Abstract
Aim: This study was to prepare the functionalized nano-graphene oxide (nano-GO) particles, and observe targeted fluorescence imaging and photothermy of U251 glioma cells under near infrared (NIR) exposure. Material and methods: The functionalized nano-GO-Tf-FITC particles were prepared and then were incubated with U251 glioma cells. Estimation of CCK8 cell activity was adopted for measurement of cytotoxicity. The effect of fluorescein imaging was detected by fluorescence microscope with anti-CD71-FITC as a control. Finally, we detected the killing efficacy with flow cytometry after an 808 nm NIR exposure. Results: Both nano-GO-Tf-FITC group and CD71-FITC group exhibited green-yellow fluorescence, while the control group without the target molecule nano-GO-FITC was negative. The nano-GO-Tf-FITC was incubated with U251 cells at 0.1 mg/ml, 1.0 mg/ml, 3.0 mg/ml and 5.0 mg/ml. After 48 h of incubation, the absorbance was 0.747 ± 0.031, 0.732 ± 0.043, 0.698 ± 0.051 and 0.682 ± 0.039, while the absorbance of control group is 0.759 ± 0.052. There is no significant difference between the nano-GO-FITC groups and control group. In addition, the apoptosis and death index of nano-GO-Tf-FITC group was significantly higher than that of nano-GO-FITC and blank control group (P < 0.05). Conclusion: The nano-GO-Tf-FITC particles with good biological compatibility and low cytotoxicity are successfully made, which have an observed effect of target imaging and photothermal therapy on glioma U251 cells.
Keywords: Glioma, graphene oxide, transferring, fluorescence imaging, photothermy
Introduction
Glioma is one of the most common malignancies in the nervous system. Due to its infiltrative growth and unclear margin with normal brain tissue around, glioma is difficult to remove completely. Even combined with postoperative radiotherapy and chemotherapy, glioma has a high recurrence rate and poor prognosis. In consequence, novel therapies that can improve the therapeutic effect and reduce side effects have always been explored [1-3]. The strong capacity of light absorption of nano-particles extends the absorption of visible light and near infrared ray in PTA (photothermal ablation therapy). This property of nano metallic material can induce protein degeneration and kill the tumor cells by localized heating [4-7]. Compared with precious metals and other types of nano-material, graphene is hailed as the best potential carbon material because of its unique electrical properties, optical properties and thermal capacity [8,9]. GO (graphene oxide) is a hot spot of research on graphene. The abundant function groups on its surface provide a variety of active sites for conjunction of organic small molecules, polymers, biomacromolecules and other functional groups [10-12]. They can couple with some specific antibodies and ligands, which make multi-functional nanoparticles possible. The present study was designed to prepare the functionalized nano-graphene oxide (nano-GO) particles, and observe targeted fluorescence imaging and photothermy of U251 glioma cells under near infrared (NIR) exposure.
Materials and methods
Reagents and equipment
Graphene (≤ 30 μm) was purchased from Sinopharm Chemical Reagent Co., Ltd. Concentrated sulfuric acid, potassium permanganate, potassium nitrate, hydrogen peroxide (30%), hydrochloric acid, ferric chloride, FITC (fluorescein isothiocyanate) and L-Lysine were purchased from Shanghai Jingchun industrial co., ltd. Cell Counting Kit-8 (CCK-8) for cell proliferation and cytotoxicity was purchased from Dojindo (Japan). Cell streaming Annexin V/PI cell apoptosis detection kit, pancreatin (1:250, containing EDTA), Tf and PBS (pH = 7.4) were purchased from Invitrogen (USA). Anti-CD71-FITC antibody was purchased from Abcam (USA), and 10% DMEM medium with high glucose and fetal bovine serum (FBS) were purchased from Gibco (USA). The cell incubator (HERA cell 240i) was purchased from Thermo (USA). The infrared laser (λ: 808 nm) was purchased from Changchun Leishi Technology Co., Ltd. Fourier Transform Infrared Spectrometer (TENSOR 27) was purchased from BRUKER (Germany); Automatic inverted fluorescence microscope (Axio Observer Z1) was purchased from Carl Zeiss (Germany). High-resolution transmission power mirror (JEM-2010) was purchased from JEOL Corporation (Japan).
Preparation of functionalized nano-GO-Tf-ITC particles
Synthesis of GO-N3
After prepared by the improved Hummers method [13], 50 mg GO was added into 20 ml DMSO. Add 108 mg DDC and 31.35 mg DMAP into the mixtures after a 30-minute-ultrasonic dispersion. 160 mg azide propylamine was added and a 48-hour-reaction was taken. The reaction liquor was dialyzed with tap water for 1 day and with distilled water for 2-3 days (MWCO = 14000), and then freeze-dried to get GO-N3.
Synthesis of PLLD-G3
1.73 g (5 mmol) Boc-Lys (Boc)-OH and 350 μL (5.5 mmol) propargylamine were dissolved in 20 ml DMF. The mixture was stirred under nitrogen for 10 min and was cooled to 0°C, and then 2.18 g (5.5 mmol) HBTU and 0.74 g (5.5 mmol) HOBt were added. After the solution was stirred for 16 h, ethyl acetate was added. The organic phase was separated and washed with saturated NaHCO3 solution, NaHSO4 solution (0.1 mol/L), saturated NaHCO3 solution and brine. The compounds was purified by column chromatography (silicone, CHCl3-CH3OH = 5:1) and then distilled with rotary evaporator to obtain the first generation of poly-lysine (G1). The synthetic method of G2 and G3 was the same with G1, and G3 was obtained by continuously reacting with G1 and G2 synthesized.
Synthesis of nano-GO-PLLD-G3
10 mg GO-N3 was dissolved in 4 mL DMSO and dispersed under sonication for 0.5 h. 10 mg PLLD-G3 was dissolved in 6 mL water and added into GO-N3 solution. 12.5 mg anhydrous cupric sulfate and 80 mg sodium ascorbate were added into the solution after 30-minute-aeration of nitrogen. Then the reaction continued for 48 h at 40°C with nitrogen aeration. The reaction liquor was dialyzed for 3 days by dialysis bag (MWCO = 14000) and was freeze-dried to obtain GO-PLLD-G3.
Synthesis of nano-GO-Tf-FITC particles
50 mg GO-PLLD-G3 was distributed well in pure water, and 5 mg Tf and FITC were dissolved in the dispersion liquid GO-PLLD-G3 respectively. The mixture was dialyzed to remove unloaded reagent after stirring the mixture at 4°C in laminar flow for 48 h. The product was freeze-dried to obtain the functionalized nano-GO-Tf-FITC. In another group, nano-GO-FITC was obtained with GO-PLLD-G3 and FITC and without transferrin.
Assignment of particle diameter and surface morphology of nano-GO-Tf-FITC
Nano-GO-Tf-FITC sample solution with the concentration of 2.0 mg/ml was prepared. A drop of sample solution was dripped on a copper net covered with carbon film. The supernatant water was dried with filter paper 1 minute later. The sample was dried naturally at room temperature and the morphology was observed under JEM-2010HR transmission electron microscopy. The accelerating voltage of TEM is 200 Kv. The instrument was vacuumized, and the morphology of the sample was observed and photographed. The particle diameter of sample was measured.
Cell culture
Human glioma U251 cell lines were purchased from ATCC Stem Cell Library (USA). Cells were cultured in high glucose DMEM medium contained 10% fetal bovine serum and 1% penicillin/streptomycin. And the cells were placed at 37°C in a humidified incubator under 5% CO2. The cells were digested with 0.25% trypsin enzyme and passaged in a 1:3. An experiment was performed for the cells in logarithmic grow phase, and the cell survival rate was over 95%.
Detection of the in vitro cytotoxicity of functionalized nano-GO-Tf-FITC particles
The cytotoxicity of functionalized nano-GO-Tf-FITC particles was detected with CCK-8 cell viability test. U251cells in logarithmic grow phase were inoculated in 96-well plate at 2 × 10 [3] each well. The capacity of a well was 200 μL. After a 24-hour incubation, they were added into NGO-Tf-FITC at concentrations of 0.1, 1.0, 3.0, 5.0 mg/ml. Four wells were set for every single concentration. After 48-hour incubation, the medium is removed. 100 μL medium and 5 μL CCK8 reagent was added into each well, and the plate was incubated at 37°C in an incubator. After 30 minutes, the absorbance of each well was measured at 450 nm using a microplate reader the well without cells was taken as control and the blank well was used for zero adjustment. The rate of cell survival in the medium above was calculated by the formulas W = (A-A0)/(A1-A0) × 100%, in which, W, A, A0 and A1 referred to cell survival rate, absorbance of experimental wells, absorbance of blank wells, and absorbance of control wells, respectively.
In vitro fluorescence imaging of functionalized nano-GO-Tf-FITC particles
U251 cells in logarithmic grow phase were inoculated in 96-well plate at 2 × 103 each well. The capacity of a well was 200 μL. The cells were divided into 3 groups after incubated for 48 hours: targeted experimental group in which 3 mg/mL nano-GO-Tf-FITC was added into each well; control group in which 5 mg/mL anti-CD71-FITC reagent was added into each well; non-targeted control group in which 3 mg/mL nano-GO-FITC was added into each well. There were four wells for each group. After incubated at 37°C for 30 minutes, the cells were rinsed with PBS buffer twice, and observed and photographed under the automatic fluorescent microscope.
Detection of the photothermy of functionalized nano-GO-Tf-FITC particles in vitro
U251cells in logarithmic grow phase were inoculated in 35-mm petri dish at 1 × 105 each well. After the cells were incubated for 48 hours, 1 ml NGO-Tf-FITC particles (3 mg/L) were added into each experimental well and control well, respectively, while no particle was added into the blank group well. There were 3 wells for each group. After culture at 37°C for 1 h, the cells were rinsed with PBS buffer twice. Each well was exposed to 808 nm near infrared ray with power of 7.5 W/cm2 (the beam: 0.4 cm2) for 5 minutes. After culture at 37°C for 2 hours, the cells were collected and detected by Annexin V/PI cell apoptosis kit following the instructions. 1 × 105 cells were detected in each sample.
Statistical analysis
Data were analyzed using SPSS 13.0 software. Comparisons of the inter-group data were performed with one-way analysis of variance. The difference with P < 0.05 was considered statistically significant.
Results
Infrared spectra characteristic of GO and GO-N3
The infrared spectra of the compositions GO and GO-N3 was tested with FT-IR (Nicolte Nexs 607, USA). -OH (3400 cm-1), C = O (1722 cm-1), C = C (1609 cm-1) and C-O (1109 cm-1) were infrared absorption peaks of pure GO functional groups. Special peak was displayed at 2100 cm-1 after azido grafting with GO (Figure 1). The results illustrated a successful grafting of azido to GO. The element analysis informed that the content of azido in 1 g GO-N3 was 4.43%.
Figure 1.
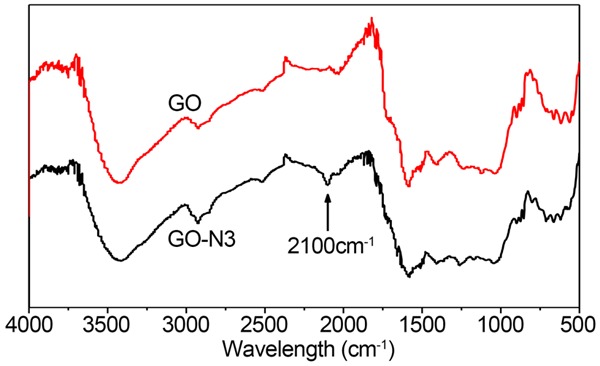
IR spectra of GO and GO-N3. The infrared spectra of the compositions GO and GO-N3 was tested with FT-IR (Nicolte Nexs 607, USA). -OH (3400 cm-1), C = O (1722 cm-1), C = C (1609 cm-1) and C-O (1109 cm-1) were infrared absorption peaks of pure GO functional groups. Special peak was displayed at 2100 cm-1 after azido grafting with GO.
Particle diameter and surface morphology of nano-GO-Tf-FITC
Nano-GO showed monolithic layer under the JEM-2010 HR high-resolution Transmission electron microscopy. The diameter of nano particles was 20-200 nm (Figure 2A, 2B). The particles diameters of Nano-GO-PLLD-G3 prepared through modification by alkynyl-polylysine were within 100nm, presented as monolithic layer. The solution of nano-GO-PLLD-G3 is bright yellow, while the transparency decreased with the increase of concentration. The dispersed phase was still stable after 3 months (Figure 2C).
Figure 2.

TEM images of nano-GO. A, B: The diameter of nano particles was 20-200 nm. C: The solution of nano-GO-PLLD-G3 is bright yellow, while the transparency decreased with the increase of concentration. The dispersed phase was still stable after 3 months.
In vitro cytotoxicity of functionalized nano-GO-Tf-FITC particles
The nano-GO-Tf-FITC was incubated with U251 cells at concentrations of 0.1 mg/ml, 1.0 mg/ml, 3.0 mg/ml and 5.0 mg/ml. After a 48-hour incubation, the absorbance of each well was measured at 450 nm using a microplate reader. The absorbances were 0.747 ± 0.031, 0.732 ± 0.043, 0.698 ± 0.051 and 0.682 ± 0.039, while the absorbance of control group was 0.759 ± 0.052. There was no significant difference between the nano-GO-FITC groups and control group (P > 0.05), suggesting that nano-Tf-FITC particles had good biological compatibility and low cytotoxicity (Figure 3).
Figure 3.

CCK8 cytotoxicity results. A1, A2, A3 and A4 for the concentrations of 0.1, 1.0, 3.0 and 5.0 mg/mL group, control for the blank control group. The absorbances were 0.747 ± 0.031, 0.732 ± 0.043, 0.698 ± 0.051 and 0.682 ± 0.039 for A1, A2, A3 and A4 group respectively, while the absorbance of control group was 0.759 ± 0.052. There was no significant difference between the nano-GO-FITC groups and control group (P > 0.05).
Analysis of fluorescent imaging of nano-GO-Tf-FITC particles to U251 cells
Both of nano-GO-Tf-FITC group and anti-CD71-FITC group exhibited yellow-green fluorescence under the fluorescent microscope (Figure 4A and 4B), while the control group without the target molecule nano-GO-FITC did not showed fluorescent image (Figure 4C). The results showed that comparing with the control group anti-CD71-FITC, the successful combination of nano-GO-Tf- FITC with cells, as well as nano-GO integrated with the target molecule and fluorescent molecule. Nano-GO-FITC was not linked with the target molecules, so that they cannot integrate with U251 cells, therefore, no fluorescence were shown.
Figure 4.

Fluorescence images of U251 combining with carriers (inverted fluorescence microscope, × 100). Both of nano-GO-Tf-FITC group (A) and anti-CD71-FITC group (B) exhibited yellow-green fluorescence under the fluorescent microscope, while the control group without the target molecule nano-GO-FITC did not showed fluorescent image (C).
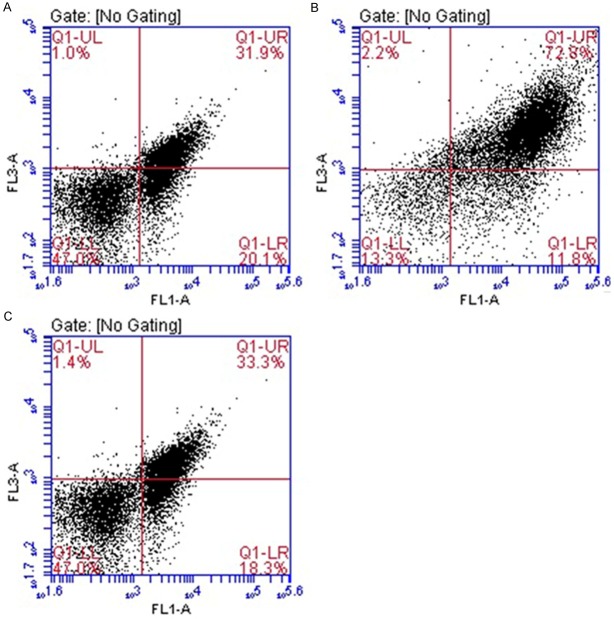
The photothermy of nano-GO-Tf-FITC particles to U251 cells in vitro
Cells apoptosis and death index (%) of nano-FITC (negative control) group, nano-Tf-FITC (experimental group) group and blank control group were 52.6 ± 2.66 (A), 73.6 ± 3.41 (B) and 51.2 ± 2.93 (C), respectively, (Figure 5). Nano-Tf-FITC group had significant differences with both NGO-FITC group and blank control group (P < 0.05), which suggested that NGO-Tf-FITC particles had remarkable effect of targeted-combination and photothermal therapy. There is no statistically significant difference between nano-FITC group and blank control group (P > 0.05) (Figure 6), indicating that NGO-FITC particles can’t integrate with U251 cells due to the lack of the target molecule Tf.
Figure 5.
The flow cytometry results after treatment by near infrared radiation. Cells apoptosis and death index (%) of nano-FITC group (negative control group), nano-Tf-FITC group (experimental group) and blank control group were 52.6 ± 2.66 (A), 73.6 ± 3.41 (B) and 51.2 ± 2.93 (C), respectively.
Figure 6.
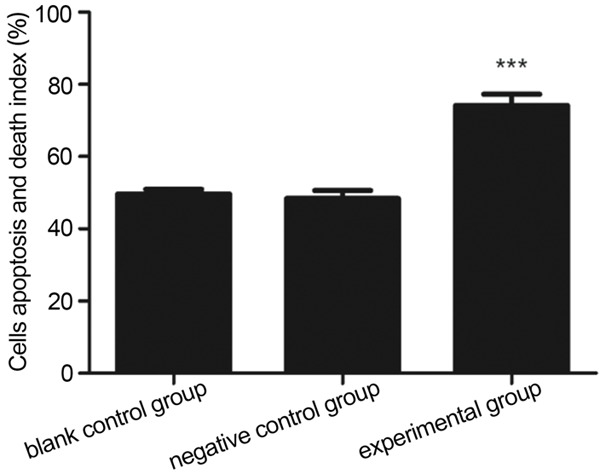
The comparison of flow cytometry results after treatment by near infrared radiation among different groups (***P < 0.05). Nano-Tf-FITC group (experimental group) had significant differences with both NGO-FITC group (negative control group) and blank control group (P < 0.05). There is no statistically significant difference between nano-FITC group (experimental group) and blank control group (P > 0.05).
Discussion
Brain glioma is the most common malignant tumor, which accounts for approximately 40-60% of intracranial tumors [14]. Five-year mortality rate of brain glioma ranked third in generalized malignancy, only below pancreatic carcinoma and lung cancer [14]. Studies show that glioma has the characteristics of high incidence, high recurrence rate, high fatality rate and low cure rate. The median survival time of malignant glioma patients (III and IV by WHO classification) is less than 18 months, with a 1-year survival rate of about 30% and 5-year survival rate of less than 5% [14-16]. The surgical treatment is still the main method for glioma, combined with local radiotherapy, chemotherapy and immunotherapy in present. The therapeutic effect is poor due to its unclear margin, remained tumor tissues after surgery, drug resistance of tumor and non-sensitivity to radiotherapy, and the mean mortality time is still less than 1 year [17,18]. In recent years, many advanced diagnosis and treatment techniques were used in the clinic as science advanced. Although many therapies for glioma have been used in the clinic, and major breakthroughs have been made on the molecule biology mechanism of its development in the last 20 years, the overall therapeutic effect is not obviously improved. It’s urgently required to find a new efficient therapy [5,17,18]. As the malignant gliomas show focal growth and seldom exhibit metastasis, it is of important significance to find a new therapy that can take a real-time imaging during operation for a more exact resection, and kill the glioma cells with hyperthermia during or after the operation without consideration of chemosensitivity and radiosensitization.
Thermotherapy is a newly developed therapy for tumor in recent years. Due to their imperfect structure and longer heat dissipation than normal tissues, the temperature of the tumor tissues is 5-100°C higher than normal tissues when heated by various kinds of heat sources. As a result, the malignant tumor cells are sensitive to hyperthermia and can be killed by hyperthermia while the normal tissues are unhurt [19-21]. Research has confirmed that the glioma cells are sensitive to temperature [22,23]: The center of tumor can be carbonized, and the peripheral tumor cells were swollen, necrotic or liquefied, and the residual cells were dying when the local temperature reach 43°C-44°C. Hyperthermia not only can cause transposed AIF of glioma cells and suppress DNA replication, transcription, synthesis and repair thus inhibiting glioma cell growth, but also can have a synergistic effect with other therapies and improve the anti-cancer effect. The sources of heat treatments can be laser, ultrasound, microwave, and electro-thermal therapy. It has special advantages compared with other therapies: it can kill the residual tumor cells after surgery and avoid the problem of drug-resistance and sensitivity to radiotherapy and chemotherapy. Clinical studies showed that the laser heat treatment and electro-thermal therapy can improve the median survival and overall survival of recurrent malignant glioma [24,25], but at the same time there are still many shortcomings difficult to solve: a. the treatment is untargeted. Both tumor cells and normal cells are destroyed in the laser and electrothermal ways. And the quality of patient’s life must be decreased if a large number of normal cells are killed. b. It requires high energy density. In the course of treatment, tens to hundreds of watt of energy is needed. c. The treatment is invasive. The tumor must be directly exposed or be inserted into center with optical fibers. d. The temperature and range of treatment is not easy to control, and special equipment was required to guide and monitor. Therefore, how to kill tumor cells selectively and targeted with hyperthermia and left normal cells unhurt is the difficult problem in current study.
In recent years, researches on photothermolysis of nano materials had make it possible to solve the problem. Photothermal ablation therapy based on nano-metallic materials has begun to be applied to the cancer treatment. The fundamentals of it is destroying the tumor cells with the hyperthermia induced by photothermal conversion under the laser irradiation, in which the strong light interception on the sites of cancer cells and high efficiency of photothermal conversion are key to the success of PTA [5]. Rather than the precious metals and other types of nano-material, graphene is hailed as the most potential carbon material because of its unique electrical properties, optical properties and thermal capacity. GO contains abundant functional groups like hydroxyl, carboxyl and epoxy and has unique optics, thermal and hydrophilic characteristics [8,9,12]. Through esterification or amide reaction of its active carboxylic groups on the surface, GO can be connected with several small organic molecules, polymers, biological molecules and other bioactive molecules to improve its biological compatibility [9,10]. With the low cytotoxicity and cost, GO may become a new nano material with biological compatibility widely used in biomedical field [10-12].
In our study, transferrin (Tf) and fluorescein isothiocyanate (FITC) were connected with GO while PLL-G3 act as a joint molecule to make functionalized nano-GO-Tf-FITC particles. We observed the targeted fluorescence imaging and photothermy of the particles for glioma U251 cells and provide basis for a novel therapy for glioma. The new therapy can turn difficulty to easy without consideration of chemosensitivity and radiosensitization. Meanwhile, this new therapy can not only take a real-time imaging during operation for a more exact resection, but also kill the glioma cells with hyperthermia during or after operation.
Tf is a member of the Iron-binding-protein family. Tf is safe and non-poisonous, and it distributes in blood vessels of human abundantly and helps plasma glycoprotein transfer iron ions to normal cells. Tf has been used as a drug carrier in the targeted therapy of tumors as the tumor cells need more iron ions than normal ones. The TfR (Transferrin receptor) is II-cross-membrane glycoprotein with a molecule weight of 90 kD. It is found currently that the two types of transferrin receptors TfR1 and TfR2 can combined with Tf and mediate the absorption of iron. They are essential proteins in iron intake and cell proliferation regulation [26,27]. TfR is over-expressed on surfaces of many kinds of malignant tumor cells including glioma, but most normal cells have a low Tf content. Therefore, TfR has been considered to be an ideal therapeutic target [28,29]. The antitumor drugs connected with Tf or TfR antibody can combine with the TfRs on the surface of tumor cells. On one hand, the antitumor drugs can block the function of TfR to directly kill the tumor cells. On the other hand, they can get inside the tumor cells through endocytosis of vesicles so as to kill the tumor cells more accurately and efficiently and overcome the drug resistance induced by P-glycoprotein-mediated drug efflux [30]. In addition, the expression of TfR in tumor cells is more stable than that of other tumor specific antigen. They are seldom modified, covered or lost. And they are endocytosis receptors that can mediate the process of transferring antibodies or substance carried by its ligand protein into cell and use TfR to bind with its specific ligands. It has been proved that Tf-mediated transcytosis can go through the Blood Brain Barrier (BBB) for transport. The nano-carrier coupling with Tf has a potential for targeted therapy of glioma, and TfR is considered to be a potential target for molecule-based therapy [28,31].
There are fewer data of preparation of functionalized nano-GO, which have an observed effect of target imaging and photothermal therapy on the brain glioma U 251 cells. In our study, alkyl was used to modify the synthesis of PLLD-G3 to modify GO, thus increasing the histocompatibility and stability of GO. The synthetic GO maintained stable dispersed phase after standing at the room temperature for 3 months. And then the preparation of functionalized GO carrier, nano-GO-Tf-FITC was achieved by connecting FITC and Tf with GO with the joint molecule PLLD-G3. FITC (fluorescein isothiocyanate) is the most widely used fluorescein currently with the maximum absorption wavelength of 490 to 495 nm and the maximum emission wavelength of 520 to 530 nm. The fluorescence of FITC is bright yellow-green to which human eye is sensitive. The nano-GO-Tf-FITC particles prepared in this way can have a targeted combination with the glioma cells and make the tumor cells exhibit yellow-green fluorescence under the fluorescent microscope. The results showed that carrier nano-GO-Tf-FITC exhibited Kelly fluorescence under the fluorescent microscope, while the control group without the target molecule nano-GO-FITC was negative. These results indicated that after the nano-GO-Tf-FITC particles were combined with U251 cells successfully, yellow-green fluorescence was showed under excitation of near-infrared light of the fluorescent microscope, nano-GO-FITC without the target molecule Tf did not show fluorescent imaging, suggesting nano-GO-Tf-FITC linking with the targeted molecule Tf successfully combined with U251 cells while nano-GO-Tf-FITC which was not linked with the targeted molecule Tf did not combine with U251 cells Therefore, the results confirmed that nano-GO-Tf-FITC had the function of targeting fluorescent imaging of brain glioma U251 cells and this new functionalized nano-GO-Tf-FITC had good biocompatibility and low cytotoxicity. The particles not only showed high affinity to U251 cells, but also could significantly kill the tumor cells with targeted photothermy under an 808nm near infrared laser exposure.
Conclusions
A new functionalized nano-GO-Tf-FITC particle was successfully prepared with Tf as a targeted ligand and FITC which is soluble in water and has a strong binding force with protein as a fluorescence indicator based on the special property of photothermal conversion of GO. The particles could have a targeted combination with glioma cells for fluorescent imaging of tumor cells and significantly kill the tumor cells with targeted photothermy under an 808nm near infrared laser exposure, thus laying a foundation for further researches on the glioma U251 cells targeted fluorescence imaging and photothermy.
Disclosure of conflict of interest
None.
References
- 1.Moliterno JA, Patel TR, Piepmeier JM. Neurosurgical approach. Cancer J. 2012;18:20–25. doi: 10.1097/PPO.0b013e3183243f6e3. [DOI] [PubMed] [Google Scholar]
- 2.Vranic A. New developments in surgery of malignant gliomas. Radiol Oncol. 2011;45:159–165. doi: 10.2478/v10019-011-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izumoto S, Tsuboi A, Oka Y, Suzuki T, Hashiba T, Kagawa N, Hashimoto N, Maruno M, Elisseeva OA, Shirakata T, Kawakami M, Oji Y, Nishida S, Ohno S, Kawase I, Hatazawa J, Nakatsuka S, Aozasa K, Morita S, Sakamoto J, Sugiyama H, Yoshimine T. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 4.Yang DP, Cui DX. Advances and prospects of gold nanorods. Chem Asian J. 2008;3:2010–2022. doi: 10.1002/asia.200800195. [DOI] [PubMed] [Google Scholar]
- 5.Rozanova N, Zhang J. Photothermal ablation therapy for cancer based on metal nanostructures. Science in China Series B: Chemistry. 2009;52:1559–1575. [Google Scholar]
- 6.El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–135. doi: 10.1016/j.canlet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 7.Van de Broek B, Devoogdt N, D’Hollander A, Gijs HL, Jans K, Lagae L, Muyldermans S, Maes G, Borghs G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano. 2011;5:4319–4328. doi: 10.1021/nn1023363. [DOI] [PubMed] [Google Scholar]
- 8.Yang K, Zhang S, Zhang G, Sun X, Lee ST, Liu Z. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10:3318–3323. doi: 10.1021/nl100996u. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Jaiswal M, Lin M, Saha S, Ozyilmaz B, Loh KP. Electronic properties of nanodiamond decorated graphene. ACS Nano. 2012;6:1018–1025. doi: 10.1021/nn204362p. [DOI] [PubMed] [Google Scholar]
- 10.Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–191. doi: 10.1038/nmat1849. [DOI] [PubMed] [Google Scholar]
- 11.Novoselov KS, Jiang D, Schedin F, Booth TJ, Khotkevich VV, Morozov SV, Geim AK. Two-dimensional atomic crystals. Proc Natl Acad Sci U S A. 2005;102:10451–10453. doi: 10.1073/pnas.0502848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahoo NG, Bao H, Pan Y, Pal M, Kakran M, Cheng HK, Li L, Tan LP. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: a comparative study. Chem Commun (Camb) 2011;47:5235–5237. doi: 10.1039/c1cc00075f. [DOI] [PubMed] [Google Scholar]
- 13.Hummers WS Jr, Offeman RE. Preparation of graphitic oxide. Journal of The American Chemical Society. 1958;80:1339–1339. [Google Scholar]
- 14.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 15.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 16.Sijben AE, McIntyre JB, Roldan GB, Easaw JC, Yan E, Forsyth PA, Parney IF, Magliocco AM, Bernsen H, Cairncross JG. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89:97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 17.Kyritsis AP, Levin VA. An algorithm for chemotherapy treatment of recurrent glioma patients after temozolomide failure in the general oncology setting. Cancer Chemother Pharmacol. 2011;67:971–983. doi: 10.1007/s00280-011-1617-9. [DOI] [PubMed] [Google Scholar]
- 18.Alavi JB, Eck SL. Gene therapy for high grade gliomas. Expert Opin Biol Ther. 2001;1:239–252. doi: 10.1517/14712598.1.2.239. [DOI] [PubMed] [Google Scholar]
- 19.Togni P, Vrba J, Vannucci L. Microwave applicator for hyperthermia treatment on in vivo melanoma model. Med Biol Eng Comput. 2010;48:285–292. doi: 10.1007/s11517-009-0563-8. [DOI] [PubMed] [Google Scholar]
- 20.Schwerdt A, Zintchenko A, Concia M, Roesen N, Fisher K, Lindner LH, Issels R, Wagner E, Ogris M. Hyperthermia-induced targeting of thermosensitive gene carriers to tumors. Hum Gene Ther. 2008;19:1283–1292. doi: 10.1089/hum.2008.064. [DOI] [PubMed] [Google Scholar]
- 21.Senthil M, Harrison LE. Simultaneous bicavitary hyperthermic chemoperfusion in the management of pseudomyxoma peritonei with synchronous pleural extension. Arch Surg. 2009;144:970–972. doi: 10.1001/archsurg.2009.166. [DOI] [PubMed] [Google Scholar]
- 22.Fukami T, Nakasu S, Baba K, Nakajima M, Matsuda M. Hyperthermia induces translocation of apoptosis-inducing factor (AIF) and apoptosis in human glioma cell lines. J Neurooncol. 2004;70:319–331. doi: 10.1007/s11060-004-9168-0. [DOI] [PubMed] [Google Scholar]
- 23.van Landeghem FK, Maier-Hauff K, Jordan A, Hoffmann KT, Gneveckow U, Scholz R, Thiesen B, Bruck W, von Deimling A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Borkamo ED, Fluge O, Mella O, Akslen LA, Bruland O, Dahl O. Hyperthermia improves the antitumour effect of metronomic cyclophosphamide in a rat transplantable brain tumour. Radiother Oncol. 2008;86:435–442. doi: 10.1016/j.radonc.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Fiorentini G, Giovanis P, Rossi S, Dentico P, Paola R, Turrisi G, Bernardeschi P. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. In Vivo. 2006;20:721–724. [PubMed] [Google Scholar]
- 26.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Mastroberardino PG, Hoffman EK, Horowitz MP, Betarbet R, Taylor G, Cheng D, Na HM, Gutekunst CA, Gearing M, Trojanowski JQ, Anderson M, Chu CT, Peng J, Greenamyre JT. A novel transferrin/TfR2-mediated mitochondrial iron transport system is disrupted in Parkinson’s disease. Neurobiol Dis. 2009;34:417–431. doi: 10.1016/j.nbd.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 29.Calvo P, Gouritin B, Chacun H, Desmaele D, D’Angelo J, Noel JP, Georgin D, Fattal E, Andreux JP, Couvreur P. Long-circulating PEGylated polycyanoacrylate nanoparticles as new drug carrier for brain delivery. Pharm Res. 2001;18:1157–1166. doi: 10.1023/a:1010931127745. [DOI] [PubMed] [Google Scholar]
- 30.Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012;1820:291–317. doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang J, Paillard A, Passirani C, Morille M, Benoit JP, Betbeder D, Garcion E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm Res. 2012;29:1495–1505. doi: 10.1007/s11095-011-0624-1. [DOI] [PubMed] [Google Scholar]