Abstract
Purpose: Pathological evidence has continually supported the prognostic value of tumour-infiltrating T lymphocytes for several solid tumours across diverse patient cohorts. To investigate the clinical relevance of tumour-infiltrating memory T lymphocytes, we investigated relevant publications to identify the significance of memory tumour-infiltrating T lymphocytes (TILs) in predicting survival in cancer patients and analysed the influence of variations in the tumour stage and the infiltrated location thereon. Methods: Relevant publications that assessed the clinical relevance of memory TILs to the patient’s overall survival (OS) were investigated. Disease-free survival (DFS) was also evaluated where possible. Hazard ratios (HR) and 95% confidence intervals (CI) were assessed using a random-effects model. Heterogeneity was investigated with stratified analysis and I2 statistics. Results: In total, 16 relevant publications, including 4248 cancer patients, were analysed. In the pooled analysis, intra-tumour accumulation of memory TILs correlated positively with favourable clinical outcomes for both OS (pooled HR, 2.06; 95% CI, 1.76-2.40) and DFS (pooled HR, 2.31; 95% CI, 1.67-3.19). Various effects of the tumour stage and the anatomical region where the cells infiltrated were identified in survival prediction, using memory TILs. Conclusions: Overall, infiltration of memory TILs can serve as a biomarker for survival prediction in cancer. Additional heterogeneous effects of the associated factors should be considered when categorizing high-risk patients.
Keywords: Cancer, memory T cells, prognosis, survival, tumour-infiltrating lymphocyte
Introduction
Malignant tumours constitute a major life-threatening health problem worldwide. Although progress in anti-cancer therapy has markedly improved survival and quality of life of cancer patients, the response to therapy and patient outcomes remain significantly variable. This variability is due to the intrinsic heterogeneity of both cancer and the affected individuals, in terms of properties such as grade, stage, pathohistological type, and sensitivity to chemotherapy [1,2]. Therefore, further investigation is needed to develop novel immunologic parameters for individualized intervention [3]. Recent studies have verified several immunological biomarkers for use in prognostic prediction and have supported the predictive significance of an effective anti-tumour response among cohorts [4]. These biomarkers include tumour-infiltrating T lymphocytes (TILs), a tumour-associated antigen (TAA)-reactive expanded T-cell population that secretes cytokines characteristic of effector cells [5-7].
The immune context of tumours has been extensively investigated over the past decade, leading to the development of a variety of approaches that may lead to novel anticancer treatments. TILs, which comprise a variety of T lymphocyte subsets [8,9], have recently gained attention for use as a prognostic marker in a number of human cancers [10,11]. T cells expressing CD45RO, which is the most suitable single marker of human memory T cells, have been demonstrated to play specific and significant roles in a number of human cancers such as colorectal cancer [12,13], gastric cancer [14], oesophageal carcinoma [15,16], and renal cell carcinoma [17]. Almost all cohort studies of tumours have reported that a favourable clinical outcome is correlated with the accumulation of memory TILs; however, heterogeneity in the quantification of infiltration and the various strategies for sampling memory TILs has limited the application of this predictor as a benchmark. Several studies have defined the entire CD45RO+ infiltrating cell population as memory TILs, whereas others have only focused on cytotoxic or effector memory TILs. Furthermore, the cut-off value used to categorize high-density and low-density memory TIL infiltration has not been consistent across studies. Questions remain as to whether these inconsistencies reflect variations in the study design or whether different clinical outcomes from diverse publications imply underlying immunological or environmental modifications of the anti-tumour immune response. It is also unclear whether associations between the quality of memory TILs and prognosis vary depending on survival-associated factors such as anatomical location and tumour stage.
In this study, we investigated the predictive value of memory TILs for overall survival (and disease-free survival where possible) across available publications with inconsistent observations. The secondary aim of the study was to establish whether there is an association between the characteristics of the tumour stage and memory TIL location with the variability in the results of previous studies.
Material and methods
Literature search strategy
Studies published prior to July 2014 were selected for further investigation. Relevant articles were included by two reviewers after trawling PubMed, EMBASE, and Cochrane databases, using the keywords “memory T cells”, “cancer”, and “prognosis”. No language restriction was considered. The reference lists of the included studies and relevant publications recommended by PubMed were also reviewed to identify additional relevant papers.
Criteria for inclusion and exclusion
The inclusion criteria of this study were the following: (1) diagnosis of patients by using clear pathological evidence; (2) identification of CD45RO+ T cells in surgically removed specimens or tissue microarrays; (3) evaluation of CD45RO+ T cell infiltration density by using an immunohistochemical method; and (4) the availability of sufficient information for estimation of the hazard ratio (HR) for high/low infiltrating density.
The following studies were excluded from this study: (1) letters, reviews, case reports, and editorials; (2) articles with insufficient data for hazard ratio calculation; (3) non-surgical treatment studies; and (4) studies based on peripheral blood specimens.
Our decisions to include or exclude the articles were not influenced by the authors’ names or the impact factors of journals in which the articles were published.
Data extraction
Data on study cohorts, methodology, and results were extracted. The author information, journal, year of publication, type of cancer, markers used to identify memory TILs, and sampling location were noted for each study. Methods used to classify high-density and low-density memory TIL infiltration were extracted. Survival plots for OS and DFS were obtained.
Measures
OS and DFS (where possible) were chosen as the index of this meta-analysis. Disease-specific or recurrence-free survival was used as a substitute if OS/DFS was lacking because these two indices are similar to OS and DFS, respectively. The cut-off value used to define high- and low-density CD45RO+ TIL infiltration in this meta-analysis is shown in Table 1.
Table 1.
Characteristics of 16 eligible clinical trials
| No | Author (s) | Year | Cancer | Location | Tissue | Stage | Definition | High/Low |
|---|---|---|---|---|---|---|---|---|
| 1 | A Oberg | 2002 | Colorectal cancer | Metastatic LN | FFPE slide | Duke’s stage C | 5 cells/500* field of vision | 36/54 |
| 2 | Franck Pages | 2005 | Colorectal cancer | Intra-tumour | TMA | All | 250 cells/square mm | 160/176 |
| 3 | Jerome Galon | 2006 | Colorectal cancer | CT/IM | TMA | All | median | 160/160 |
| 4 | HE Lee | 2008 | Gastric cancer | Intra-tumour | TMA | All | Other | 65/155 |
| 5 | RA de Jong | 2009 | Endometrial cancer | Intra-tumour | FFPE slide | All | Present/Absent | 181/117 |
| 6 | Ninke Liffers | 2009 | Ovarian cancer | primary/metastasis | TMA | All | Other | 123/109 |
| 7 | Franck Pages | 2009 | Colorectal cancer | CT/IM | TMA | Stage I, II | Other | 188/27 |
| 8 | Paul Salama | 2009 | Colorectal cancer | Intra-tumour/normal | TMA | Stage II, III | Median | |
| 9 | Sandra Rauser | 2010 | Oesophageal adenocarcinoma | Intra-tumour | TMA | All | 2.0 labelling index | |
| 10 | Won-Suk Lee | 2010 | Colorectal cancer | Intra-tumour | FFPE slide | Stage II | median | 25/29 |
| 11 | Rui-Qing Peng | 2010 | Colon cancer | Intra-tumour | FFPE slide | Stage IIIB | 24 cells per high-power field | 51/14 |
| 12 | Rui-Qing Peng | 2010 | Colon cancer | Intra-tumour | FFPE slide | Stage IIIB | 26 cells per high-power field | 51/17 |
| 13 | K Hotta | 2011 | Renal cell carcinoma | Intra-tumour | FFPE slide | All | 49.8 cells/400* magnification | 52/53 |
| 14 | K Enomoto | 2012 | Oesophageal squamous cell carcinoma | Intra-tumour | FFPE slide | All | 113 cells/400* magnification | 54/51 |
| 15 | Qiang Gao | 2012 | Hepatocellular carcinoma | Intra-/peri-tumour | TMA | All | median | |
| 16 | Kohei Wakatsuki | 2013 | Gastric cancer | Intra-tumour | FFPE slide | Advanced | 133 cells/400* magnification | 37/37 |
CT: central tumor; IM: infiltrative margin; LN: lymph node; FFPE: formalin-fixed paraffin-embedded; TMA: tissue microarray.
Statistical analysis
The effects of memory TIL infiltration were evaluated by hazard ratio (HR), which was defined as the risk of the poor prognosis ratio of low/high density infiltration. In studies that calculated HRs by using the ratio of low/high density memory TIL infiltration, the reciprocal of the HR and 95% CI was used for our analysis. Analyses were performed using SPSS Version 19.0 (SPSS, Chicago, IL, USA) and Review Manager Version 5.2.
Results
Study selection and characteristics
Sixteen eligible clinical trials that met the inclusion criteria for survival analysis were selected for further evaluation (Table 1) [12-27]. Figure 1 provides a summary of the included studies. The primary assessment of the included publications demonstrated relatively high quality scores (median quality score: 0.82; range: 0.69-0.91). All of the studies used immunohistochemical methods for detecting memory TILs, and half of the studies (8/16) used tissue microarrays as a high-throughput method to evaluate memory TILs. The threshold used to define high and low infiltration densities is shown in Table 1.
Figure 1.
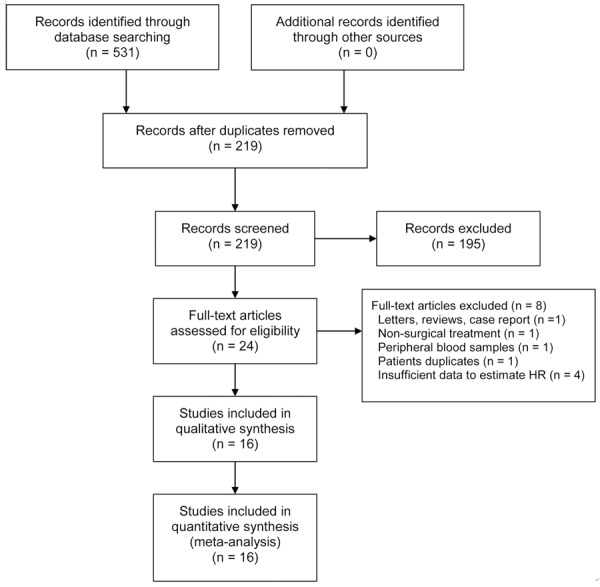
Flowchart of study identification and inclusion.
Pooled analysis
We performed this investigation under the assumption of homogeneity, and stratified by the pathological stage of the tumour and the anatomical location in which the memory TILs were counted. All studies in this analysis used CD45RO as a memory phenotype marker for TILs, except for Galon et al., who defined CD3+CD45RO+ double-positive TILs as memory T cells. All of the studies reported that patients with a high density of memory TILs showed a prolonged survival time with respect to both OS (Figure 2) and DFS (Figure 3). The favourable clinical outcome for OS was significant (Figure 2; HR: 2.06; 95% CI: 1.76-2.40). Furthermore, the DFS analysis, which was conducted using eight publications [12,13,15,16,20,23], revealed the same association bet-ween high-density memory TIL infiltration and survival (HR > 1), and the HR for DFS was higher than that for OS (Figure 3; HR: 2.31; 95% CI: 1.67-3.19). A moderate heterogeneity of OS estimation among the studies was noted (I2 = 50%). In contrast, for DFS estimation, we a highly heterogeneous distribution of HRs was reported for the cohorts (HR range: 1.42-6.16, I2 = 87%), suggesting that the effects of memory TIL-mediated anti-tumour immunity on the prevention of recurrence are complex.
Figure 2.
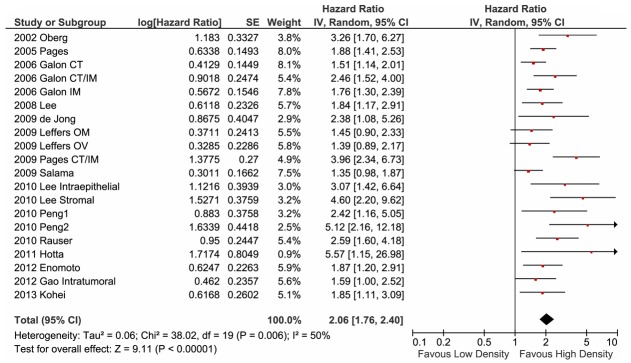
Forest plot of hazard ratio of memory tumour-infiltrating T lymphocyte (TIL) density for overall survival. Hazard ratios (HR) and 95% confidence intervals (CI) from each paper are shown as horizontal black bars with red dots. The pooled estimation is illustrated with a black diamond; the centre represents the pooled HR and the horizontal range indicates the 95% CI. The HRs are defined as the ratio of lower CD45RO density to higher CD45RO density, except for the 2006 Galon evaluation, which defined CD3+CD45RO+ cells as memory TILs. Therefore, a HR greater than unity represents a prolonged overall survival associated with a higher density of CD45RO+ TIL infiltration. CT, centre of tumour site; IM, invasive margin; CT/IM, both sites measured. OM, evaluated with tissue from omental metastasis; OV, tissue from primary lesion of ovarian cancer.
Figure 3.
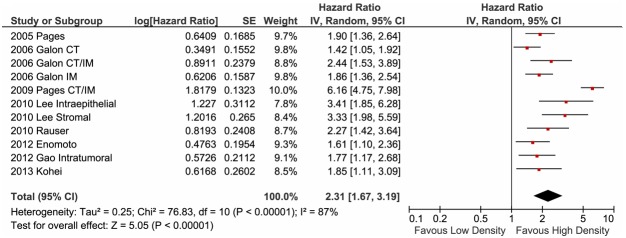
Forest plot of hazard ratio of memory tumour-infiltrating T lymphocyte (TIL) density for disease-free survival. Hazard ratios and 95% confidence intervals (CI) for disease-free survival associated with low density versus high density of CD45RO memory TIL infiltration. CT, centre of tumour site; IM, invasive margin; CT/IM, both sites measured.
Stratified analysis
In the case of CD45RO+ memory TILs for all cancer patients, we confirmed a significant association between a lack of memory T cell infiltration and an increased risk of clinical relapse. However, the prognostic value of this association may vary, based on the properties of the tumour and the host. Thus, we next evaluated the effects of the location of TIL infiltration and tumour stage on survival.
First, we examined the influence of the anatomical region of memory T cell infiltration on the HR for OS. Five studies [12,19-21,26] that evaluated the clinical relevance of memory TILs in certain niches were included in this analysis. Five studies reported that high-density intra-tumour memory TIL infiltration was a significant but less overt indicator of favourable outcomes (Figure 4; HR: 1.73; 95% CI: 1.37-2.17). However, in the remaining three studies, which reported the presence of extra-tumour memory T cell infiltration [19,21,26], almost no influence on patient prognosis was observed (Figure 4; HR: 1.02; 95% CI: 0.77-1.35). These findings demonstrate that CD45RO+ T cells may contribute to the anti-tumour immune response within the tumour microenvironment, but not within the surrounding area. Despite this distinct difference in the biological effect of memory TILs on patient outcome, we identified pooled evidence that supports the beneficial impact of a dense infiltration of memory TILs (Figure 4; HR: 1.49; 95% CI: 1.17-1.89).
Figure 4.
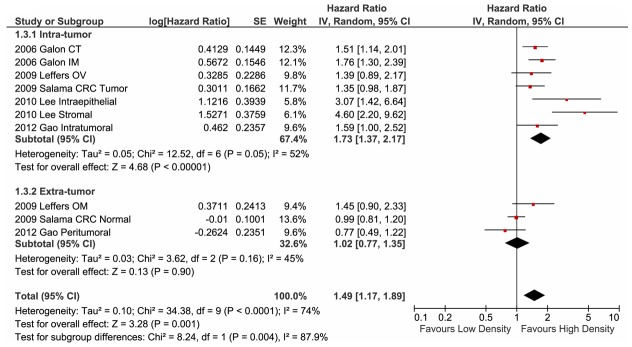
Forest plot of hazard ratios for studies on the location of memory TILs. Pooled hazard ratios and 95% confidence intervals (CI) for the association of memory T cells and overall survival. The density of memory T cells was evaluated for primary tumour sites or other sites as indicated. CT, centre of tumour site; IM, invasive margin; CT/IM, both sites measured; OM, evaluated with tissue from omental metastasis; OV, tissue from primary lesion of ovarian cancer; CRC, colorectal cancer.
The close association of cancer patient prognosis with the stage of tumour progression has been clearly established. This prompted us to investigate whether the tumour stage is a factor in the prognostic effects of memory TILs on OS. Thus, we investigated the prognostic significance of memory TIL infiltration in cohorts with stage I+II and stage III+IV cancer patients. For this analysis, we employed six studies that reported sufficient information to estimate the HRs [14,16,20,23-25], and separated them into two subgroups: stage I+II and stage III+IV. The clinical presentation and outcome of stage IIB oesophageal cell carcinoma are highly similar to those of the advanced stage; therefore, we classified stage IIB-IV oesophageal tumours into the stage III+IV subgroup. Using this stratified analysis, the most significant pooled protection effect of memory TILs was observed for patients with stage I or stage II tumours (Figure 5; HR: 3.31; 95% CI: 2.46-4.46). This indicated that anti-tumour immunity is effective in the restriction of tumour progression during the early stages. A much less marked (HR: 1.39; 95% CI: 1.01-2.82) and heterogeneous (HR range: 0.97-5.12, I2 = 75%) level of protection was observed for patients with advanced-stage tumours (Figure 5). Despite this difference in HRs for cancer patients with different tumour stages, the overall effect of memory TIL infiltration was favourable for the clinical outcome (HR: 2.28; 95% CI: 1.47-3.53), consistent with our earlier findings for all patients (shown in Figure 2).
Figure 5.
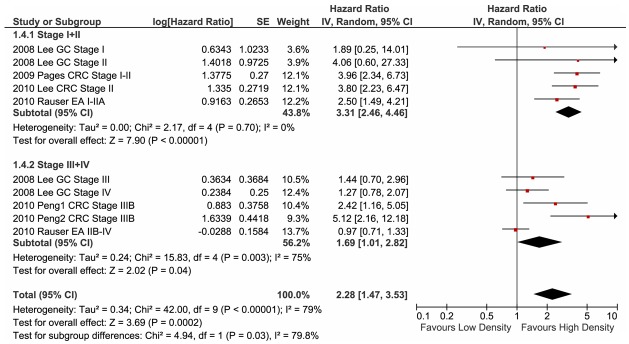
Forest plot of hazard ratios for studies on the staging of tumours. Pooled hazard ratios and 95% confidence intervals (CI) for cancer patients in stage I+II and those in stage III+IV. GC, gastric cancer, IUAC stage; EA, oesophageal adenocarcinoma, UICC stage; CRC, colorectal cancer, IUAC stage.
Publication bias
In this systematic review, published studies may not truly represent all related studies undertaken. Bias may be introduced when non-significant findings remain inaccessible, thus distorting the apparent magnitude of certain factors. In our study, OS and DFS following high- and low-density memory T cell infiltration in patients with several cancer types were calculated using the random-effect model. Using this analysis, we generated consistent results with high reliability. The funnel plots for survival and recurrence following high- and low-density memory T cell infiltration showed basic symmetry, suggesting that no significant publication bias existed (Figure 6).
Figure 6.
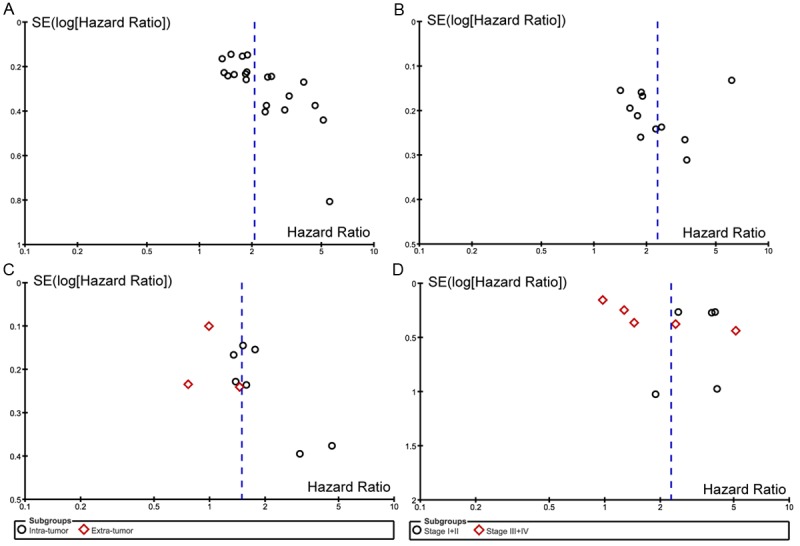
Funnel plot of hazard ratios and standard errors (SE) for the individual studies. No significant publication bias was observed.
Discussion
Our systematic review indicated that memory T lymphocyte infiltration of a tumour site could serve as a robust indicator for OS and DFS prediction in patients with malignant tumours. This observation was reproducible across diverse cohorts, despite the significant heterogeneity among studies in terms of both patient characteristics and categorization criteria. CD45RO, an isoform of the human leukocyte common antigen, was broadly accepted for specifically assaying the infiltration of memory TILs in the majority of the studies (15/16) included in this meta-analysis. CD45RO staining for identification of memory T lymphocytes is a superior benchmark for the evaluation of T cell infiltration in cancer patient specimens than CD3 staining alone, which has been used in previous reports [10]. Despite identifying no substantial difference in the pooled analysis for OS prediction (pooled HR: 2.06; 95% CI: 1.76-2.40) and DFS (pooled HR: 2.31; 95% CI: 1.67-3.19), CD45RO+ memory TIL infiltration showed a stronger although less consistent association with DFS than with OS. Furthermore, significant heterogeneity in the HRs was observed when the patients were separated based on different thresholds for defining high- and low-density infiltration. Since the infiltration of memory T lymphocytes represents a universal pathological observation in tumours, a standard approach for measuring positivity of memory TILs would facilitate future clinical trials. We noted that the median memory TIL values used to determine a positive score were significantly heterogeneous across studies of distinct types of cancer. Therefore, we propose that cancer type-specific criteria should be established for defining memory TIL-positive tumours.
Even though numerous studies have confirmed the significant influence of memory TILs in a number of cancer types, the prognostic value of this biomarker is still not entirely accepted, hampering its application in the guidance of treatment and individual patient prognostication. This may be due to the heterogeneous nature of memory TILs within the definition of CD45RO-positivity and the inconsistence in the methodology used in such studies. The major purpose of our meta-analysis was to integrate the heterogeneous observations from currently available publications to support the further adoption of this predictive biomarker as a benchmark. Additionally, continuing accumulation of knowledge on the overall impact of chemotherapeutics on the immune context of the tumour microenvironment may further promote direct screening of the infiltration of memory T cells and evaluation of chemical compounds in future [28].
Clinical association of intra-tumour memory TILs with a favourable prognosis strongly implies that immune-mediated mechanisms may spontaneously function in at least some patients with cancer, and that these mechanisms have a profound impact on the suppression of tumour growth and metastasis. Future studies should focus on elucidating the underlying immunomechanism by which subpopulations of memory TILs contribute to tumour suppression, reduced clinical relapse, and prolonged survival.
It is well-known that the interplay between emergent tumour cells and the immune context plays a critical role throughout the various stages of tumourigenesis [29]. Surprisingly, our analysis revealed that the prognostic significance of memory TILs varied markedly with distinct tumour stages. In comparison to tumours in the advanced stage, the protective effects of memory T cell infiltration was significantly reduced at the early stage in many types of cancers (Figure 5), suggesting a preferential functional effect on early tumourigenesis. Since most studies on memory TILs have been based on surgical-debulking specimens, our findings suggest that infiltrating memory T lymphocytes may play a crucial role in avoiding tumour recurrence in post-surgery patients.
In terms of the effect of the location of TIL infiltration, greater advantageous effects were observed among diverse cohorts showing intra-tumour memory T cells than among those showing infiltration of extra-tumour sites. It has been shown that the gastrointestinal tract mucosa is a well-studied niche that harbours viral antigen-specific resident memory T cells [30-32]. When forced into circulation, the T cells isolated from the mucosa niche were severely impaired in terms of survival and proliferation during antigen re-challenge [33,34]. Thus, our result suggests that the intra-tumour microenvironment might be a favourable niche for maintaining the surveillance function of memory TILs to against clinical recurrence. In conclusion, a prolonged survival time was consistently identified among cohort with dense infiltration of memory T cells within the tumour site, regardless of the progress stage of the tumour. Besides, further study is needed to identify the impact of other confounding factors such as genetic, behavioural, or environmental modifications to memory TILs in prognostic prediction.
The study had some limitations. Firstly, only 16 eligible publications were selected for this investigation and all the publications were retrospective studies. Although our results were drawn from studies measuring different lymphocyte populations (CD3+CD45RO+ and CD45RO+) and different grouping strategies impeded the direct comparison across studies, our findings still support the observation that memory TIL infiltration is a favourable indicator in survival prediction. Secondly, due to lack of information, some confounding factors such as the infiltration of effector-like memory T cells or FoxP3+ regulatory T cells could not be taken into consideration in this study. These lymphocytes may contribute to additional cytotoxic effects or may regulate the anti-tumour immune response, thereby influencing the predictive value of memory TILs. Finally, most of the publications included in our analysis did not provide sufficient information to investigate the treatment response to adjuvant therapy. Further randomized controlled trials will be required to confirm the prognostic significance of memory TIL status in the efficacy of this treatment strategy.
In summary, we have demonstrated that the finding of intra-tumour infiltration by CD45RO+ memory T cells is a robust immunological indicator of a favourable survival time in cancer patients, and that CD45RO is more sensitive than CD3 for categorizing patients, based on clinical outcomes. In our study, the predictive significance of memory TILs was reproducible among cohorts with diverse tumour types, regardless of the detailed characteristics of the patients. The variability that we observed based on the location of TIL infiltration suggests that some environmental factors may modulate the anti-tumour immune response. Additionally, we identified a poor-protective effect of memory TILs in advanced stage patients, implying that an immunosurveillance mechanism may contribute to preventing clinical recurrence. Taken together, quantification of memory T lymphocytes in a tumour site could provide feasible criteria for patient selection in clinical trials aimed at evaluating the efficacy of immunotherapy. Enhancement of the quantity and quality of memory TILs in situ may provide a novel strategy to improve the prognosis of cancer patients.
Acknowledgements
We thank Dr. Qian Zhang, Dr. YY Li and Dr. Jun Niu for useful suggestions.
Disclosure of conflict of interest
None.
References
- 1.Nguyen TT, Wright JD, Powell MA, Gibb RK, Rader JS, Allsworth JE, Mutch DG. Prognostic factors associated with response in platinum retreatment of platinum-resistant ovarian cancer. Int J Gynecol Cancer. 2008;18:1194–1199. doi: 10.1111/j.1525-1438.2007.01184.x. [DOI] [PubMed] [Google Scholar]
- 2.Winter WE 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 3.Bindea G, Mlecnik B, Angell HK, Galon J. The immune landscape of human tumors: Implications for cancer immunotherapy. Oncoimmunology. 2014;3:e27456. doi: 10.4161/onci.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halapi E, Yamamoto Y, Juhlin C, Jeddi-Tehrani M, Grunewald J, Andersson R, Hising C, Masucci G, Mellstedt H, Kiessling R. Restricted T cell receptor V-beta and J-beta usage in T cells from interleukin-2-cultured lymphocytes of ovarian and renal carcinomas. Cancer Immunol Immunother. 1993;36:191–197. doi: 10.1007/BF01741091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi K, Yonamine K, Masuko-Hongo K, Iida T, Yamamoto K, Nishioka K, Kato T. Clonal expansion of T cells that are specific for autologous ovarian tumor among tumor-infiltrating T cells in humans. Gynecol Oncol. 1999;74:86–92. doi: 10.1006/gyno.1999.5430. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto Y, Backlin K, Nakagomi H, Halapi E, Juhlin C, Bucht A, Kiessling R. Cytotoxic activity and T cell receptor repertoire in tumor-infiltrating lymphocytes of adrenal cell carcinomas. Cancer Immunol Immunother. 1993;37:163–168. doi: 10.1007/BF01525430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiraoka N. Tumor-infiltrating lymphocytes and hepatocellular carcinoma: molecular biology. Int J Clin Oncol. 2010;15:544–551. doi: 10.1007/s10147-010-0130-1. [DOI] [PubMed] [Google Scholar]
- 9.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124:192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 14.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enomoto K, Sho M, Wakatsuki K, Takayama T, Matsumoto S, Nakamura S, Akahori T, Tanaka T, Migita K, Ito M, Nakajima Y. Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol. 2012;168:186–191. doi: 10.1111/j.1365-2249.2012.04565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauser S, Langer R, Tschernitz S, Gais P, Jutting U, Feith M, Hofler H, Walch A. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I-IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10:608. doi: 10.1186/1471-2407-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–1196. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q, Zhou J, Wang XY, Qiu SJ, Song K, Huang XW, Sun J, Shi YH, Li BZ, Xiao YS, Fan J. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:455–466. doi: 10.1245/s10434-011-1864-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee WS, Park S, Lee WY, Yun SH, Chun HK. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer. 2010;116:5188–5199. doi: 10.1002/cncr.25293. [DOI] [PubMed] [Google Scholar]
- 21.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberg A, Samii S, Stenling R, Lindmark G. Different occurrence of CD8+, CD45R0+, and CD68+ immune cells in regional lymph node metastases from colorectal cancer as potential prognostic predictors. Int J Colorectal Dis. 2002;17:25–29. doi: 10.1007/s003840100337. [DOI] [PubMed] [Google Scholar]
- 23.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J. Clin. Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 24.Peng RQ, Chen YB, Ding Y, Zhang R, Zhang X, Yu XJ, Zhou ZW, Zeng YX, Zhang XS. Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol. 2010;16:2428–2434. doi: 10.3748/wjg.v16.i19.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng RQ, Wu XJ, Ding Y, Li CY, Yu XJ, Zhang X, Pan ZZ, Wan DS, Zheng LM, Zeng YX, Zhang XS. Co-expression of nuclear and cytoplasmic HMGB1 is inversely associated with infiltration of CD45RO+ T cells and prognosis in patients with stage IIIB colon cancer. BMC Cancer. 2010;10:496. doi: 10.1186/1471-2407-10-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 27.Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO(+) memory T cells on human gastric cancer. Oncol Rep. 2013;29:1756–1762. doi: 10.3892/or.2013.2302. [DOI] [PubMed] [Google Scholar]
- 28.Kandalaft LE, Singh N, Liao JB, Facciabene A, Berek JS, Powell DJ Jr, Coukos G. The emergence of immunomodulation: combinatorial immunochemotherapy opportunities for the next decade. Gynecol Oncol. 2010;116:222–233. doi: 10.1016/j.ygyno.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 30.Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 34.Isakov D, Dzutsev A, Belyakov IM, Berzofsky JA. Non-equilibrium and differential function between intraepithelial and lamina propria virus-specific TCRalphabeta(+) CD8alpha-beta(+) T cells in the small intestinal mucosa. Mucosal Immunol. 2009;2:450–461. doi: 10.1038/mi.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]


