Abstract
Objective: The aim of the present study was to investigate whether pterostilbene could modulate the TLR4/NF-κB signaling, reduce neutrophil accumulation and TNF-α induction in an ischemia/reperfusion injured rat heart model. Materials and Methods: Rats were randomly exposed to sham operation, myocardial ischemia and reperfusion (MI/R), MI/R + pterostilbene, MI/R + pterostilbene + L-NAME. And myocardial infarct size, apoptosis, TLR4 expression, NF-κB expression, MPO level and TNF-α level were detected. Results: The results demonstrated that after MI/R, the expressions of myocardial TLR4, NF-κB and caspase-3 increased significantly in ischemia area. Compared with MI/R, pterostilbene significantly attenuated the expressions of TLR4, NF-κB and caspase-3. In addition, it also reduced myeloperoxidase (MPO) levels, both serum and myocardial TNF-α production, myocardial infarct sizes (INF/AAR%) and myocardial apoptosis induced by MI/R. All the effects of pterostilbene were abolished by L-NAME, a nitric oxide synthase inhibitor. Conclusion: These data provide evidence that pterostilbene inhibits TLR4/NF-κB signaling and apoptosis in the rat heart subjected to MI/R, which is associated with NO production.
Keywords: Pterostilbene, ischemia/reperfusion injury, apoptosis, TLR4/NF-κB signaling, neutrophil, TNF-α
Introduction
The inflammatory reaction induced by ischemia/reperfusion is one of the most significant links in the myocardial ischemia-reperfusion injury [1]. In the process of inflammation, various cytokines are released, including tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and IL-8, etc [2]. TNF-α can trigger the inflammatory reaction caused by myocardial ischemia-reperfusion. In addition, vascular endothelial cell injury, and inflammatory cells, such as neutrophils, activated by cytokines and adhesion molecules are also included in inflammation. So TNF-α activity and the amount of neutrophil infiltration can be considered as the indicators of inflammatory reaction. Besides, it has been demonstrated that the TLR4/NF-κB signaling is activated during MI/R, which in turn results in TNF-α induction, and TNF-α in turn triggers the inflammatory reaction during MI/R [3,4].
The TLRs are a family of molecules that play a pivotal role in innate immunity [5,6]. Activation of the corresponding TLRs triggers an inflammatory response, alerting the host to the presence of microbial invasion and initiating an immune response. Recent studies have demonstrated that some TLR family members also alert the host to the presence of tissue damage and become activated by endogenous molecules released from damaged tissues [7,8]. Besides, heparan sulfate, hyaluronic acid and other endogenous molecules have been shown to initiate inflammatory pathways through TLR4 [9-11]. Studies have also suggested that TLR4-mediated signaling induces myocardial dysfunction during MI/R [12].
Pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene, Pte) is a naturally derived compound found primarily in blueberries and Pterocarpus marsupium heartwood [13,14]. It is structurally similar to resveratrol, a compound found in red wine that has comparable antioxidant, anti-inflammatory, and anticarcinogenic properties; however, pterostilbene exhibits increased bioavailability due to the presence of two methoxy groups which cause it to exhibit increased lipophilic and oral absorption [15-17]. Substantial evidence suggests that pterostilbene may have numerous preventive and therapeutic properties in a vast range of human diseases that include neurological, metabolic, and hematologic disorders [18]. Further benefits of pterostilbene have been reported in preclinical trials, in which pterostilbene was shown to be a potent anticancer agent in several malignancies [19]. Though previous study has suggested that Pte has anti-inflammatory effect, its role in mediating inflammation is not well understood. Therefore, the present study aims to investigate the role of TLR4/NF-κB signaling pathway in the anti-inflammatory effect of Pte in a rat model of myocardial ischemia-reperfusion (MI/R).
Materials and methods
Reagents
Pte was purchased from DiBai Chemistry Company (Shanghai, China). MPO assay kit was purchased from JianCheng Bioengineering Institute (Nanjing, China). TNF-α ELISA kit was purchased from R&D Corporation (USA). L-NAME was purchased from Sigma Chemical Co. (St. Louis, MO, USA). BCA protein quantification kit was purchased from Bio-Rad (USA). NF-κB p65 Rabbit polyclonal (ab16502) was purchased from Abcam (USA). Anti-TLR4 antibody (ab13556) and goat anti-rabbit IgG were purchased from Abcam (USA). The Bcl2, Caspase3, antibodies were purchased from Cell Signaling Company, USA.
EnVison + was purchased from Gene Tech (China). Cell death detection kit was purchased from Roche Molecular Biochemicals (Germany).
Animals
Forty adult male Sprague-Dawley rats (250-300 g) were purchased from the Center of Experimental Animal in the Shandong University, China. All animals used in this study were cared for in accordance with the Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health (NIH publication no. 85-23, revised 1996), and all procedures were approved by the Committee of Experimental Animals of the Fourth Military Medical University.
Myocardial ischemia-reperfusion model and experimental protocol
Male Sprague-Dawley rats (250-300 g) were anesthetized i.p. with sodium pentobarbital (Sigma, St. Louis, USA, 40 mg/kg). Myocardial ischemia was produced by exteriorizing the heart with a left thoracic incision followed by a slipknot (5-0 silk) around the left anterior descending coronary artery (LAD). After 30 min of ischemia, the slipknot was released and the animal received 120 min of reperfusion.
Rats were randomly assigned to four experimental groups. There were 10 rats in each group: (1) sham group: silk was drilled underneath the LAD but the LAD was not ligated; (2) MI/R group: LAD was ligated for 30 min and then allowed 120 min reperfusion with receiving vehicle (0.9% NaCl i.v.); (3)MI/R + Pte group: Pte (100 μmol/L, i.v.) was administered 5 min before reperfusion; (4) MI/R + Pte + L-NAME group: L-NAME (1 mmol/L, i.v.), a nitric oxide synthase inhibitor, was administered 20 min before reperfusion. Fifteen minutes after the administration of L-NAME, Pte (100 μmol/L, i.v.) was given.
Assay of myocardial infarct area
Each treated heart was rapidly excised and serially sectioned along the long axis into six slices. This step was followed by incubation in 1% TTC for 10 min at 37°C to demarcate the viable and nonviable myocardium. The six myocardial sections were weighed individually. An observer who was blinded to the protocol assessed the percentage of infarcted area using computer-assisted planimetry (OPTIMAS v5.2).
Determination of myocardial apoptosis
At the end of reperfusion, myocardial apoptosis was analyzed by terminal deoxynu-cleotidyl transferase dUTP nick end labeling assay (TUNEL) using an in situ cell death detection kit. A doublestaining technique was used, in brief, TUNEL staining for apoptotic cell nuclei and 4,6-diamino-2-phenylindole (DAPI) staining for all myocardial cell nuclei as described previously [20]. The index of apoptosis was expressed by the number of positively stained apoptotic myocytes/the total number of myocytes counted ×100%.
Immunohistochemical analysis of myocardial TLR4 expression
Before immunohistochemical examination, 3 μm slices from pretreated myocardium tissue were placed in a bathing solution of 3% H2O2 and 60% methanol PBS for 30 min and then treated with 0.01 mol/L sodium citrate buffer at 95°C in a microwave oven for 13 min (antigen retrieval). Thereafter, specimens were treated with 5% normal goat serum and 5% bovine serum albumin in PBS. Before each step, sections were rinsed three times in PBS buffer. Incubation with primary Anti-TLR4 antibodies was performed in a PBS-based solution of 1% bovine serum albumin for 12 h at 4°C in the recommended dilutions. After rinsing with PBS, sections were incubated with the corresponding secondary biotinylated goat anti-rabbit antibodies EnVison + for 1 h at room temperature. A streptavidin/horseradish peroxidase complex was then applied as a detection system (1:100 dilutions) for 1 h. Finally, staining was developed with 3,3-diaminobenzidine tetra-hydrochloride in 0.05 mol/L Tris-HCl buffer and 0.1% H2O2. Negative control sections were incubated without the primary antibody. All dates in this study were analyzed by software “Image Pro Plus” (Media Cybernetics Corporation, DC).
Western blotting analysis of myocardial NF-κB, Bcl-2 and caspase-3 expression
At the end of the reperfusion period, the protein samples were prepared. Briefly, the cardiac tissues were homogenized in lysis buffer containing 50 mmol/L Tris-HCl (pH 7.3), 150 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L dithiothreitol, 1% Triton X-100, and 1% protease inhibitor cocktail. The lysates were centrifuged for 15 min at 12,000×g, and the resulting supernatant was transferred to a new tube and stored at -70°C. The protein concentrations were determined using a BCA protein assay kit, and the proteins were separated by electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked for 1 h in Tris-buffered saline and Tween 20 (TBST, pH 7.6) that contained 5% non-fat dry milk and then incubated overnight at 4°C with antibodies against NF-κB (1:500 dilution), Bcl-2 (1:1000 dilution) and caspase-3 (1:1000 dilution) followed by washes with TBST. The membranes were then probed with goat anti-rabbit IgG secondary antibody (1:5,000 dilutions) at room temperature for 90 min, followed by washes with TBST. The blots were visualized with ECL-Plus reagent and were quantified using the Quantity One software package (Bio-Rad Laboratories, UK).
Determination of myeloperoxidase (MPO) level
After reperfusion, the myocardial tissue was placed at -70°C for preservation. MPO test kit was used to detect level of MPO in the myocardial tissue according to manufacturer’s instruction.
Detection of TNF-α level
After reperfusion, the levels of TNF-α in myocardial tissue homogenate and serum were detected in strict accordance with manufacturer’s instructions. BCA kit was used to detect the protein quantization.
Statistical analysis
Data is presented as means ± SEM. The significance of differences among groups was evaluated by a Student’s t-test for unpaired data or Dunnett’s t-test for multiple comparisons preceded by one-way analysis of variance (ANOVA). For all test, P < 0.05 was considered as statistically significant.
Results
Pterostilbene reduced the myocardial infarction area induced by MI/R
MI/R induced a significant infarction area. Compared with the MI/R group, pterostilbene reduced myocardial infarction area significantly. The effect of pterostilbene was blocked by L-NAME, a nitric oxide synthase (NOS) inhibitor (Figure 1).
Figure 1.
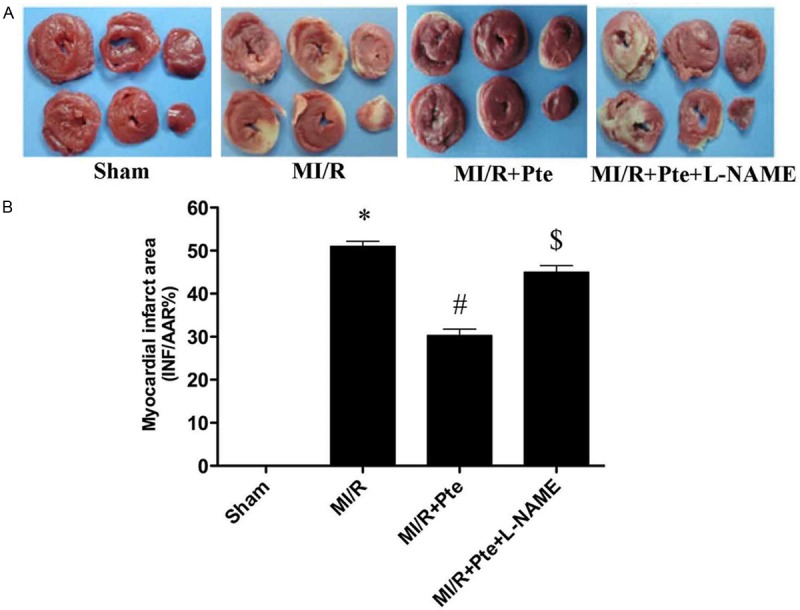
The comparison of myocardial infarct size in each group. A: Representative TTC staining of samples from rat ventricles subjected to different treatment groups; B: Quantitative analysis of myocardial infarct sizes (INF/AAR%). *P < 0.05 vs. Sham, #P < 0.05 vs. MI/R, $P < 0.05 vs. MI/R + Pte. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Pterostilbene reduced myocardial apoptosis in the heart subjected to MI/R
In the sham group, a very low level of TUNEL-positive staining was detected. A significant number of TUNEL-positive cells were observed in the MI/R group. Administration of pterostilbene exerted a significant anti-apoptotic effect, which was abolished by L-NAME (Figure 2).
Figure 2.
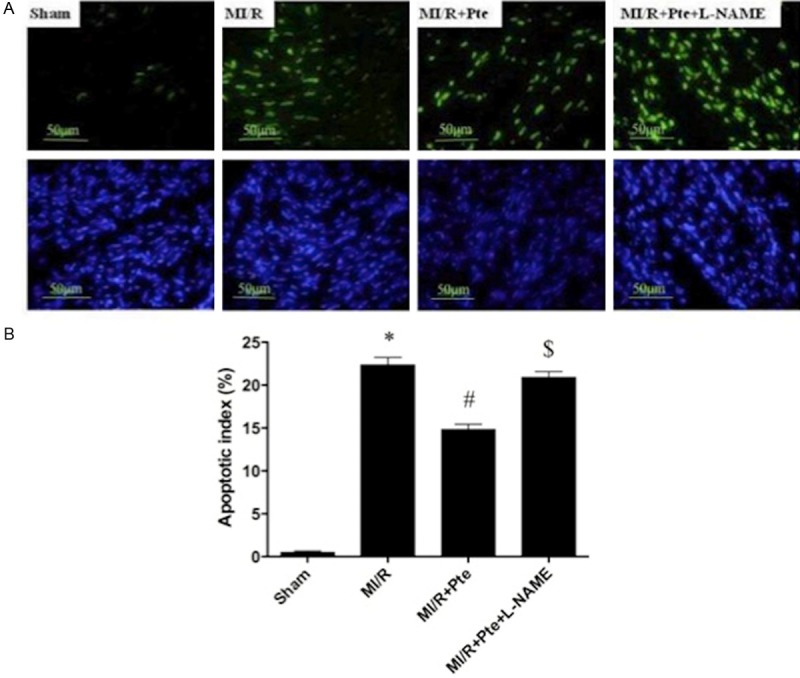
Essay of apoptosis. A: TUNEL staining; B: Quantitative analysis of percentage of cardiomyocyte apoptosis. *P < 0.05 vs. Sham, #P < 0.05 vs. MI/R, $P < 0.05 vs. MI/R + Pte. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Pterostilbene reduced TLR4 protein expression in the heart subjected to MI/R
In the MI/R group, immunohistochemical analysis showed that the expression of TLR4 highly increased compared with the sham group. With the administration of pterostilbene, the expression of TLR4 was significantly reduced, which was abolished by L-NAME (Figure 3).
Figure 3.
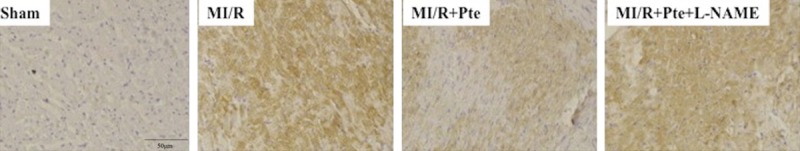
Immunohistochemical analysis of myocardial TLR4 expression. TLR4 is brown staining. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Pterostilbene decreased NF-κB expression in the heart subjected to MI/R
To further study the effect of pterostilbene on TLR4 mediated signaling, NF-κB, a downstream molecule of TLR4 was determined. The results from Western blotting showed that the expression of NF-κB was up-regulated in MI/R group. Administration of pterostilbene significantly decreased NF-κB expression, the effect of pterostilbene was abolished by L-NAME (Figure 4).
Figure 4.
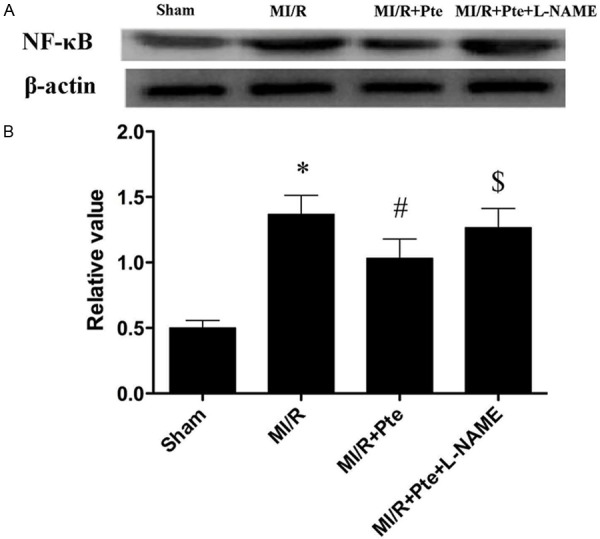
Effect of pterostilbene on NF-κB expression. A: NF-κB expression detected by Western blot. B: Quantitative analysis of NF-κB. *P < 0.05 vs. Sham, #P < 0.05 vs. MI/R, $P < 0.05 vs. MI/R + Pte. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Effects of pterostilbene on expressions of Bcl2 and Caspase3 in MI/R
MI/R significantly decreased the expression of Bcl-2 (P < 0.05). However, pterostilbene increased Bcl-2 expression (P < 0.05), which was abolished by L-NAME (P < 0.05). In contrast, MI/R significantly increased the expression of caspase-3 (P < 0.05). The expression of caspase-3 was decreased by pterostilbene treatment (P < 0.05), which was blocked by L-NAME (P < 0.05) (Figure 5).
Figure 5.
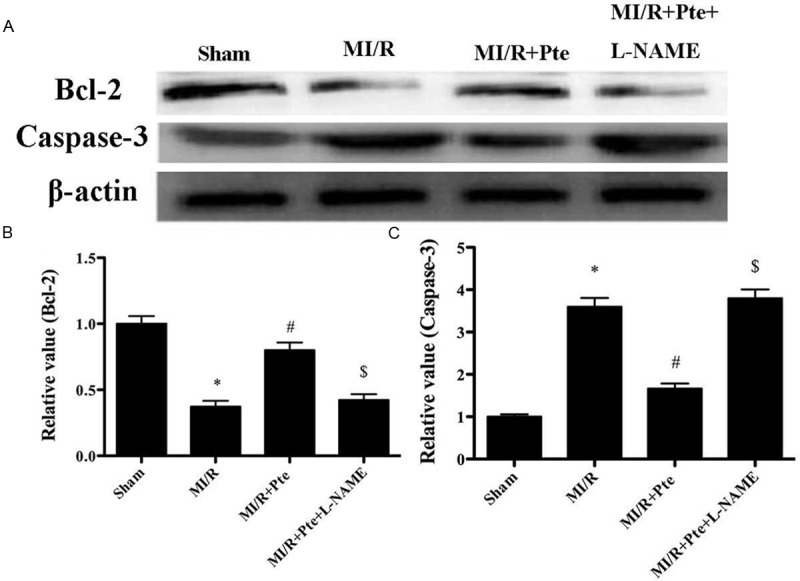
Effects of pterostilbene on the expressions of Bcl-2 and caspase-3 in MI/R. A: Bcl-2 and caspase-3 expression detected by Western blot. B: Quantitative analysis of Bcl-2 and caspase-3 expression. *P < 0.05 vs. Sham, #P < 0.05 vs. MI/R, $P < 0.05 vs. MI/R + Pte. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Pterostilbene inhibited neutrophil infiltration in MI/R tissue
Neutrophil contains a certain amount of myeloperoxidase (MPO), accounting for 5% of dry cell weight. So the activity of MPO in the myocardium can be considered as the indication of neutrophil infiltration. As shown in Figure 6, the MPO activity in the sham group was relatively lower, while the MPO activity in MI/R group was significantly increased. Pterostilbene significantly decreased myocardial MPO activity, while L-NAME attenuated the effect of pterostilbene.
Figure 6.
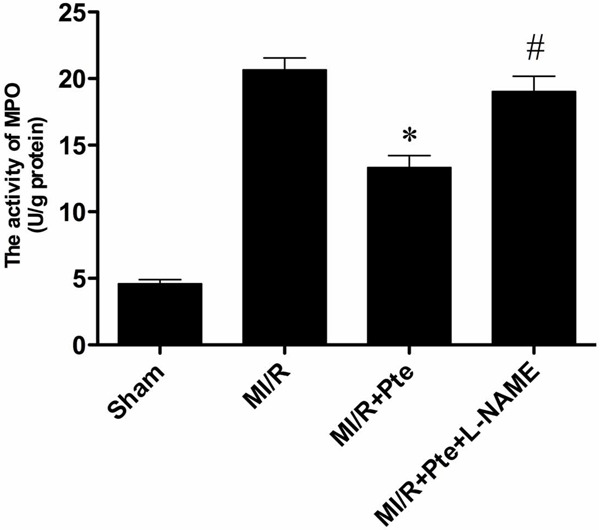
The comparison of MPO activity in each group. Compared with MI/R group, the MPO activity in MI/R + Pte group reduced significantly. L-NAME eliminated the effect of pterostilbene. *P < 0.05 vs. MI/R group; #P < 0.05 vs. MI/R + Pte group. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Pterostilbene reduced TNF-α level in serum and MI/R tissue
The MI/R injury results in production of large amount of TNF-α. Thus, myocardial and serum TNF-α levels were examined. In Figure 7, compared with the MI/R group, pterostilbene significantly decreased the levels of TNF-α in both myocardium and serum, which was eliminated by L-NAME.
Figure 7.
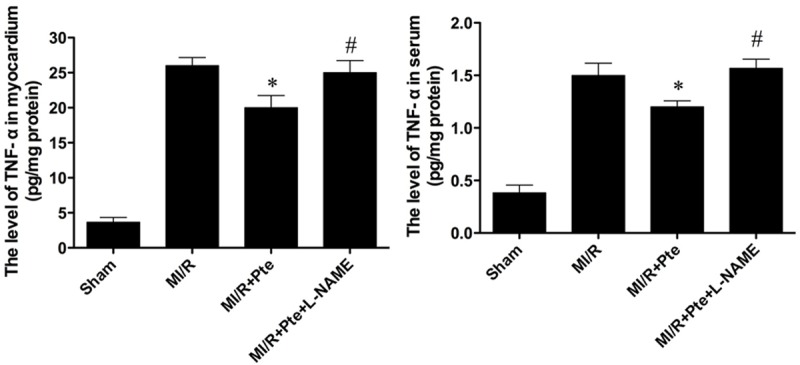
The comparison of levels of TNF-α in myocardium and serum in each group. Compared with the MI/R group, pterostilbene reduced TNF-α level significantly. L-NAME abolished the effect of pterostilbene. *P < 0.05 vs. MI/R group; #P < 0.05 vs. MI/R + Res group. Sham: sham group; MI/R: MI/R group; MI/R + Pte: MI/R + pterostilbene group; MI/R + Pte + L-NAME: MI/R + pterostilbene + L-NAME group, n=10 in each group.
Discussion
The major findings in the present study are: (1) Pte attenuates inflammatory reaction induced by I/R injury by inhibiting TLR4/NF-κB signaling. (2) Pte attenuates inflammatory reaction induced by I/R injury through inhibiting neutrophil infiltration and TNF-α production. (3) NO may play an important role in the protective effect of Pte.
Inflammatory reaction plays an important role in myocardial ischemia/reperfusion injury (1). The release of inflammatory cytokines and the aggregation and infiltration of inflammatory cells are the key steps in inflammation [21].
TNF-α is secreted mainly by macrophages, which is likely to promote inflammatory cascade by increasing releases of other proinflammatory cytokines and influencing neutrophil recruitment [22]. TNF-α, as an important cytokine in inflammation, plays an important role of initiation in the inflammation induced by MI/R [3]. TNF-α can induce the release of other inflammatory mediators, increase the expression of cell adhesion factor, and promote neutrophil adhesion to endothelial cells. In addition, TNF-α has a negative inotropic effect, which can inhibit myocardial contractility, and lower blood pressure. TNF-α can also induce cardiomyocyte apoptosis and participate in ventricular remodeling [23]. Previous studies suggest that the level of TNF-α increases significantly after MI/R [24], while the administration of TNF-α monoclonal antibody attenuated edema, and is conducive to cardiac function recovery [25].
MI/R injury seems to be induced in part by neutrophil activation. The underlying mechanisms include: (1) Cell damage caused by the release of oxygen free radicals, proteolytic enzymes, and cytotoxic substances. (2) The released inflammatory mediators cause vascular endothelial cell damage, increased vascular permeability, and edema. (3) Further activation of inflammatory cells increase further the inflammatory response [26]. (4) Neutrophil adhesion to vascular endothelium and small blood vessels occlusion result in no-reflow phenomenon. Previous studies have demonstrated the link between neutrophil and ischemia/reperfusion injury. Removal of neutrophils or drug inhibition of neutrophil activity has been shown to reduce ischemia/reperfusion injury [27,28]. In the present study, we found that neutrophil accumulation and TNF-α production in the MI/R group increased significantly. And pterostilbene reduces neutrophil accumulation and TNF-α production, indicating that pterostilbene inhibits neutrophil accumulation and TNF-α production and therefore it attenuated neutrophil-mediated ischemia/reperfusion injury.
TLRs are present both in immune and nonimmune cells, and their expression is rapidly altered in response to pathogens, cytokines, and environmental stressors [29]. Roles of TLRs, particularly TLR4 are responsible for the genesis of a variety of cardiovascular disorders as well as activation of NF-κB and inflammatory cytokines such as TNF-α, leading to cell death [30,31]. TLR4 is expressed normally in many tissues including the heart, where the receptor is up-regulated in response to ischemia. TLR4 expression levels in cardiomyocytes are enhanced by either LPS or IL-1 [32]. TLR4-mediated signaling has been well established, it is understood that up-regulation of TLR4 results in activation of NF-κB in cardiomyocytes. This can augment the release of cytokines including TNF-α, IL-1 and MCF as well as the infiltration of leukocytes [33].
It is believed that NO is closely related to MI/R induced inflammation [34]. Endothelial-derived NO can inhibit cell adhesion factors, such as P-selectin and ICAM-1 levels, thereby inhibiting leukocyte adhesion and inward membrane migration [35]. Endothelial-derived NO can also inhibit the expression of TNF-α and other pro-inflammatory factors. It can also increase levels of IL-10 and other anti-inflammatory factor, and indirectly inhibit inflammatory cells to aggregation of local inflammation, thereby reducing the inflammatory response [36]. In the present study, when L-NAME, a nitric oxide synthase inhibitor, was added, the protective effect of Pte was abolished, suggesting that NO plays a pivotal role in the protective role of Pte.
In conclusion, the present study demonstrates that pterostilbene attenuates inflammation induced by myocardial ischemia/reperfusion injury through TLR4/NF-κB signaling pathway, and pterostilbene decreases apoptosis induced by myocardial ischemia/reperfusion injury. The protective effects of pterostilbene are closely associated with the increase of NO production and the inhibition of neutrophil infiltration and TNF-α production.
Acknowledgements
This study was supported by the Independent Innovation Foundation of Shandong University, IIFSDU, NO. 2012TS171 and the Research Award Fund for outstanding young scientists of Shandong Province in China, NO.2006BS03014.
Disclosure of conflict of interest
None.
References
- 1.Xiong J, Xue FS, Yuan YJ, Wang Q, Liao X, Wang WL. Cholinergic anti-inflammatory pathway: a possible approach to protect against myocardial ischemia reperfusion injury. Chin Med J (Engl) 2010;123:2720–2726. [PubMed] [Google Scholar]
- 2.Naidu BV, Farivar AS, Woolley SM, Grainger D, Verrier ED, Mulligan MS. Novel broad-spectrum chemokine inhibitor protects against lung ischemia-reperfusion injury. J Heart Lung Transplant. 2004;23:128–134. doi: 10.1016/s1053-2498(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 3.Batista ML Jr, Rosa JC, Lopes RD, Lira FS, Martins E Jr, Yamashita AS, Brum PC, Lancha AH Jr, Lopes AC, Seelaender M. Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine. 2010;49:102–108. doi: 10.1016/j.cyto.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Shimamoto A, Chong AJ, Yada M, Shomura S, Takayama H, Fleisig AJ, Agnew ML, Hampton CR, Rothnie CL, Spring DJ, Pohlman TH, Shimpo H, Verrier ED. Inhibition of Toll-like receptor 4 with eritoran attenuates myocardial ischemia-reperfusion injury. Circulation. 2006;114:I270–274. doi: 10.1161/CIRCULATIONAHA.105.000901. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- 7.Arumugam TV, Okun E, Tang SC, Thundyil J, Taylor SM, Woodruff TM. Toll-like receptors in ischemia-reperfusion injury. Shock. 2009;32:4–16. doi: 10.1097/SHK.0b013e318193e333. [DOI] [PubMed] [Google Scholar]
- 8.Kaczorowski DJ, Nakao A, McCurry KR, Billiar TR. Toll-like receptors and myocardial ischemia/reperfusion, inflammation, and injury. Curr Cardiol Rev. 2009;5:196–202. doi: 10.2174/157340309788970405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 10.Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 11.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, Kelly RA. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–280. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roupe KA, Remsberg CM, Yanez JA, Davies NM. Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol. 2006;1:81–101. doi: 10.2174/157488406775268246. [DOI] [PubMed] [Google Scholar]
- 14.Lin HS, Yue BD, Ho PC. Determination of pterostilbene in rat plasma by a simple HPLC-UV method and its application in pre-clinical pharmacokinetic study. Biomed Chromatogr. 2009;23:1308–1315. doi: 10.1002/bmc.1254. [DOI] [PubMed] [Google Scholar]
- 15.Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68:593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perecko T, Drabikova K, Rackova L, Ciz M, Podborska M, Lojek A, Harmatha J, Smidrkal J, Nosal R, Jancinova V. Molecular targets of the natural antioxidant pterostilbene: effect on protein kinase C, caspase-3 and apoptosis in human neutrophils in vitro. Neuro Endocrinol Lett. 2010;31(Suppl 2):84–90. [PubMed] [Google Scholar]
- 17.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCormack D, McFadden D. A review of pterostilbene antioxidant activity and disease modification. Oxid Med Cell Longev. 2013;2013:575482. doi: 10.1155/2013/575482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack D, McFadden D. Pterostilbene and cancer: current review. J Surg Res. 2012;173:e53–61. doi: 10.1016/j.jss.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 20.Su H, Sun X, Ma H, Zhang HF, Yu QJ, Huang C, Wang XM, Luan RH, Jia GL, Wang HC, Gao F. Acute hyperglycemia exacerbates myocardial ischemia/reperfusion injury and blunts cardioprotective effect of GIK. Am J Physiol Endocrinol Metab. 2007;293:E629–635. doi: 10.1152/ajpendo.00221.2007. [DOI] [PubMed] [Google Scholar]
- 21.Speyer CL, Ward PA. Role of endothelial chemokines and their receptors during inflammation. J Invest Surg. 2011;24:18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 22.Khimenko PL, Bagby GJ, Fuseler J, Taylor AE. Tumor necrosis factor-alpha in ischemia and reperfusion injury in rat lungs. J Appl Physiol (1985) 1998;85:2005–2011. doi: 10.1152/jappl.1998.85.6.2005. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, Liu M, Kennedy RH, Liu SJ. TNF-alpha-induced impairment of mitochondrial integrity and apoptosis mediated by caspase-8 in adult ventricular myocytes. Cytokine. 2006;34:96–105. doi: 10.1016/j.cyto.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Meldrum DR, Cleveland JC Jr, Cain BS, Meng X, Harken AH. Increased myocardial tumor necrosis factor-alpha in a crystalloid-perfused model of cardiac ischemia-reperfusion injury. Ann Thorac Surg. 1998;65:439–443. doi: 10.1016/s0003-4975(97)01297-6. [DOI] [PubMed] [Google Scholar]
- 25.Gurevitch J, Frolkis I, Yuhas Y, Lifschitz-Mercer B, Berger E, Paz Y, Matsa M, Kramer A, Mohr R. Anti-tumor necrosis factor-alpha improves myocardial recovery after ischemia and reperfusion. J Am Coll Cardiol. 1997;30:1554–1561. doi: 10.1016/s0735-1097(97)00328-8. [DOI] [PubMed] [Google Scholar]
- 26.Lefer AM, Ma XL, Weyrich A, Lefer DJ. Endothelial dysfunction and neutrophil adherence as critical events in the development of reperfusion injury. Agents Actions Suppl. 1993;41:127–135. [PubMed] [Google Scholar]
- 27.Ma XL, Lefer DJ, Lefer AM, Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992;86:937–946. doi: 10.1161/01.cir.86.3.937. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 29.Otsui K, Inoue N, Kobayashi S, Shiraki R, Honjo T, Takahashi M, Hirata K, Kawashima S, Yokoyama M. Enhanced expression of TLR4 in smooth muscle cells in human atherosclerotic coronary arteries. Heart Vessels. 2007;22:416–422. doi: 10.1007/s00380-007-1001-1. [DOI] [PubMed] [Google Scholar]
- 30.Ha T, Li Y, Hua F, Ma J, Gao X, Kelley J, Zhao A, Haddad GE, Williams DL, William Browder I, Kao RL, Li C. Reduced cardiac hypertrophy in toll-like receptor 4-deficient mice following pressure overload. Cardiovasc Res. 2005;68:224–234. doi: 10.1016/j.cardiores.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi T. Toll-like receptors and myocardial contractile dysfunction. Cardiovasc Res. 2008;78:3–4. doi: 10.1093/cvr/cvn044. [DOI] [PubMed] [Google Scholar]
- 32.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 33.Liu YY, Cai WF, Yang HZ, Cui B, Chen ZR, Liu HZ, Yan J, Jin W, Yan HM, Xin BM, Yuan B, Hua F, Hu ZW. Bacillus Calmette-Guerin and TLR4 agonist prevent cardiovascular hypertrophy and fibrosis by regulating immune microenvironment. J Immunol. 2008;180:7349–7357. doi: 10.4049/jimmunol.180.11.7349. [DOI] [PubMed] [Google Scholar]
- 34.Liu P, Hock CE, Nagele R, Wong PY. Formation of nitric oxide, superoxide, and peroxynitrite in myocardial ischemia-reperfusion injury in rats. Am J Physiol. 1997;272:H2327–2336. doi: 10.1152/ajpheart.1997.272.5.H2327. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Wu F, Zhang H, Fu F, Ji L, Dong L, Li Q, Liu W, Zhang Y, Lv A, Wang H, Ren J, Gao F. Insulin inhibits leukocyte-endothelium adherence via an Akt-NO-dependent mechanism in myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2009;47:512–519. doi: 10.1016/j.yjmcc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Zhang H, Wu F, Nan Y, Ma H, Guo W, Wang H, Ren J, Das UN, Gao F. Insulin inhibits tumor necrosis factor-alpha induction in myocardial ischemia/reperfusion: role of Akt and endothelial nitric oxide synthase phosphorylation. Crit Care Med. 2008;36:1551–1558. doi: 10.1097/CCM.0b013e3181782335. [DOI] [PubMed] [Google Scholar]


