorphism is associated with ischaemic and haemorrhagic stroke. Clin Sci (Lond) 2009;116:335–40. doi: 10.1042/CS20080162. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Huang Y, Chen X, Liu Y, Lu X, Shi Y, Tang W, Yang J, Chen W, Zhao X, Gao L, Li S, Feng G, He L. Association between the PDE4D gene and ischaemic stroke in the Chinese Han population. Clin Sci (Lond) 2009;117:265–72. doi: 10.1042/CS20080471. [DOI] [PubMed] [Google Scholar]
- 29.Munshi A, Babu MS, Kaul S, Shafi G, Anila AN, Alladi S, Jyothy A. Phosphodiesterase 4D (PDE4D) gene variants and risk of ischemic stroke in a South Indian population. J Neurol Sci. 2009;285:142–5. doi: 10.1016/j.jns.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Li N, He Z, Xu J, Liu F, Deng S, Zhang H. Association of PDE4D and IL-1 gene polymorphism with ischemic stroke in a Han Chinese population. Brain Res Bull. 2010;81:38–42. doi: 10.1016/j.brainresbull.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Kalita J, Somarajan BI, Kumar B, Kumar S, Mittal B, Misra UK. Phosphodiesterase 4 D gene polymorphism in relation to intracranial and extracranial atherosclerosis in ischemic stroke. Dis Markers. 2011;31:191–7. doi: 10.3233/DMA-2011-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang XN, Du HB, Wang J, et al. Investigation on the single-nucleotide polymorphism of phosphodiesterase 4D gene in Uygur and Han patients with ischemic stroke in Xinjiang district. J Clin Neurol. 2012:25. [Google Scholar]
- 33.He Y, Bai JY, Song B, Tan S, Chang YS, Li T, Shi CC, Zhang H, Feng QC, Qi H, Song GY, Zheng H, Xu YM. Sex-dependent association of phosphodiesterase 4D gene polymorphisms with ischemic stroke in Henan Han population. Chin Med J (Engl) 2012;125:2255–9. [PubMed] [Google Scholar]
- 34.He Y, Yang DZ, Yu H, Li MY, Feng QC, Zheng H. Genetic variants of phosphodiesterase 4D gene are associated with an enhanced risk for ischemic stroke in young Chinese population. Neurol India. 2013;61:21–5. doi: 10.4103/0028-3886.108131. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Wang Y, Qu Y, Qi R, Lu D, Li C, Yan L. The cAMP-mediated protein kinase signal transduction pathway is involved in the pyrogenic effect of CRH in rats. Chin Med J (Engl) 2001;114:1064–7. [PubMed] [Google Scholar]
- 36.Bevan S, Dichgans M, Gschwendtner A, Kuhlenbäumer G, Ringelstein EB, Markus HS. Variation in the PDE4D gene and ischemic stroke risk: a systematic review and meta-analysis on 5200 cases and 6600 controls. Stroke. 2008;39:1966–71. doi: 10.1161/STROKEAHA.107.509992. [DOI] [PubMed] [Google Scholar]
- 37.Domingues-Montanari S, Fernández-Cadenas I, del Rio-Espinola A, Corbeto N, Krug T, Manso H, Gouveia L, Sobral J, Mendioroz M, Fernández-Morales J, Alvarez-Sabin J, Ribó M, Rubiera M, Obach V, Martí-Fàbregas J, Freijo M, Serena J, Ferro JM, Vicente AM, Oliveira SA, Montaner J. Association of a genetic variant in the ALOX5AP with higher risk of ischemic stroke: a case-control, meta-analysis and functional study. Cerebrovasc Dis. 2010;29:528–37. doi: 10.1159/000302738. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Li X, Li J, Ou R, Sheng W. Meta-analysis of association between variation in the PDE4D gene and ischemic cerebral infarction risk in Asian populations. Neurogenetics. 2010;11:327–33. doi: 10.1007/s10048-010-0235-8. [DOI] [PubMed] [Google Scholar]
- 39.Yoon D, Park SK, Kang D, Park T, Park JW. Meta-analysis of homogeneous subgroups reveals association between PDE4D gene variants and ischemic stroke. Neuroepidemiology. 2011;36:213–22. doi: 10.1159/000327915. [DOI] [PubMed] [Google Scholar]


