Abstract
Objective: In published studies, Y-box binding protein-1 (YB-1) correlated with the prognosis of patients with breast cancer (BC), but the specific role of YB-1 is still unclear. Our study aimed to evaluate the prognostic value of YB-1 in BC patients using meta-analysis based on the published studies. Methods: We searched the relevant literatures deadline for June 2014 in databases, including PubMed, Embase, Medline and Cochrane library, and finally 8 studies were included in our study. Our study contained 1094 BC patients with 398 YB-1 positive and 696 YB-1 negative. Results: Our results showed that YB-1 abnormal expression did not correlated with the lymph node status [OR = 1.258, 95% CI = 0.895-1.769, P = 0.186], high histological grade [OR = 2.709, 95% CI = 0.861-8.530, P = 0.089], histological type [OR = 0.837, 95% CI = 0.526-1.331, P = 0.452], P53 status [OR = 2.006, 95% CI 0.686-5.865, P = 0.203] and PR [OR = 0.607, 95% CI = 0.347-1.061, P = 0.080] in BC patients. But YB-1 over-expression was associated with other unfavorable factors: ER negativity [OR = 0.604, 95% CI = 0.388-0.941, P = 0.026], HER2 positivity [OR = 3.841, 95% CI = 2.637-5.594, P = 0.000], and high tumorous T stage [OR = 2.169, 95% CI = 1.295-3.632, P = 0.003]. In addition, our data suggested that high YB-1 expression had an adverse impact on 5-year OS [RR = 2.767, 95% CI = 2.054-3.727, P = 0.000] in BC patients. Conclusions: Our findings implied that YB-1 might a novel biomarker to predict the prognosis of BC, and could be a potential direction for developing diagnostic and therapeutic approaches in BC.
Keywords: YB-1, breast cancer, prognosis, meta-analysis
Introduction
Breast cancer (BC) is the most common cancer among women. In 2013, according to the American Cancer Society statistics, approximately 232,340 new cases of invasive BC and 39,620 cases of death were expected to occur among US women, and the incidence is now up to 1 in 8 women [9,38]. There are four main challenges in managing BC patients, including the inexact risk factors, advanced stage BC with metastases, tumor relapse and drug resistance. So it is significant for early diagnosis, prognostic evaluation and individual treatment scheme of BC. Until now, gene expression analysis for some traditional molecular markers, such as ER, PR, HER-2, has been used to classify the sub-type of BC in order to predict the prognosis and therapy in clinic. But other novel and potential markers are also urgently needed to develop in BC.
YB-1 is a member of the cold-shock super-family and plays an important role in some cellular process, including DNA repair and transcription, mRNA splicing and translation, the regulation of cell cycle, oxidative stress and multidrug resistance [4,7,10,24]. Recent studies had shown that YB-1 expression was higher in some malignant tumors than those in normal tissues, including in osteosarcoma [14], head and neck squamous cell carcinoma [23], multiple myeloma [2], colon carcinoma [41], prostate cancer and breast cancer [25]. It played an important role in inducing the expressions of some putative oncogenes, such as EGFR, HER2, c-Met, and MMP-2 [12,21,39,43]. Moreover, YB-1 could also promote the cell growth of cancer stem cells (CSCs) through inducing the expressions of some CSCs-associated genes, such as CD44 and CD49f [40]. These findings suggested powerfully that YB-1 might be an oncogene in many cancers.
In BC, though there are several clinical researches which had investigated the relationships between YB-1 and some clinicopathological parameters, their conclusions were still controversial. Therefore, this meta-analysis is aimed to assess the clinical value of YB-1 in BC patients.
Materials and methods
Literatures search
We searched the literatures from the electronic databases: PubMed, Embase, Medline and Cochrane library, and the searching deadline was June 2014. The keywords were searched by using the following terms and their combinations: Y-box binding protein-1, YB-1, breast, mammary gland, carcinoma, cancer.
Studies must meet the following criteria: (a) immunohistochemistry (IHC) was only method to detect the expression of YB-1 in BC tissues, (b) literatures must provide the original data of YB-1 expression, clinicopathological parameters and OS rate, or were able to obtain the above information by calculation, (c) studies were published as original researches, (d) available full texts could be obtained, (e) language of the publications was English.
The excluded criteria: (a) letters to the editor, reviews or some articles published in a book or paper, (b) articles in non-English language, (c) we could not get the original data of YB-1 expression, clinicopathological parameters or OS rate.
Data extraction
The eligible articles included are assessed by two investigators (Xu Wang and Xiaofeng Xue). General information extracted from the eligible articles include: author, the publication of the year, the source of the BC patients, the methods to measure the YB-1 expression in BC tissues, the total number of BC patients in each study, the number of YB-1 positive and YB-1 negative in BC patients, the correlations between YB-1 and clinicopathological parameters and the 5-year OS rate.
Statistical analysis
We used OR and 95% CI to evaluate the associations between YB-1 expression and clinicopathological parameters in BC (including the histological grade, histological type, lymph node status, tumorous T stage, P53 status, ER, PR and HER-2 status). To expand the number of samples and studying index, we divided the histological grade into high histological grade (3 grade) and low histological grade (1-2 grade), and the histological type was divided into invasive breast cancer (IDC) and other histological type. Similarly, we divided tumorous T stage into two groups: high T stage (3-4 stage) and low T stage (1-2 stage). In addition, we combined ERα and ERβ as ER group. For the overall survival results, we calculated the RR and 95% CI about the impact of YB-1 expression on 5-year OS rate.
The heterogeneity was evaluated by the Q-test using I2-value and P-values. We defined that I2 = 0-50% or P-values greater than 0.05 meant no statistically significant heterogeneity. If statistical results have not significant homogeneity, a fixed effect model was used for analysis; if not, a random-effect model was used. To prove the reliability and evaluate the influence of individual study to overall results, sensitivity analysis was performed. Begg’ test and Funnel plots were used to measure the publication bias for each study and we defined that P-value greater than 0.05 or doing not have apparent asymmetry for the shape of graphics was regarded as a lack of statistically significant publication bias. All of the statistical calculations were performed by STATA version 12.0 (STATA Corporation, College Station, TX).
Results
Study characteristics
86 studies were reviewed from the above electronic databases. The process of retrieving and eliminating literatures was showed in Figure 1. Finally, eight eligible literatures published from 2003 to 2013 were eligible in our study [5,13,15,19,28,29,34,45]. Of which, seven literatures provided complete original data about YB-1 expression and clinicopathological parameters [5,13,15,19,29,34,45]. Five literatures assessed the prognostic value of YB-1 expression for OS in BC patients by the Kaplan-Meier method [5,13,15,28,45].
Figure 1.
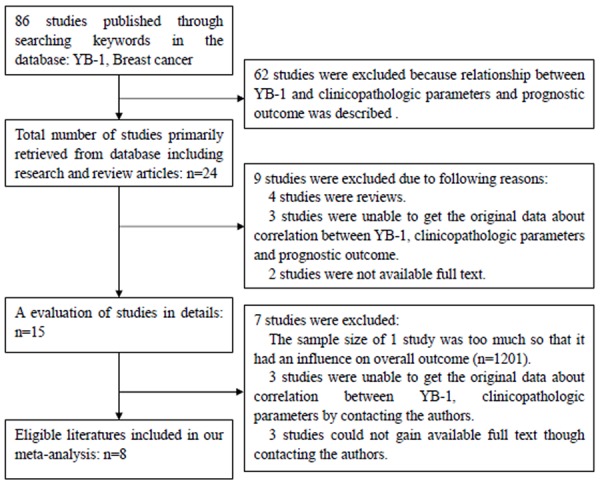
Flow diagram showing inclusion and exclusion of studies.
We obtained relevant information by directly extracting original data or get original data by indirectly calculating from the eight studies. These eight eligible articles were showed in Table 1. A total of 1094 BC patients were included in this meta-analysis, with 398 YB-1 positive and 696 YB-1 negative BC patients. All patients came from five countries (Germany, Greece, Japan, China and Poland). The method using to assess the expression of YB-1 in BC tumors was IHC.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Method | Clinical stage | Total | YB-1 positive (n) | YB-1 negative (n) |
|---|---|---|---|---|---|---|---|
| E. Mylona | 2013 | Greece | IHC | I-III | 225 | 52 | 173 |
| Edgar Dahl | 2009 | Germany | IHC | I-III | 159 | 42 | 117 |
| Hisashi Saji | 2003 | Japan | IHC | - | 63 | 14 | 49 |
| Maciejczyk | 2012 | Poland | IHC | I-III | 101 | 38 | 63 |
| Oleg Gluz | 2009 | Germany | IHC | I-III | 211 | 125 | 86 |
| Teruhiko Fujii | 2008 | Japan | IHC | I-III | 73 | 30 | 43 |
| Tokiko Ito | 2012 | Japan | IHC | - | 23 | 9 | 14 |
| Wenxiu Xie | 2012 | China | IHC | I-III | 239 | 88 | 151 |
Relationships between YB-1 expression and clinicopathological parameters in BC patients
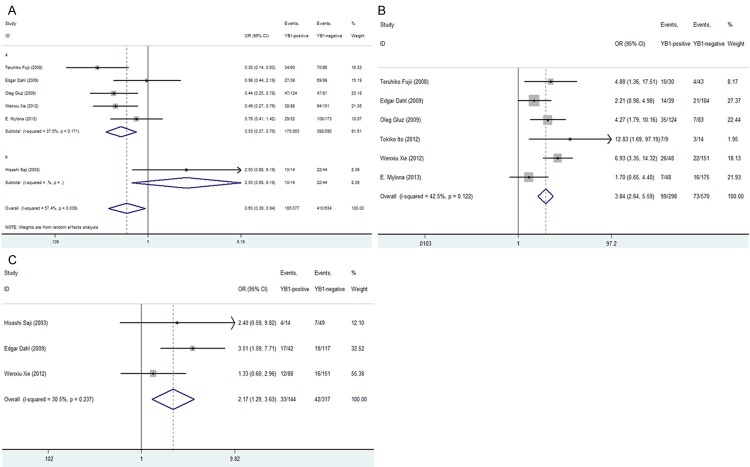
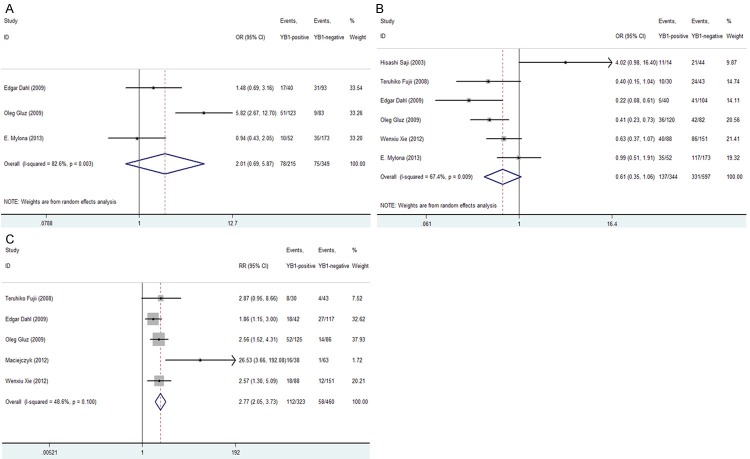
Our results demonstrated that YB-1 over-expression was significantly associated with following clinicopathological parameters: ER negativity [OR = 0.604, 95% CI = 0.388-0.941, P = 0.026, random-effect], HER2 positivity [OR = 3.841, 95% CI = 2.637-5.594, P = 0.000, fixed-effect] and high tumorous T stage [OR = 2.169, 95% CI = 1.295-3.632, P = 0.003, fixed-effect] (Figure 2A-C).
Figure 2.
Forrest plot of ORs for the association of YB-1 expression with the (A) ER status; (B) HER2 status; (C) tumorous T stage.
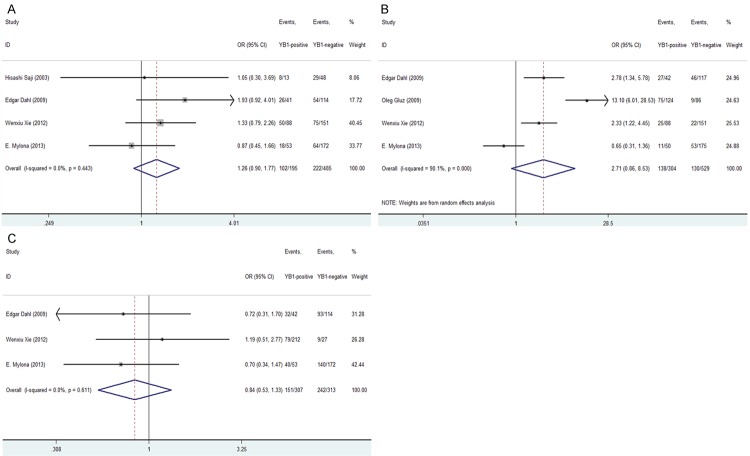
In addition, we also found that high YB-1 expression was not statistically associated with lymph node status [OR = 1.258, 95% CI = 0.895-1.769, P = 0.186, fixed-effect], high histological grade [OR = 2.709, 95% CI = 0.861-8.530, P = 0.089, random-effect], histological type [OR = 0.837, 95% CI = 0.526-1.331, P = 0.452, fixed-effect], P53 status [OR = 2.006, 95% CI = 0.686-5.865, P = 0.203, random-effect] or PR [OR = 0.607, 95% CI = 0.347-1.061, P = 0.080, random-effect] (Figures 3A-C, 4A and 4B).
Figure 3.
Forrest plot of ORs for the association of YB-1 expression with the (A) lymph node status; (B) histological grade; (C) histological type.
Figure 4.
Forrest plot of ORs or RRs for the association of YB-1 expression with the (A) P53 status; (B) PR status (C); Overall survival rate.
Impact of YB-1 expression on 5-year OS in BC patients
Due to the RR of OS not given directly, we calculated the RR of 5-year OS rate through extracting the original information from five studies included. The pooled RRs of five studies containing 783 patients were analyzed. Our results showed that high YB-1 expression was statistically significant with the poor 5-year OS rate in BC patients [RR = 2.767, 95% CI = 2.054-3.727, P = 0.000, fixed-effect] (Figure 4C). Meanwhile, in the process of evaluation of five related studies, no significant heterogeneity was observed among the studies on YB-1 over-expression in BC patients (Q = 7.78, I2 = 48.6%, P = 0.100).
Sensitivity analysis
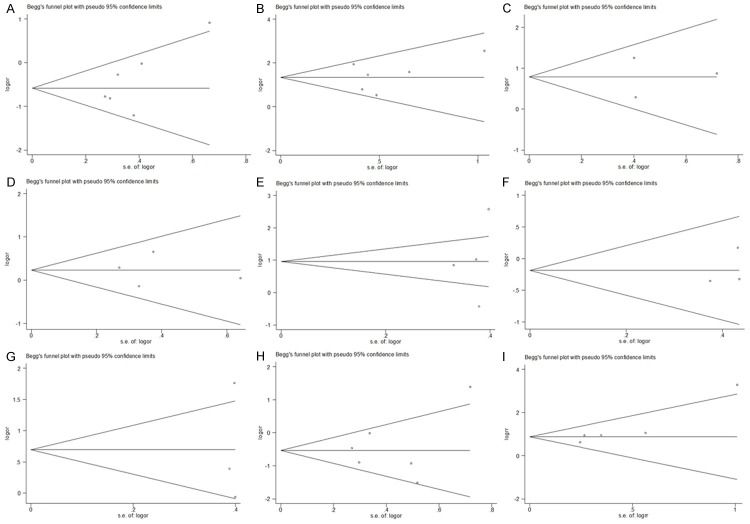
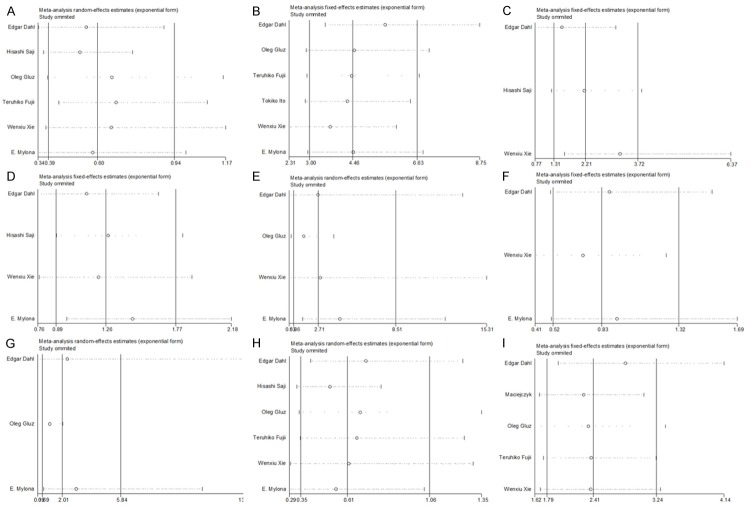
We used sensitivity analysis to test whether the inclusion criteria of the meta-analysis affected the final results, and further confirmed whether the entire study was not affected by the results regarding of related clinicopathological parameters and OS by any single study. The results of sensitivity analysis were showed in Figure 5. We found that no study had an obvious impact on our overall results, and verified the stability and reliability of our results.
Figure 5.
Sensitivity analyses of all the studies. (A: ER status; B: HER2 status; C: Tumorous T stage; D: Lymph node status; E: Histological grade; F: Histological type; G: P53 status; H: PR status; I: Overall survival rate).
Publication bias
Begg’s funnel plot and homologous P-values were used to assess the publication bias in our selected literatures. We defined that the shape of graphics had apparent asymmetry or P-values greater than 0.05 meant significant publication bias. Our results showed that the relationships between YB-1 over-expression and all clinicopathological parameters had no published bias (Figure 6A-H). Similar results were also found in OS analysis (Begg’s test score of P = 0.086) (Figure 6I).
Figure 6.
Funnel plots for publication bias. All the graphical funnel plots appeared to be symmetrical. (A: ER status; B: HER2 status; C: Tumorous T stage; D: Lymph node status; E: Histological grade; F: Histological type; G: P53 status; H: PR status; I: Overall survival rate).
Discussion
In general, the traditional indicators related to the prognosis of BC could be divided into two classes as follows: (i) pathological indicators, such as tumor diameter, position, histological grade and lymphatic metastasis, (ii) hormone receptor, for example ER, PR, HER-2 and EGFR. However, these indicators could not totally and effectively reflect the tumor biological characteristics and drug response after treatment. Therefore, exploring some other novel and potential biological markers of BC is very necessary. Recently, some other potential molecular indicators such as multidrug resistance 1 (MDR1), enhancer of zeste homolog 2 (EZH2), microsatellite instability (MSI), tumor metastasis suppressor (Nm23) and Trefoil factor 1 (TFF1), had been found to influence the prognosis of BC [3,27,31,32,35]. Recently, Habibi et al showed that YB-1 was also a biological marker of poor prognosis in BC patients with a large cohort of 4049 cases over an observation period of 20 years. They pointed that YB-1 could be the universal biomarker that distinguished patients at high risk across all subtypes of BC [17].
In our meta-analysis, we did not found that YB-1 abnormal expression was related to lymph node status, high histological grade, histological type, human tumor suppressor gene P53 status and PR in BC patients. But our data showed that YB-1 over-expression was associated with ER negativity, HER2 positivity and high tumorous T stage, and led to a poorer prognosis in BC patients. The risk of 5-year OS rate in patients with positive YB-1 was 2.767 times higher than that of negative YB-1 patients [OS 95% CI = 2.054-3.727]. Except for BC, YB-1 overexpression was also a sign of poor prognosis in non-small cell lung cancer, ovarian cancer, melanoma and prostate cancer [30,33,36]. But the potential and specific mechanisms how YB-1 regulates the development of cancer is still unclear. In our study, according to BC, the possible mechanisms might be related to the negative expression of ER and positive expression of HER2. Ito et al. proved that knockdown of YB-1 could increase the expression of ERα in wild-type MCF7 cells [19]. They also found that the expression of HER2 was increased in MCF7 cells that stably expressed YB-1, and silencing the expression of YB-1 using siRNA led to the down-regulation of HER2 [37]. These results were affirmed by clinical BC samples (Wu et al., 2006). Davies et al. suggested that YB-1 expression led to the clonal selection and expansion of HER2 positive cells through affecting the process of cell divisions [8]. YB-1 was the promoter of HER2 [45]. Patients with HR-/HER2+ tumors had an unfavorable prognosis and higher risk of recurrence than patients with HR+/HER2- tumors [6,42]. Those characteristics of BC were controlled by YB-1 expression via HER2-Akt-dependent pathway [13]. So, the YB-1-HER2 axis was very important for personalized therapeutics against BC by YB-1 targeted drugs.
Moreover, YB-1 was correlated with BC aggressiveness. Janz et al. [20] found that patients with low expressions of YB-1 were still free of disease, whereas the 5-year relapse in those with high expression of YB-1 was 30%. The mechanisms might be as follows: (i) YB-1 increased the expression of EGFR and Ki67, which coupled with the growth and proliferation of tumor cells [44]; (ii) YB-1 could enhance the presentation of the membrane type I-matrix metalloproteinase (MT1-MMP) [26]; (iii) YB-1 induced an epithelial-mesenchymal transition (EMT) [11]. Those findings implied that YB-1 might contribute to the development of an invasive, metastatic potential of BC.
Besides, Janz et al. also found that YB-1 associated with drug resistance and acted as a biomarker for predicting the efficacy of high-dose chemotherapy in BC [20,33]. The proof was that in patients who received postoperative chemotherapy, the 5-year relapse was 66% in patients with high expression of YB-1. In contrast, no relapse was observed in patients with low YB-1. Patients with high YB-1 were at high risk for recurrence, especially those who were subjected to chemo- and radiotherapy [29]. In consequence, YB-1 was proved to be prognostic and predictive significant independent of tumor-biological factors. We know that MDR1 is a major ATP-binding cassette (ABC) transporter linked to multidrug resistance in cancer cells. Exposure to cytotoxic anticancer agents or UV irradiation, the expression of MDR1 and YB-1 was up-regulated [1,16,18]. YB-1, interacted with APE1 (a central enzyme acting as a transcriptional regulator), could led to the activation of MDR1 [4]. YB-1 also increased the expression of MDR1 through VEGF-VEGFR pathway [1]. But Kaszubiak and Hu et al. had reported that YB-1 was not involved or sufficient in the regulation of MDR1 gene or the development of drug-resistance phenotype in MDR cancer cells [18,22]. So, the role of YB-1 regulating the drug resistance is still not clear so far.
Though larger well-designed studies with more ethnic groups and larger population studies are required, our present meta-analysis indicated that elevated YB-1 over-expression was associated with ER negativity, HER2 positivity and high tumorous T stage, and led to a poorer patient survival in BC. To our knowledge, this meta-analysis is the first one to evaluate the prognostic role of YB-1 expression in BC. These results suggested that YB-1 might a novel biomarker to predict the prognosis of BC, and could be a potential direction for developing diagnostic and therapeutic approaches in BC.
Acknowledgements
This study was supported by grants from the National youthful Science Foundation of China (No. 81101858 and 81302147), the National Science Foundation of Jiangsu Province, China (No. BK20130270), the Science and Education Youth Health Foundation, Suzhou, China No. JXW2012005), and the Special Subject of Diagnosis Treatment of Key Clinical Diseases of Shuzhou City Sci-tech Bureau (LCZX201401).
Disclosure of conflict of interest
None.
References
- 1.Akiyama K, Ohga N, Hida Y, Kawamoto T, Sadamoto Y, Ishikawa S, Maishi N, Akino T, Kondoh M, Matsuda A, Inoue N, Shindoh M, Hida K. Tumor endothelial cells acquire drug resistance by MDR1 up-regulation via VEGF signaling in tumor microenvironment. Am J Pathol. 2012;180:1283–93. doi: 10.1016/j.ajpath.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Bommert KS, Effenberger M, Leich E, Küspert M, Murphy D, Langer C, Moll R, Janz S, Mottok A, Weissbach S, Rosenwald A, Bargou R, Bommert K. The feed-forward loop between YB-1 and MYC is essential for multiple myeloma cell survival. Leukemia. 2013;27:441–50. doi: 10.1038/leu.2012.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calaf GM, Echiburu-Chau C, Roy D. Organophosphorous pesticides and estrogen induce transformation of breast cells affecting p53 and c-Ha-ras genes. Int J Oncol. 2009;35:1061–8. doi: 10.3892/ijo_00000421. [DOI] [PubMed] [Google Scholar]
- 4.Chattopadhyay R, Das S, Maiti AK, Boldogh I, Xie J, Hazra TK, Kohno K, Mitra S, Bhakat KK. Regulatory role of human AP-endonuclease (APE1/Ref-1) in YB-1-mediated activation of the multidrug resistance gene MDR1. Mol Cell Biol. 2008;28:7066–80. doi: 10.1128/MCB.00244-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahl E, En-Nia A, Wiesmann F, Krings R, Djudjaj S, Breuer E, Fuchs T, Wild PJ, Hartmann A, Dunn SE, Mertens PR. Nuclear detection of Y-box protein-1 (YB-1) closely associates with progesterone receptor negativity and is a strong adverse survival factor in human breast cancer. BMC Cancer. 2009;9:410. doi: 10.1186/1471-2407-9-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darb-Esfahani S, Loibl S, Muller BM, Roller M, Denkert C, Komor M, Schlüns K, Blohmer JU, Budczies J, Gerber B, Noske A, du Bois A, Weichert W, Jackisch C, Dietel M, Richter K, Kaufmann M, von Minckwitz G. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res. 2009;11:R69. doi: 10.1186/bcr2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das S, Chattopadhyay R, Bhakat KK. Stimulation of NEIL2-mediated oxidized base excision repair via YB-1 interaction during oxidative stress. J Biol Chem. 2007;282:28474–84. doi: 10.1074/jbc.M704672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies AH, Barrett I, Pambid MR, Hu K, Stratford AL, Freeman S, Berquin IM, Pelech S, Hieter P, Maxwell C, Dunn SE. YB-1 evokes susceptibility to cancer through cytokinesis failure, mitotic dysfunction and HER2 amplification. Oncogene. 2011;30:3649–60. doi: 10.1038/onc.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 10.Eliseeva IA, Kim ER, Guryanov SG, Ovchinnikov LP, Lyabin DN. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Mosc) 2011;76:1402–33. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 11.Evdokimova V, Tognon C, Ng T, Ruzanov P, Melnyk N, Fink D, Sorokin A, Ovchinnikov LP, Davicioni E, Triche TJ, Sorensen PH. Translational activation of snail1 and other developmentally regulated transcription factors by YB-1 promotes an epithelial-mesenchymal transition. Cancer Cell. 2009;15:402–15. doi: 10.1016/j.ccr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Finkbeiner MR, Astanehe A, To K, Fotovati A, Davies AH, Zhao Y, Jiang H, Stratford AL, Shadeo A, Boccaccio C, Comoglio P, Mertens PR, Eirew P, Raouf A, Eaves CJ, Dunn SE. Profiling YB-1 target genes uncovers a new mechanism for MET receptor regulation in normal and malignant human mammary cells. Oncogene. 2009;28:1421–31. doi: 10.1038/onc.2008.485. [DOI] [PubMed] [Google Scholar]
- 13.Fujii T, Kawahara A, Basaki Y, Basaki Y, Hattori S, Nakashima K, Nakano K, Shirouzu K, Kohno K, Yanagawa T, Yamana H, Nishio K, Ono M, Kuwano M, Kage M. Expression of HER2 and estrogen receptor alpha depends upon nuclear localization of Y-box binding protein-1 in human breast cancers. Cancer Res. 2008;68:1504–12. doi: 10.1158/0008-5472.CAN-07-2362. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara-Okada Y, Matsumoto Y, Fukushi J, Setsu N, Matsuura S, Kamura S, Fujiwara T, Iida K, Hatano M, Nabeshima A, Yamada H, Ono M, Oda Y, Iwamoto Y. Y-box binding protein-1 regulates cell proliferation and is associated with clinical outcomes of osteosarcoma. Br J Cancer. 2013;108:836–47. doi: 10.1038/bjc.2012.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gluz O, Mengele K, Schmitt M, Kates R, Diallo-Danebrock R, Neff F, Royer HD, Eckstein N, Mohrmann S, Ting E, Kiechle M, Poremba C, Nitz U, Harbeck N. Y-box-binding protein YB-1 identifies high-risk patients with primary breast cancer benefiting from rapidly cycled tandem high-dose adjuvant chemotherapy. J. Clin. Oncol. 2009;27:6144–51. doi: 10.1200/JCO.2008.19.6261. [DOI] [PubMed] [Google Scholar]
- 16.Guay D, Garand C, Reddy S, Schmutte C, Lebel M. The human endonuclease III enzyme is a relevant target to potentiate cisplatin cytotoxicity in Y-box-binding protein-1 overexpressing tumor cells. Cancer Sci. 2008;99:762–9. doi: 10.1111/j.1349-7006.2008.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habibi G, Leung S, Law JH, Gelmon K, Masoudi H, Turbin D, Pollak M, Nielsen TO, Huntsman D, Dunn SE. Redefining prognostic factors for breast cancer: YB-1 is a stronger predictor of relapse and disease-specific survival than estrogen receptor or HER-2 across all tumor subtypes. Breast Cancer Res. 2008;10:R86. doi: 10.1186/bcr2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Z, Jin S, Scotto KW. Transcriptional activation of the MDR1 gene by UV irradiation. Role of NF-Y and Sp1. J Biol Chem. 2000;275:2979–85. doi: 10.1074/jbc.275.4.2979. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Kamijo S, Izumi H, Kohno K, Amano J, Ito K. Alteration of Y-box binding protein-1 expression modifies the response to endocrine therapy in estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2012;133:145–59. doi: 10.1007/s10549-011-1731-8. [DOI] [PubMed] [Google Scholar]
- 20.Janz M, Harbeck N, Dettmar P, Berger U, Schmidt A, Jürchott K, Schmitt M, Royer HD. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int J Cancer. 2002;97:278–82. doi: 10.1002/ijc.1610. [DOI] [PubMed] [Google Scholar]
- 21.Kashihara M, Azuma K, Kawahara A, Basaki Y, Hattori S, Yanagawa T, Terazaki Y, Takamori S, Shirouzu K, Aizawa H, Nakano K, Kage M, Kuwano M, Ono M. Nuclear Y-box binding protein-1, a predictive marker of prognosis, is correlated with expression of HER2/ErbB2 and HER3/ErbB3 in non-small cell lung cancer. J Thorac Oncol. 2009;4:1066–74. doi: 10.1097/JTO.0b013e3181ae2828. [DOI] [PubMed] [Google Scholar]
- 22.Kaszubiak A, Kupstat A, Muller U, Hausmann R, Holm PS, Lage H. Regulation of MDR1 gene expression in multidrug-resistant cancer cells is independent from YB-1. Biochem Biophys Res Commun. 2007;357:295–301. doi: 10.1016/j.bbrc.2007.03.145. [DOI] [PubMed] [Google Scholar]
- 23.Kolk A, Jubitz N, Mengele K, Bissinger O, Schmitt M, Kremer M, Holm PS. Expression of Y-box-binding protein YB-1 allows stratification into long- and short-term survivors of head and neck cancer patients. Br J Cancer. 2011;105:1864–73. doi: 10.1038/bjc.2011.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lasham A, Print CG, Woolley AG, Dunn SE, Braithwaite AW. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013;449:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 25.Law JH, Li Y, To K, Wang M, Astanehe A, Lambie K, Dhillon J, Jones SJ, Gleave ME, Eaves CJ, Dunn SE. Molecular decoy to the Y-box binding protein-1 suppresses the growth of breast and prostate cancer cells whilst sparing normal cell viability. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lovett DH, Cheng S, Cape L, Pollock AS, Mertens PR. YB-1 alters MT1-MMP trafficking and stimulates MCF-7 breast tumor invasion and metastasis. Biochem Biophys Res Commun. 2010;398:482–8. doi: 10.1016/j.bbrc.2010.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, Diederichs S, Eichmüller SB. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–75. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 28.Maciejczyk A, Szelachowska J, Ekiert M, Matkowski R, Hałoń A, Lage H, Surowiak P. Elevated nuclear YB1 expression is associated with poor survival of patients with early breast cancer. Anticancer Res. 2012;32:3177–84. [PubMed] [Google Scholar]
- 29.Mylona E, Melissaris S, Giannopoulou I, Theohari I, Papadimitriou C, Keramopoulos A, Nakopoulou L. Y-box-binding protein 1 (YB1) in breast carcinomas: relation to aggressive tumor phenotype and identification of patients at high risk for relapse. Eur J Surg Oncol. 2014;40:289–96. doi: 10.1016/j.ejso.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Panupinthu N, Yu S, Zhang D, Zhang F, Gagea M, Lu Y, Grandis JR, Dunn SE, Lee HY, Mills GB. Self-reinforcing loop of amphiregulin and Y-box binding protein-1 contributes to poor outcomes in ovarian cancer. Oncogene. 2014;33:2846–56. doi: 10.1038/onc.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelden S, Insawang T, Thuwajit C, Thuwajit P. The trefoil factor 1 (TFF1) protein involved in doxorubicininduced apoptosis resistance is upregulated by estrogen in breast cancer cells. Oncol Rep. 2013;30:1518–26. doi: 10.3892/or.2013.2593. [DOI] [PubMed] [Google Scholar]
- 32.Roh S, Park SY, Ko HS, Sohn JS, Cha EJ. EZH2 expression in invasive lobular carcinoma of the breast. World J Surg Oncol. 2013;11:299. doi: 10.1186/1477-7819-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybalkina E, Stromskaia TP, Ovchinnikov LP, Stavrovskaia AA. [Connection of intracellular protein YB-1 localization in cell cultures of human tumors with multidrug resistance] . Vopr Onkol. 2013;59:623–8. [PubMed] [Google Scholar]
- 34.Saji H, Toi M, Saji S, Koike M, Kohno K, Kuwano M. Nuclear expression of YB-1 protein correlates with P-glycoprotein expression in human breast carcinoma. Cancer Lett. 2003;190:191–7. doi: 10.1016/s0304-3835(02)00590-6. [DOI] [PubMed] [Google Scholar]
- 35.Shi JF, Yang N, Ding HJ, Zhang JX, Hu ML, Leng Y, Han X, Sun YJ. ERalpha directly activated the MDR1 transcription to increase paclitaxel-resistance of ERalpha-positive breast cancer cells in vitro and in vivo. Int J Biochem Cell Biol. 2014;53:35–45. doi: 10.1016/j.biocel.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Shibahara K, Sugio K, Osaki T, Uchiumi T, Maehara Y, Kohno K, Yasumoto K, Sugimachi K, Kuwano M. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin Cancer Res. 2001;7:3151–5. [PubMed] [Google Scholar]
- 37.Shibata T, Kan H, Murakami Y, Ureshino H, Watari K, Kawahara A, Kage M, Hattori S, Ono M, Kuwano M. Y-box binding protein-1 contributes to both HER2/ErbB2 expression and lapatinib sensitivity in human gastric cancer cells. Mol Cancer Ther. 2013;12:737–46. doi: 10.1158/1535-7163.MCT-12-1125. [DOI] [PubMed] [Google Scholar]
- 38.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 39.Stratford AL, Habibi G, Astanehe A, Jiang H, Hu K, Park E, Shadeo A, Buys TP, Lam W, Pugh T, Marra M, Nielsen TO, Klinge U, Mertens PR, Aparicio S, Dunn SE. Epidermal growth factor receptor (EGFR) is transcriptionally induced by the Y-box binding protein-1 (YB-1) and can be inhibited with Iressa in basal-like breast cancer, providing a potential target for therapy. Breast Cancer Res. 2007;9:R61. doi: 10.1186/bcr1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.To K, Fotovati A, Reipas KM, Law JH, Hu K, Wang J, Astanehe A, Davies AH, Lee L, Stratford AL, Raouf A, Johnson P, Berquin IM, Royer HD, Eaves CJ, Dunn SE. Y-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res. 2010;70:2840–51. doi: 10.1158/0008-5472.CAN-09-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsofack SP, Garand C, Sereduk C, Chow D, Aziz M, Guay D, Yin HH, Lebel M. NONO and RALY proteins are required for YB-1 oxaliplatin induced resistance in colon adenocarcinoma cell lines. Mol Cancer. 2011;10:145. doi: 10.1186/1476-4598-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SY, Shamliyan T, Virnig BA, Kane R. Tumor characteristics as predictors of local recurrence after treatment of ductal carcinoma in situ: a meta-analysis. Breast Cancer Res Treat. 2011;127:1–14. doi: 10.1007/s10549-011-1387-4. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Stratford AL, Astanehe A, Dunn SE. YB-1 is a Transcription/Translation Factor that Orchestrates the Oncogenome by Hardwiring Signal Transduction to Gene Expression. Transl Oncogenomics. 2007;2:49–65. [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Lee C, Yokom D, Jiang H, Cheang MC, Yorida E, Turbin D, Berquin IM, Mertens PR, Iftner T, Gilks CB, Dunn SE. Disruption of the Y-box binding protein-1 results in suppression of the epidermal growth factor receptor and HER-2. Cancer Res. 2006;66:4872–9. doi: 10.1158/0008-5472.CAN-05-3561. [DOI] [PubMed] [Google Scholar]
- 45.Xie W, Yang J, Cao Y, Peng C, Ning H, Zhang F, You J. Expression of Y-Box-binding protein 1 in Chinese patients with breast cancer. Tumour Biol. 2012;33:63–71. doi: 10.1007/s13277-011-0246-6. [DOI] [PubMed] [Google Scholar]